Abstract
Background
Although body mass index (BMI) is the most widely accepted parameter for defining obesity, recent studies have indicated a unique set of patients who exhibit normal BMI and excess body fat (BF), which is termed as normal weight obesity (NWO). Increased BF is an established risk factor for atherosclerosis. However, it is unclear whether NWO subjects already have a higher degree of vascular inflammation compared to normal weight lean (NWL) subjects; moreover, the association of BF with vascular inflammation in normal weight subjects is largely unknown.
Methods
NWO and NWL subjects (n = 82 in each group) without any history of significant vascular disease were identified from a 3-year database of consecutively recruited patients undergoing 18 F-fluorodeoxyglucose positron emission tomography/computed tomography (18 F-FDG-PET/CT) at a self-referred Healthcare Promotion Program. The degree of subclinical vascular inflammation was evaluated using the mean and maximum target-to-background ratios (TBRmean and TBRmax) of the carotid artery, which were measured by 18 F-FDG-PET/CT (a noninvasive tool for assessing vascular inflammation).
Results
We found that metabolically dysregulation was greater in NWO subjects than in NWL subjects, with a significantly higher blood pressure, higher fasting glucose level, and worse lipid profile. Moreover, NWO subjects exhibited higher TBR than NWL subjects (TBRmean: 1.33 ± 0.16 versus 1.45 ± 0.19, p < 0.001; TBRmax: 1.52 ± 0.23 versus 1.67 ± 0.25, p < 0.001). TBR was significantly associated with total BF (TBRmean: r = 0.267, p = 0.001; TBRmax: r = 0.289, p < 0.001), age (TBRmean: r = 0.170, p = 0.029; TBRmax: r = 0.165, p = 0.035), BMI (TBRmean: r = 0.184, p = 0.018; TBRmax: r = 0.206, p = 0.008), and fasting glucose level (TBRmean: r = 0.157, p = 0.044; TBRmax: r = 0.182, p = 0.020). In multiple linear regression analysis, BF was an independent determinant of TBRmean and TBRmax, after adjusting for age, BMI, and fasting glucose level (TBRmean: regression coefficient = 0.020, p = 0.008; TBRmax: regression coefficient = 0.028, p = 0.005). Compared to NWL, NWO was also independently associated with elevated TBRmax values, after adjusting for confounding factors (odds ratio = 2.887, 95% confidence interval 1.206–6.914, p = 0.017).
Conclusions
NWO is associated with a higher degree of subclinical vascular inflammation, of which BF is a major contributing factor. These results warrant investigations for subclinical atherosclerosis in NWO patients.
Keywords: Normal weight obesity, Fat, Atherosclerosis, 18 F-FDG-PET/CT, Vascular inflammation
Background
According to National Health and Nutrition Examination Survey (NHANES) and Korean National Health and Nutrition Examination Survey (KNHANES) data, the prevalence of obesity has increased to 30% in USA [1] and 30.9% in Korea [2]. Obesity is a well-known cause of cardiovascular disease (CVD) and all-cause mortality [3-5]. Classically, obesity has been defined as an excess of body weight compared to the height, which has been simplified as a single value, termed as the body mass index (BMI) [6].
The limitation of BMI is that it can misclassify subjects who have lower lean mass but higher fat mass as non-obese [7]. This inherent limitation of BMI in the accurate diagnosis of obesity is observed to a greater extent among Asians and the elderly, who commonly have lower lean body mass [8-10]. Consequently, the new concept of normal weight obesity (NWO) had been proposed. NWO subjects have normal BMI and an elevated body fat percentage (BF%) [11], and may be at a high risk for cardiometabolic disease [12-16]. Considering that fat itself is a major contributing factor on CVD in obese subjects [5], methods to estimate the risk of CVD associated with BF% or absolute fat mass can be accurate and helpful for assessing early cardiometabolic disease risk in the NWO population.
Inflammation is a major driving factor of atherosclerosis progression [17] and a major determinant of plaque rupture [18], the results of which may be devastating. Therefore, accurate assessment of vascular inflammation rather than the extent of luminal stenosis may be more essential for preventing CVD [19]. The uptake of 18 F-fluorodeoxyglucose (18 F-FDG) in the vessel correlates very well with the degree of macrophage infiltration [20], because the macrophages preferentially utilize glucose, particularly under anaerobic conditions [21,22]. Based on these findings, the usefulness of 18 F-FDG positron emission tomography/computed tomography (18 F-FDG-PET/CT) has been demonstrated when evaluating vascular inflammation [23-26]. Therefore, in the present study, we aimed to determine the degree of subclinical vascular inflammation in NWO subjects and the contributing factors associated with the inflammation. Hence, we used a large database consecutively recruited from the patients undergoing 18 F-FDG-PET/CT from a self-referred healthcare promotion program to identify patients without any history of clinical cerebrovascular disease.
Methods
Study design and population
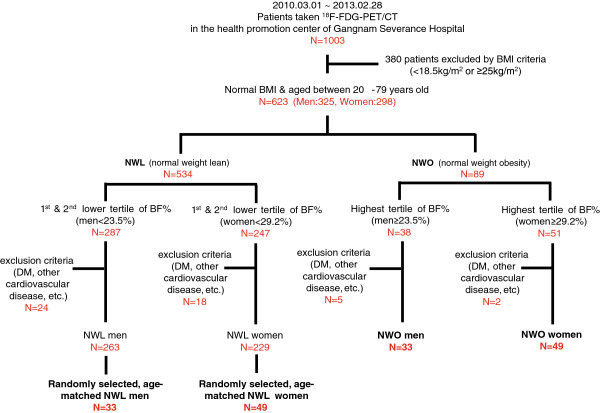
This cross-sectional study was designed to evaluate the effect of BF on vascular inflammation of the carotid arteries in subjects with a normal BMI. Asymptomatic subjects self-referred for 18 F-FDG-PET/CT examination from March 2010 to February 2013 in a health checkup program at the Health Promotion Center, Gangnam Severance Hospital, were consecutively registered (N = 1003; Men: n = 602; Women: n = 401). All subjects underwent a medical examination, including clinical history, physical examination, blood analysis, abdominal ultrasound, and 18 F-FDG-PET/CT.
We divided the whole population by gender-specific BF% tertiles and defined NWO subjects as those with a normal BMI (18.5–24.9 kg/m2) and the highest tertile of BF% (Men, ≥23.5%, Women, ≥29.2%) in line with a previous study15, thus yielding 89 NWO subjects (Men: N = 38; Women: N = 51). Subjects who were previously diagnosed with diabetes mellitus and/or taking anti-diabetic medication were excluded. Moreover, subjects with previously diagnosed malignancy within 5 years of PET/CT examination, chronic inflammatory disease, or other previous disease of the carotid artery were excluded. Finally, 82 NWO subjects were included in the present study. Moreover, 82 control normal weight lean (NWL) subjects (Men, <23.5%; Women, <29.2%)—i.e., normal BMI subjects who were in the lower two tertiles for gender-specific BF%—were randomly selected from sex-matched subjects using random numbers uniformly distributed between 0 and 1 (Figure 1). In addition, we constructed another database comprising 63 subjects each in the NWL and NWO groups who were matched for sex and BMI values using greedy matching techniques. The exclusion criteria of the NWL subjects were identical to those for the NWO subjects. The protocol of this study was approved by the institutional review board of Gangnam Severance Hospital, and all subjects provided written informed consent for the 18 F-FDG-PET/CT examination.
Figure 1.
Schematic diagram and the process of the registration of subjects. 18 F-FDG-PET/CT, 18 F-fludeoxyglucose positron emission tomography/computed tomography; BMI, body mass index; NWL, normal weight lean; NWO, normal weight obesity; BF, body fat; DM, diabetes mellitus.
Questionnaire
The current and past medical history of disease, medication, presence of CVD risk factors (smoking, alcohol, and family history of premature CVD), and other information on medico-social history were self-reported via a routine questionnaire before the health checkup.
Anthropometric measurement
All subjects were requested to fast for more than 8 hours before all biochemical and anthropometric examinations. Body weight was measured by electronic load to the nearest 0.01 kg, with the parents wearing a light gown without shoes for the health examination. Height was measured to the nearest 0.1 cm. BMI was calculated as the weight in kilograms divided by the square of height in meters (kg/m2). The BF%, total body fat, visceral fat, subcutaneous fat, and muscle mass were measured using the bioelectrical impedance (BI) body composition analyzer X-scan plusII (Jawon Medical, Seoul, Korea) [27]. In brief, the tetra-polar electrode method with 8 touch electrodes placed on both hands and feet facilitated the estimation of the BI value in the abdominal area, which was evaluated by the current intercrossing between each hand and foot. By using the waist-to-hip ratio, body weight, height, age, sex, and the BI value of the abdominal area, the Jawon Medical Company created a formula specific for Korean individuals to calculate the visceral fat amount. The subcutaneous fat amount was estimated by excluding the visceral fat from the total fat amount [28,29]. Systolic and diastolic blood pressure was measured using BP203RV II (Colin Corp., Aichi, Japan) after resting for more than 15 min in the sitting position. The presence of fatty liver was assessed using a Philips IU-22 Ultrasound system (Philips Healthcare, Andover, MA) by an expert radiologist.
Biochemical measurement
Glucose was measured by the glucose oxidase method using a 747 Automatic Analyzer (Hitachi, Tokyo, Japan). Glycated hemoglobin A1c (HbA1c) level was measured by high-performance liquid chromatography (Cobas Integra 800, Roche, Mannheim, Germany). The levels of total cholesterol, high-density lipoprotein cholesterol (HDL-cholesterol), triglyceride, C-reactive protein (CRP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured by an enzymatic colorimetric method (Daiichi, Hitachi). Low-density lipoprotein cholesterol (LDL-cholesterol) content was calculated according to the Friedewald formula.
18 F-FDG-PET/CT imaging and image analysis
All patients fasted at least 8 h before the injection of 18 F-FDG, after ensuring that serum glucose levels at this time point did not exceed 150 mg/dL. The injection dose of 18 F-FDG was 5.18 MBq/kg (0.14 mCi/kg). 18 F-FDG-PET images were obtained using a standard PET/CT scanner (Biograph TruePoint 40, Siemens Healthcare, Erlangen, Germany) and all data were acquired in the 3D mode with a 1.5-min scan time per bed. Tomographic PET images were reconstructed by using an iterative method involving ordered subset expectation maximization, under the condition of 2 iterations and 21 subsets. The matrix of reconstructed PET images using this method was 128 × 128, and the slice thickness was 5 mm. Low-dose CT scans for attenuation correction and identification of anatomical landmarks were obtained immediately before the PET scan.
Image analysis was conducted using a dedicated PET/CT workstation (Syngo 2009A, Siemens Healthcare) according to previous reports [20,24-26]. In detail, for exact measurement of the carotid artery and jugular vein, the overall diameter, course, and variation of cervical vasculature were first reviewed using CT images, after which the metabolic activity of the artery and vein was estimated by using PET/CT fusion images prior to region of interest (ROI) measurements. A circular ROI was used in most slices of the carotid arteries, whereas an oval or circular ROI was used for the jugular vein depending on its contour. Each ROI was intended to include the entire wall of the artery and vein. The measurement of the uptake value (SUV) was started from the highest level of the common carotid artery before bifurcation to the external and internal carotid branches (mostly around the level of the hyoid bone), and 10 ROIs were consecutively measured from the inferior level up to the origin of the carotid artery from the aortic arch. Eight ROIs were used to measure the jugular vein SUV, and their locations were similar to those of the carotid artery. The maximal (SUVmax) and mean SUV (SUVmean) were measured in the carotid artery and the jugular vein, respectively, after which the target-to-background ratio (TBR) was calculated using the following equations: Mean TBR (TBRmean) = (mean SUV of carotid artery)/(mean SUV of jugular vein); Maximum TBR (TBRmax) = (maximum SUV of carotid artery)/(mean SUV of jugular vein) [20,24-26].
Statistical analyses
Anthropometric, clinical, and biochemical data are presented as the mean ± standard deviation (SD) for continuous variables and absolute numbers (percentage) for categorical variables. An independent two-sample t-test or paired sample t-test was used to evaluate the differences in the continuous variables between NWL and NWO subjects after testing each variable for normality with the Shapiro-Wilk test. The χ2 test or Fisher’s exact test was used to compare categorical variables between the NWL and NWO groups, as appropriate. The degree of a relationship between putative CVD risk factors and TBRmean or TBRmax was expressed by Pearson’s correlation coefficient (r). Multiple stepwise linear regression analysis was used to determine the independent predictors of TBRmean and TBRmax. Logistic regression models were analyzed to evaluate the odds ratio (OR) for TBRmax elevation in the NWO group compared to the NWL group. All statistical analyses were performed using adequate software (SPSS version 20.0, Chicago, IL). A p value of <0.05 was considered to be statistically significant.
Results
Clinical and biochemical characteristics of the study population
The mean age of the subjects was 54 years, and 18% of the subjects had hypertension. The NWO subjects had a higher BMI, larger waist-to-hip ratio, and greater amount of visceral and subcutaneous fat. There was no difference in total muscle mass between the groups. NWO subjects had significantly higher systolic and diastolic BP than NWL subjects. Although there was no difference in HbA1c levels, the fasting plasma glucose level was significantly higher in the NWO group. In addition, NWO subjects had higher serum triglyceride and lower HDL-cholesterol levels. No differences in current anti-hypertensive drug, statin, and anti-platelet use were noted between the groups. Detailed comparisons of each parameter are summarized in Table 1. Moreover, we analyzed these clinical and biochemical parameters after dividing the study population based on gender. The difference in the metabolic profile tended to be significant between the female subjects in the NWO and NWL groups, whereas certain parameters such as blood pressure and CRP were not significantly different in the male subjects between the NWO and NWL groups (Table 2).
Table 1.
Clinical and biochemical characteristics of the study subjects
| Total (N = 164) | NWL (N = 82) | NWO (N = 82) | p-value | |
|---|---|---|---|---|
| Age (years) |
54.1 ± 9.4 |
52.9 ± 9.3 |
55.3 ± 9.4 |
0.101 |
| Gender (N, (%)) |
66 (40) |
33 (40) |
33 (40) |
>0.999 |
| Body mass index (kg/m2) |
22.7 ± 1.7 |
21.7 ± 1.6 |
23.8 ± 0.9 |
<0.001 |
| Waist to hip ratio |
0.87 ± 0.08 |
0.84 ± 0.08 |
0.89 ± 0.07 |
<0.001 |
| Body fat (%) |
27.1 ± 5.0 |
24.1 ± 4.5 |
30.1 ± 3.3 |
<0.001 |
| Body fat (kg) |
16.2 ± 3.0 |
14.0 ± 2.6 |
18.4 ± 1.7 |
<0.001 |
| Visceral fat (kg) |
2.0 ± 0.6 |
1.6 ± 0.4 |
2.5 ± 0.4 |
<0.001 |
| Subcutaneous fat (kg) |
14.1 ± 2.7 |
12.3 ± 2.3 |
15.9 ± 1.6 |
<0.001 |
| Muscle mass (kg) |
40.5 ± 6.5 |
41.2 ± 6.9 |
39.7 ± 6.1 |
0.147 |
| Systolic BP (mmHg) |
121.9 ± 14.9 |
118.7 ± 14.7 |
125.1 ± 14.4 |
0.005 |
| Diastolic BP (mmHg) |
76.3 ± 9.4 |
74.7 ± 9.4 |
78.0 ± 9.1 |
0.022 |
| Fasting plasma glucose (mmol/L) |
5.24 ± 0.66 |
4.98 ± 0.46 |
5.51 ± 0.72 |
<0.001 |
| HbA1c (%) |
5.6 ± 0.5 |
5.5 ± 0.4 |
5.7 ± 0.6 |
0.094 |
| Total cholesterol (mmol/L) |
5.12 ± 0.89 |
5.11 ± 0.90 |
5.13 ± 0.89 |
0.859 |
| Triglyceride (mmol/L) |
1.14 ± 0.57 |
1.01 ± 0.51 |
1.27 ± 0.61 |
0.003 |
| HDL-cholesterol (mmol/L) |
1.37 ± 0.32 |
1.46 ± 0.35 |
1.29 ± 0.261 |
0.001 |
| LDL-cholesterol (mmol/L) |
3.14 ± 0.80 |
3.09 ± 0.80 |
3.19 ± 0.80 |
0.397 |
| CRP (mg/L) |
1.6 ± 3.1 |
1.2 ± 3.2 |
2.0 ± 2.9 |
0.096 |
| AST (IU/L) |
22.4 ± 8.8 |
22.3 ± 10.6 |
22.4 ± 6.7 |
0.916 |
| ALT (IU/L) |
22.9 ± 14.5 |
20.7 ± 11.6 |
25.2 ± 16.7 |
0.049 |
| Fatty Liver (N, (%)) |
53 (32.3) |
14 (17.1) |
39 (47.6) |
<0.001 |
| Antihypertension drug (N, (%)) |
24 (17.1) |
10 (12.2) |
14 (17.1) |
0.377 |
| Statin (N, (%)) |
14 (8.6) |
8 (9.8) |
6 (7.3) |
0.576 |
| Antiplatelet agent (N, (%)) | 15 (9.0) | 7 (8.5) | 8 (9.8) | 0.786 |
NWL, normal weight lean; NWO, normal weight obesity; N, number; BP, blood pressure; HbA1c, glycated hemoglobin A1c; HDL, high density lipoprotein; LDL, low density lipoprotein; AST/ALT, aspartate/alanine aminotransferase; CRP, C-reactive protein. Data are presented as mean ± SD or number (percentages). The p values represent differences between groups determined by an independent two-sample t-test for continuous variables and the χ2 test or the Fisher’s exact test for categorical variables.
Table 2.
Clinical and biochemical characteristics of the study subjects by gender
|
Men |
p |
Women |
p | |||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 66) | NWL (N = 33) | NWO (N = 33) | Total (N = 98) | NWL (N = 49) | NWO (N = 49) | |||
| Age (years) |
56.0 ± 9.1 |
55.5 ± 8.8 |
56.4 ± 9.6 |
0.699 |
52.9 ± 9.5 |
51.2 ± 9.3 |
54.6 ± 9.4 |
0.071 |
| Body mass index (kg/m2) |
23.3 ± 1.3 |
22.4 ± 1.3 |
24.2 ± 0.6 |
<0.001 |
22.3 ± 1.8 |
21.1 ± 1.5 |
23.5 ± 1.0 |
<0.001 |
| Waist to hip ratio |
0.94 ± 0.04 |
0.91 ± 0.04 |
0.97 ± 0. 02 |
<0.001 |
0.82 ± 0.05 |
0.79 ± 0.05 |
0.84 ± 0.02 |
<0.001 |
| Body fat (%) |
23.4 ± 4.1 |
20.2 ± 3.6 |
26.5 ± 1.0 |
<0.001 |
29.6 ± 3.8 |
26.6 ± 2.9 |
32.6 ± 1.4 |
<0.001 |
| Body fat (kg) |
15.5 ± 3.2 |
13.2 ± 2.6 |
17.9 ± 1.5 |
<0.001 |
16.6 ± 3.0 |
14.5 ± 2.5 |
18.7 ± 1.7 |
<0.001 |
| Visceral fat (kg) |
2.3 ± 0.6 |
1.8 ± 0.5 |
2.8 ± 0.2 |
<0.001 |
1.8 ± 0.5 |
1.4 ± 0.4 |
2.3 ± 0.3 |
<0.001 |
| Subcutaneous fat (kg) |
13.1 ± 2.6 |
11.3 ± 2.2 |
15.0 ± 1.3 |
<0.001 |
14.7 ± 2.6 |
13.0 ± 2.2 |
16.5 ± 1.5 |
<0.001 |
| Muscle mass (kg) |
47.0 ± 4.6 |
48.1 ± 4.7 |
45.9 ± 4.2 |
0.047 |
36.1 ± 3.1 |
36.6 ± 3.4 |
35.6 ± 2.8 |
0.122 |
| Systolic BP (mmHg) |
125.0 ± 13.7 |
122.5 ± 13.6 |
127.6 ± 13.4 |
0.134 |
119.8 ± 15.3 |
116.1 ± 14.9 |
123.5 ± 15.0 |
0.017 |
| Diastolic BP (mmHg) |
78.9 ± 8.2 |
77.9 ± 8.3 |
79.9 ± 8.2 |
0.319 |
74.6 ± 9.7 |
72.5 ± 9.6 |
76.7 ± 9.5 |
0.031 |
| Fasting plasma glucose (mmol/L) |
5.43 ± 0.66 |
5.09 ± 0.48 |
5.76 ± 0.66 |
<0.001 |
5.12 ± 0.62 |
4.90 ± 0.42 |
5.33 ± 0.71 |
<0.001 |
| HbA1c (%) |
5.7 ± 0.5 |
5.6 ± 0.4 |
5.8 ± 0.6 |
0.245 |
5.6 ± 0.5 |
5.5 ± 0.5 |
5.6 ± 0.5 |
0.213 |
| Total cholesterol (mmol/L) |
5.03 ± 0.95 |
5.14 ± 1.01 |
4.93 ± 0.91 |
0.364 |
5.19 ± 0.95 |
5.10 ± 0.84 |
5.29 ± 0.86 |
0.283 |
| Triglyceride (mmol/L) |
1.36 ± 0.71 |
0.19 ± 0.68 |
1.53 ± 0.71 |
0.048 |
1.36 ± 0.71 |
1.19 ± 0.68 |
1.53 ± 0.71 |
0.010 |
| HDL-cholesterol (mmol/L) |
1.22 ± 0.26 |
1.31 ± 0.29 |
1.12 ± 0.20 |
0.004 |
1.48 ± 0.31 |
1.56 ± 0.35 |
1.41 ± 0.24 |
0.016 |
| LDL-cholesterol (mmol/L) |
3.14 ± 0.85 |
3.18 ± 0. 89 |
3.11 ± 0.83 |
0.756 |
3.15 ± 0.77 |
0.04 ± 0.75 |
3.26 ± 0.79 |
0.155 |
| CRP (mg/L) |
2.4 ± 4.50 |
1.8 ± 5.1 |
2.9 ± 4.0 |
0.322 |
1.1 ± 1.3 |
0.8 ± 0.7 |
1.4 ± 1.6 |
0.026 |
| AST (IU/L) |
24.9 ± 11.6 |
25.7 ± 14.7 |
24.1 ± 7.5 |
0.593 |
20.6 ± 5.7 |
20.0 ± 5.6 |
21.3 ± 5.9 |
0.271 |
| ALT (IU/L) |
27.9 ± 15.0 |
24.7 ± 12.2 |
31.1 ± 16.9 |
0.083 |
19.6 ± 13.2 |
18.0 ± 10.5 |
21.2 ± 15.4 |
0.239 |
| Fatty Liver (N,%) |
29 (44.0) |
7 (21.2) |
22 (66.7) |
<0.001 |
24 (24.5) |
7 (14.7) |
17 (34.7) |
0.019 |
| Antihypertension drug (N, (%)) |
15 (22.7) |
6 (18.2) |
9 (27.3) |
0.378 |
9 (9.2) |
4 (8.1) |
5 (10.2) |
>0.999 |
| Statin (N,%) |
7 (10.6) |
2 (6.1) |
5 (15.2) |
0.427 |
7 (7.1) |
6 (12.2) |
1 (2.0) |
0.111 |
| Antiplatelet agent (N,%) | 7 (10.6) | 1 (21.2) | 6 (12.1) | 0.105 | 8 (8.1) | 6 (12.2) | 2 (4.1) | 0.268 |
NWL, normal weight lean; NWO, normal weight obesity; N, number; BP, blood pressure; HbA1c, glycated hemoglobin A1c; HDL, high density lipoprotein; LDL, low density lipoprotein; AST/ALT, aspartate/alanine aminotransferase; CRP, C-reactive protein. Data are presented as mean ± SD or number (percentages). The p values represent differences between groups determined by an independent two-sample t-test for continuous variables and the χ2 test or the Fisher’s exact test for categorical variables.
Evaluation of vascular inflammation by 18 F-FDG-PET/CT between NWL and NWO subjects
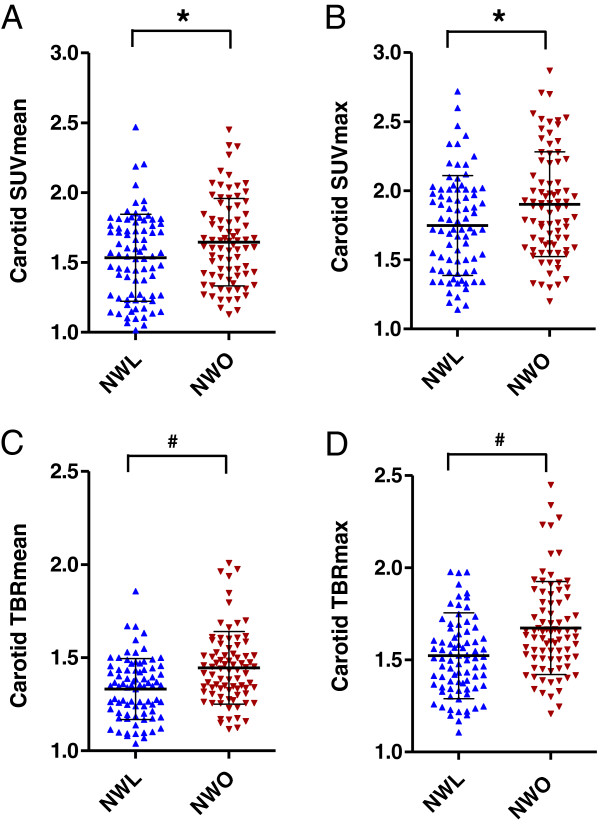
Compared to the NWL group, the NWO group had a significantly higher SUVmean and SUVmax at the carotid artery (SUVmean: 1.53 ± 0.31 versus 1.64 ± 0.31, p = 0.024; SUVmean: 1.75 ± 0.36 versus 1.90 ± 0.38, p = 0.009) (Table 3, Figures 2A and B, Figure 3). However, there was no difference in the SUVmean at the jugular vein between the two groups. TBRmean and TBRmax—the corrected values of carotid SUVmean and SUVmax divided by the SUVmean of the jugular vein in each individual, respectively—were also higher in the NWO population (TBRmean: 1.33 ± 0.16 versus 1.45 ± 0.19, p < 0.001; TBRmax: 1.52 ± 0.23 versus 1.67 ± 0.25, p < 0.001) (Table 3, Figures 2C and D). As the degree of vascular inflammation may be affected by the coexistence of hypertension or statin use, we also analyzed the degree of carotid inflammation after excluding subjects with these factors. However, the difference was consistently significant even in patients without anti-hypertensive or statin use (TBRmean: 1.33 ± 0.16 versus 1.44 ± 0.21, p < 0.001; TBRmax: 1.52 ± 0.24 versus 1.67 ± 0.03, p < 0.001) (NWL: n = 67; NWO: n = 64). We also analyzed the difference in vascular inflammation between NWL and NWO subjects from the total population who were equally matched by sex and BMI. We observed a significant difference in the degree of carotid inflammation after matching for BMI (TBRmean: 1.24 ± 0.25 versus 1.44 ± 0.21, p < 0.001; TBRmax: 1.42 ± 0.29 versus 1.68 ± 0.32, p < 0.001) (n = 63 in each group). Moreover, we analyzed the vascular inflammation according to sex. We observed a significant difference in vascular inflammation in male subjects between the NWL and NWO groups (TBRmean: 1.33 ± 0.15 versus 1.47 ± 0.16, p = 0.001; TBRmax: 1.50 ± 0.18 versus 1.71 ± 0.22, p < 0.001) and in female subjects between the NWL and NWO groups (TBRmean: 1.33 ± 0.20 versus 1.43 ± 0.22, p = 0.015; TBRmax: 1.54 ± 0.26 versus 1.65 ± 0.27, p = 0.038) (Table 4).
Table 3.
Comparison of 18 F-FDG-PET/CT results between the NWL and NWO subjects
| Total (N = 164) | NWL (N = 82) | NWO (N = 82) | p-value | |
|---|---|---|---|---|
| SUVmean, jugular vein |
1.15 ± 0.22 |
1.16 ± 0.23 |
1.15 ± 0.22 |
0.745 |
| SUVmean, carotid artery |
1.59 ± 0.32 |
1.53 ± 0.31 |
1.64 ± 0.31 |
0.024 |
| SUVmax, carotid artery |
1.83 ± 0.38 |
1.75 ± 0.36 |
1.90 ± 0.38 |
0.009 |
| TBRmean, carotid artery |
1.39 ± 0.19 |
1.33 ± 0.16 |
1.45 ± 0.19 |
<0.001 |
| TBRmax, carotid artery | 1.60 ± 0.25 | 1.52 ± 0.23 | 1.67 ± 0.25 | <0.001 |
NWL, normal weight lean; NWO, normal weight obesity; N, number; SUVmean/max, mean/maximum standardized uptake value; TBRmean/max, mean/maximum target-to-background ratio. Data are presented as mean ± SD. The p values represent differences between groups determined by the independent two-sample t-test.
Figure 2.
Scatter plot and comparison of the 18 F-FDG-PET/CT results between NWL and NWO subjects. A scatter plot of the 18 F-FDG-PET/CT results was drawn, and a comparison between the NWL and NWO subjects was performed. (A) Carotid mean SUV, (B) carotid maximum SUV, (C) carotid mean TBR, and (D) carotid maximum TBR. 18 F-FDG-PET/CT, 18 F-fludeoxyglucose positron emission tomography/computed tomography; SUV, standardized uptake value; TBR, target-to-background ratio; NWL, normal weight lean; NWO, normal weight obesity. Horizontal lines in the graph indicate means, whereas perpendicular lines denote the standard deviation. *p < 0.05 versus NWL. #p < 0.001 versus NWL.
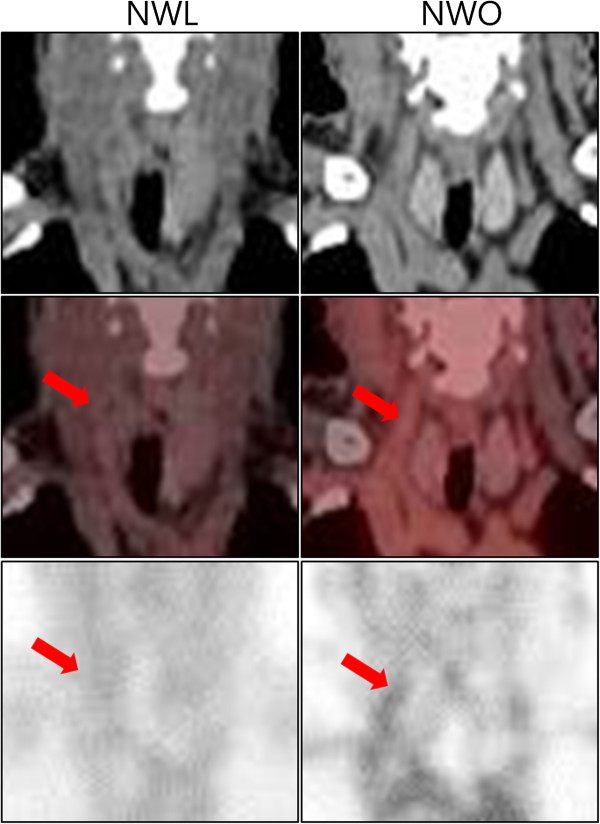
Figure 3.
Representative figures indicating the SUV uptake at the carotid artery in NWL and NWO subjects. Coronal image of 18 F-FDG-PET/CT around the carotid artery is presented as an unenhanced CT image, a fusion image of CT and PET, and a maximum intensity projection (MIP) image, from the upper panel to the lower panel. The red arrow indicates the carotid artery. 18 F-FDG-PET/CT, 18 F-fludeoxyglucose positron emission tomography/computed tomography; SUV, standardized uptake value; NWL, normal weight lean; NWO, normal weight obesity.
Table 4.
Comparison of 18 F-FDG-PET/CT results between the NWL and NWO subjects by gender
| |
Men |
p |
Women |
P | ||||
|---|---|---|---|---|---|---|---|---|
| |
Total |
NWL |
NWO |
Total |
NWL |
NWO |
||
| (N = 66) | (N = 33) | (N = 33) | (N = 98) | (N = 49) | (N = 49) | |||
| SUVmean, jugular vein |
1.15 ± 0.19 |
1.18 ± 1.19 |
1.12 ± 0.19 |
0.267 |
1.15 ± 0.24 |
1.15 ± 0.25 |
1.16 ± 0.24 |
0.736 |
| SUVmean, carotid artery |
1.60 ± 0.26 |
1.56 ± 0.28 |
1.63 ± 0.25 |
0.232 |
1.59 ± 0.35 |
1.52 ± 0.33 |
1.65 ± 0.35 |
0.058 |
| SUVmax, carotid artery |
1.83 ± 0.33 |
1.76 ± 0.31 |
1.90 ± 0.33 |
0.067 |
1.82 ± 0.41 |
1.74 ± 0.40 |
1.90 ± 0.41 |
0.057 |
| TBRmean, carotid artery |
1.40 ± 0.17 |
1.33 ± 0.15 |
1.47 ± 0.16 |
0.001 |
1.38 ± 0.22 |
1.33 ± 0.20 |
1.43 ± 0.22 |
0.015 |
| TBRmax, carotid artery | 1.60 ± 0.23 | 1.50 ± 0.18 | 1.71 ± 0.22 | <0.001 | 1.59 ± 0.27 | 1.54 ± 0.26 | 1.65 ± 0.27 | 0.038 |
NWL, normal weight lean; NWO, normal weight obesity; N, number; SUVmean/max, mean/maximum standardized uptake value; TBRmean/max, mean/maximum target-to-background ratio. Data are presented as mean ± SD. The p values represent differences between groups determined by the independent two-sample t-test.
Correlation between the degree of vascular inflammation and various CVD risk factors
The correlation between vascular inflammation and various CVD risk factors was analyzed. Age, BMI, and the fasting plasma glucose level were significantly associated with both TBRmean and TBRmax (Table 5 and Figures 4A-C, 4E-G). We also analyzed the correlation between vascular inflammation and fat mass. Total, visceral, and subcutaneous fat mass were all significantly correlated with the degree of vascular inflammation; this degree of correlation was better than that observed for the traditional CVD risk factors of age, BMI, and fasting plasma glucose level (Table 5 and Figure 4D and H). However, none of the other anthropometric or biochemical characteristics correlated with TBRmean or TBRmax.
Table 5.
Correlation between the degree of TBR by 18 F-FDG-PET/CT and various CVD risk factors
|
TBRmean |
TBRmax |
|||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Age |
0.170 |
0.029 |
0.165 |
0.035 |
| Body mass index |
0.184 |
0.018 |
0.206 |
0.008 |
| Waist to hip ratio |
0.130 |
0.097 |
0.123 |
0.117 |
| Body fat (%) |
0.169 |
0.030 |
0.200 |
0.010 |
| Body fat (kg) |
0.267 |
0.001 |
0.289 |
<0.0001 |
| Visceral fat (kg) |
0.264 |
0.001 |
0.278 |
<0.0001 |
| Subcutaneous fat (kg) |
0.240 |
0.002 |
0.266 |
0.001 |
| Muscle mass (kg) |
0.056 |
0.476 |
0.034 |
0.668 |
| Systolic BP |
0.083 |
0.292 |
0.030 |
0.702 |
| Diastolic BP |
0.123 |
0.117 |
0.090 |
0.253 |
| FPG |
0.157 |
0.044 |
0.182 |
0.020 |
| HbA1c |
−0.050 |
0.597 |
−0.021 |
0.825 |
| Total cholesterol |
0.064 |
0.417 |
0.082 |
0.296 |
| Triglyceride |
0.057 |
0.467 |
0.070 |
0.372 |
| HDL-Cholesterol |
−0.017 |
0.832 |
−0.016 |
0.843 |
| LDL-Cholesterol |
0.050 |
0.524 |
0.065 |
0.408 |
| CRP |
0.031 |
0.700 |
0.077 |
0.347 |
| ALT |
−0.073 |
0.353 |
−0.075 |
0.342 |
| Fatty liver | 0.122 | 0.119 | 0.103 | 0.189 |
SUVmean/max, mean/maximum standardized uptake value; TBRmean/max, mean/maximum target-to-background ratio; BP, blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; HDL, high density lipoprotein; LDL, low density lipoprotein; AST/ALT, aspartate/alanine aminotransferase; CRP, C-reactive protein. Data are presented as Pearson’s correlation coefficient (r). p < 0.05 was regarded as statistically significant.
Figure 4.
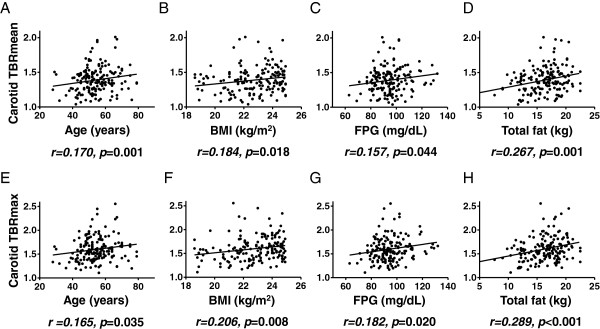
Correlation between carotid TBR, various clinical factors, and body fat mass. A correlation graph was drawn between the carotid (A-D) mean or (E-H) maximum TBR and age, BMI, fasting plasma glucose levels, and total body fat. The degree of relationship between putative CVD risk factors and TBRmean or TBRmax was expressed using Pearson’s correlation coefficient (r). TBR, target-to-background ratio; BMI, body mass index; FPG, fasting plasma glucose. p < 0.05 was regarded as statistically significant.
Independent predictors of vascular inflammation
Univariate linear regression analysis followed by multivariate linear regression analysis was performed to determine the independent predictors of vascular inflammation. For multivariate linear regression analysis, only the total fat among other fat parameters was tested together with other significant parameters because of the multicollinearity. Among the significant parameters identified by univariate linear regression analysis (age, BMI, fasting plasma glucose level, and total fat amount), the total body fat amount was the only significant independent predictor of both TBRmean and TBRmax (TBRmean: regression coefficient = 0.020, p = 0.008; TBRmax: regression coefficient = 0.028, p = 0.005) (Table 6).
Table 6.
Determinants of carotid TBRmean and TBRmax
|
TBRmean |
TBRmax |
|||||
|---|---|---|---|---|---|---|
| Regression coefficient | SE | p-value | Regression coefficient | SE | p-value | |
| Age |
0.003 |
0.002 |
0.088 |
0.003 |
0.002 |
0.124 |
| BMI |
0.014 |
0.014 |
0.339 |
−0.018 |
0.019 |
0.351 |
| FPG |
0.001 |
0.001 |
0.421 |
0.002 |
0.002 |
0.279 |
| Body fat | 0.020 | 0.007 | 0.008 | 0.028 | 0.010 | 0.005 |
TBRmean/max, mean/maximum target-to-background ratio; SE, standard error; BMI, body mass index; FPG, fasting plasma glucose. Data are presented as the regression coefficient and standard error. p < 0.05 was regarded as statistically significant.
Association of NWO with the highest risk of subclinical vascular inflammation
To assess the risk of subclinical atherosclerosis in the subjects between the NWO and NWL groups, we performed multiple logistic regression analysis using a TBRmax value equal to or greater than the highest quartile as the endpoint. After adjusting for basic clinical factors such as age, fasting plasma glucose level, systolic BP, HDL-cholesterol level, LDL-cholesterol level, and triglyceride level, NWO subjects were 3.1-fold more likely to display subclinical vascular inflammation (OR = 3.071, 95% confidence interval (CI) = 1.292–7.297, p = 0.011). Moreover, the NWO subjects were 2.9-fold more likely to exhibit subclinical vascular inflammation, even after adjusting for age, fasting plasma glucose level, systolic BP, HDL-cholesterol level, LDL-cholesterol level, triglyceride level, and all medication history concerning CVD (OR = 2.887, 95% CI = 1.206–6.914, p = 0.017); no other variables significantly contributed to the vascular inflammation, except for NWO in these models (Table 7).
Table 7.
Odds ratio for the highest TBRmax (≥ highest quartile TBRmax)
|
NWL |
NWO |
p-value | |
|---|---|---|---|
| odds ratio (95% CI) | odds ratio (95% CI) | ||
| Model 1 |
1.00 (reference) |
2.384 (1.141–4.982) |
0.021 |
| Model 2 |
1.00 (reference) |
3.071 (1.292–7.297) |
0.011 |
| Model 3 | 1.00 (reference) | 2.887 (1.206–6.914) | 0.017 |
Model 1, not adjusted; Model 2, adjusted for age, fasting plasma glucose level, systolic BP, HDL-cholesterol level, LDL-cholesterol level, and triglyceride level; Model 3, adjusted for age, fasting plasma glucose level, systolic BP, HDL-cholesterol level, LDL-cholesterol level, triglyceride level, antihypertension drug use, statin use, and antiplatelet agent use. NWL, normal weight lean; NWO, normal weight obesity; CI, confidence interval. Data are presented as odds ratios with the NWL group as a reference (1). p < 0.05 was regarded as statistically significant.
Discussion
To our knowledge, this is the first study to demonstrate that compared to NWL subjects, NWO subjects have a higher degree of vascular inflammation using 18 F-FDG-PET/CT. Furthermore, the findings also reveal the significant contribution of body fat to subclinical vascular inflammation, even in the normal BMI population. Thus, the main finding of the present study is that more active and earlier surveillance of subclinical vascular inflammation may be required to prevent clinical vascular events, even in patients who were not defined as obese by the classical definition.
BMI is calculated as the weight divided by the square of height. Body weight is mainly composed of muscle mass and fat mass. It is well known that muscle is a type of endocrine organ, exercise has an anti-inflammatory effect on vessels, and excess fat mass exerts harmful effects on vascular inflammation [30,31]. Obesity is principally defined as the presence of excess fat mass with elevated cardiometabolic risk. Therefore, although BMI is the most easily used and widely accepted criteria for defining obesity [6], BMI can miscategorize a significant proportion of subjects who have lower muscle mass content and higher body fat levels as those having a same cardiovascular risk as healthy, non-obese subjects [7-10]. Previous studies have indicated the elevation of carotid artery TBR in obese subjects with BMI values of ≥25 kg/m2[32] or BMI values of ≥30 kg/m2[33]. In addition, several studies identified a persistent positive correlation between TBR and BMI even after adjusting for confounding factors [32,34]. However, none of these studies assessed the possibility of NWO, previously classified as not obese, as a potential risk for vascular inflammation. The importance of our study lies in identifying NWO as a novel risk factor for increased vascular inflammation and in determining that body fat itself, rather than BMI, is more important as a driving force for this subclinical inflammation.
Several reports have indicated that visceral fat may have a greater harmful effect on vascular inflammation than subcutaneous fat [35]. When we analyzed the degree of vascular inflammation in subjects with excess visceral fat or in subjects with excess subcutaneous fat by generating new parameters such as “the ratio of visceral-to-subcutaneous fat amount” or “the ratio of subcutaneous-to-visceral fat amount,” the value of TBRmean and TBRmax were found to be higher in the subjects with higher visceral-to-subcutaneous fat ratio and lower in the subjects with higher subcutaneous-to-visceral fat ratio. This suggests that varying ratios of depot-specific fat amount may have different effects on vascular inflammation (Data not shown). Moreover, we suggest that the ratio of visceral-to-subcutaneous adipose tissue may be essential for vascular inflammation, even in normal weight subjects. However, it should be considered that an increment in the absolute amount of total fat, regardless of whether it involves visceral or subcutaneous fat, may definitely exert a harmful effect on vascular inflammation, even in normal weight subjects, as shown in the present data. Although we could not extensively analyze the depot-specific effect of body fat on vascular inflammation in normal weight subjects in the present study, the findings obtained in the present study indicate that this topic warrants further investigation.
De Lorenzo et al. reported that the levels of inflammatory cytokines interleukin-6 and tumor necrosis factor-α, which are secreted by adipose tissue, are elevated in NWO subjects compared to their NWL counterparts [12]. These fat-derived inflammatory factors can affect the development of atherosclerosis [36,37]. In addition, Yoo et al. suggested that circulating adipocyte fatty acid binding protein, primarily expressed in adipocytes, is an independent determinant of vascular inflammation measured by 18 F-FDG-PET/CT [38]. Moreover, the NWO population in the present study also exhibited higher levels of the inflammation marker CRP, although the difference was not significant. Thus, these findings demonstrate that subclinical vascular inflammation may be caused by direct or indirect inflammatory signals from adipose tissue in NWO subjects. NWO subjects may be genetically predisposed to obesity and elevated cardiovascular risk [39], whereas the regulation of caloric excess may prohibit the development of obesity and elevation of cardiovascular risk [40]. As subclinical atherosclerosis can finally be presented as a clinically significant cardiovascular event [41], future strategies to identify susceptible subjects (such as NWO individuals in the present study) and to develop interventions to prohibit the development of a cardiovascular event in the population with subclinical atherosclerosis are warranted. These findings also emphasize that it is critical to identify a useful biomarker for selecting the population at risk for adipose tissue-associated vascular inflammation, including subjects with a normal BMI. The usefulness of the biomarkers may be validated with the non-invasive 18 F-FDG-PET/CT imaging technique as conducted in our study.
Before the introduction of 18 F-FDG-PET/CT as a non-invasive imaging tool for visualizing the early inflammation of atherosclerosis in the vessel, there was no definite method to detect subclinical atherosclerosis [42,43]. The current methods such as intima-media thickness, coronary computed tomography angiography, and coronary angiography mainly evaluate the structural change of the vessels, which is a manifestation of progressed atherosclerosis [44]. In the present analysis, importantly, some patients in the NWO group had a very high degree of vascular inflammation (TBRmax > 2.0), a finding that has not been observed in NWL subjects thus far. Severe inflammatory lesions may result in a clinically meaningful CVD event [45,46]. In addition, studies involving non-invasive functional imaging of vascular inflammation using 18 F-FDG-PET/CT indicated that these highly inflammatory lesions can serve as targets of earlier intervention in NWO subjects, the results of which were promising in a small population [34,47-50]. However, further studies would need to investigate whether the use of this technique results in a clinically significant reduction of future vascular events.
This study has several limitations. Our study used an arbitrary cutoff value of BF% based on the tertiles of BF% to define NWO. However, there is no established cutoff value for delineating the harmful effect of body fat on a cardiometabolic event. Therefore, although arbitrary, we followed the method used in a previously published paper [14]. Determination of the optimal cutoff value of BF% for discriminating the subjects at high risk for future CVD events will be necessary. Second, we used a relatively simple BI method to evaluate the body fat. However, there are concerns that the BI method might underestimate upper body obesity [51], and dual energy X-ray absorptiometry might be more accurate for evaluating BF%. In contrast, the BI method has several advantages such as the avoidance of radiation exposure, simplicity, and low cost, suggesting the utility of BF estimation in large-scale studies [52]. Third, this study is a cross-sectional study that cannot confirm a direct causal relationship between BF and vascular inflammation. Nevertheless, to our knowledge, this is the first study to target the NWO population specifically in a community-oriented cohort. Furthermore, this study has a relatively large sample size among studies on vascular inflammation using 18 F-FDG-PET/CT. Nevertheless, a prospective study with a longer study period is warranted in the future to confirm whether vascular inflammation assessed by 18 F-FDG-PET/CT is actually associated with CVD events.
Conclusions
To our knowledge, the present study is the first study to indicate that NWO subjects, who have increased BF% compared to NWL subjects, are associated with a higher degree of vascular inflammation using 18 F-FDG-PET/CT, which enables the noninvasive detection of functional/subclinical vascular inflammation. Furthermore, we suggested that BF may be a major contributing factor of vascular inflammation even in normal weight patients. NWO subjects, formerly classified as non-obese with the conventional BMI criteria, may represent a unique subset of patients who should not be excluded from high-risk groups of future CVD events. The findings of the present study warrant future clinical investigations into the uniqueness of NWO patients, particularly regarding the potential for future CVD events.
Abbreviations
BMI: Body mass index; BF: Body fat; NOW: Normal weight obesity; NWL: Normal weight lean; 18 F-FDG-PET/CT: 18 F-fludeoxyglucose positron emission tomography/computed tomography; TBRmean and TBRmax: Mean and maximum target-to-background ratios; NHANES: National Health and Nutrition Examination Survey; KNHANES: Korean National Health and Nutrition Examination Survey; CVD: Cardiovascular disease; BF%: Body fat percentage; BI: Bioelectrical impedance; HbA1c: Glycated hemoglobin A1c; HDL-cholesterol: High-density lipoprotein cholesterol; CRP: C-reactive protein; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; LDL-cholesterol: Low-density lipoprotein cholesterol; ROI: Region of interest; SUV: Standardized uptake value; SUVmax: Maximal SUV; TBR: Target-to-background ratio; SD: Standard deviation; OR: Odds ratio; CI: Confidence interval.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SK designed the study, contributed to acquisition of data, performed statistical analysis, and wrote the manuscript. CK, JSP, and SHK have contributed to the design of study, acquisition of data, and interpretation of data. SPL participated in the design of the study, interpretation of data, and helped to draft the manuscript. MKK and HKK participated in acquisition of data and interpretation of data. KRK contributed to interpretation of data. TJJ contributed to the design of the study, acquisition of data, and extensive interpretation of data. CWA coordinated the study, and contributed to the acquisition and interpretation of data. All authors read and approved the final manuscript.
Contributor Information
Shinae Kang, Email: SHINAE95@yuhs.ac.
Chanhee Kyung, Email: ITNUE7942@yuhs.ac.
Jong Suk Park, Email: PJS00@yuhs.ac.
Sohee Kim, Email: SSOI23@yuhs.ac.
Seung-Pyo Lee, Email: SPLEE0624@gmail.com.
Min Kyung Kim, Email: MKKIM83@yuhs.ac.
Hye Kyung Kim, Email: HYEKKIM@yuhs.ac.
Kyung Rae Kim, Email: KIMKR96@yuhs.ac.
Tae Joo Jeon, Email: TJEONNM@yuhs.ac.
Chul Woo Ahn, Email: ACW@yuhs.ac.
Acknowledgments
We thank Kyung Hwa Han, MS, Biostatistical Collaboration Unit, Gangnam Medical Research Center, Yonsei University College of Medicine. We acknowledge Gangnam Severance Health Promotion Resaerch Team for supporting the construction of the registry of data from the Health Promotion Center. We also acknowledge to the Editiage Company for English editing. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (A1061486) and by a faculty research grant from Yonsei University College of Medicine for 2011 (6-2011-0152).
References
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35(6):561–566. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Krauss RM. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation. 1998;97(21):2099–2100. doi: 10.1161/01.CIR.97.21.2099. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. National institutes of health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32(6):959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- Goh VH, Tain CF, Tong TY, Mok HP, Wong MT. Are BMI and other anthropometric measures appropriate as indices for obesity? A study in an Asian population. J Lipid Res. 2004;45(10):1892–1898. doi: 10.1194/jlr.M400159-JLR200. [DOI] [PubMed] [Google Scholar]
- Low S, Chin MC, Ma S, Heng D, Deurenberg-Yap M. Rationale for redefining obesity in Asians. Ann Acad Med Singapore. 2009;38(1):66–69. [PubMed] [Google Scholar]
- De Lorenzo A, Martinoli R, Vaia F, Di Renzo L. Normal weight obese (NWO) women: an evaluation of a candidate new syndrome. Nutr Metab Cardiovasc Dis. 2006;16(8):513–523. doi: 10.1016/j.numecd.2005.10.010. [DOI] [PubMed] [Google Scholar]
- De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: early inflammation? Am J Clin Nutr. 2007;85(1):40–45. doi: 10.1093/ajcn/85.1.40. [DOI] [PubMed] [Google Scholar]
- Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circ Cardiovasc Imaging. 2012;5(3):349–356. doi: 10.1161/CIRCIMAGING.111.969956. [DOI] [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen MD, Parati G, Lopez-Jimenez F. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31(6):737–746. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Vidal P, Pecoud A, Hayoz D, Paccaud F, Mooser V, Waeber G, Vollenweider P. Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc Dis. 2010;20(9):669–675. doi: 10.1016/j.numecd.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Di Renzo L, Galvano F, Orlandi C, Bianchi A, Di Giacomo C, La Fauci L, Acquaviva R, De Lorenzo A. Oxidative stress in normal-weight obese syndrome. Obesity (Silver Spring) 2010;18(11):2125–2130. doi: 10.1038/oby.2010.50. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Davies MJ. Acute coronary thrombosis--the role of plaque disruption and its initiation and prevention. Eur Heart J. 1995;16(Suppl L):3–7. doi: 10.1093/eurheartj/16.suppl_L.3. [DOI] [PubMed] [Google Scholar]
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–671. doi: 10.1161/01.CIR.92.3.657. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Ishino S, Mukai T, Asano D, Teramoto N, Watabe H, Kudomi N, Shiomi M, Magata Y, Iida H, Saji H. (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med. 2004;45(7):1245–1250. [PubMed] [Google Scholar]
- Leppanen O, Bjornheden T, Evaldsson M, Boren J, Wiklund O, Levin M. ATP depletion in macrophages in the core of advanced rabbit atherosclerotic plaques in vivo. Atherosclerosis. 2006;188(2):323–330. doi: 10.1016/j.atherosclerosis.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Garedew A, Henderson SO, Moncada S. Activated macrophages utilize glycolytic ATP to maintain mitochondrial membrane potential and prevent apoptotic cell death. Cell Death Differ. 2010;17(10):1540–1550. doi: 10.1038/cdd.2010.27. [DOI] [PubMed] [Google Scholar]
- Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, Rafique A, Hargeaves R, Farkouh M, Fuster V, Fayad ZA. Atherosclerosis inflammation imaging with 18 F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49(6):871–878. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12(3):294–301. doi: 10.1016/j.nuclcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. In vivo 18 F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- Bucerius J, Duivenvoorden R, Mani V, Moncrieff C, Rudd JH, Calcagno C, Machac J, Fuster V, Farkouh ME, Fayad ZA. Prevalence and risk factors of carotid vessel wall inflammation in coronary artery disease patients: FDG-PET and CT imaging study. JACC Cardiovasc Imaging. 2011;4(11):1195–1205. doi: 10.1016/j.jcmg.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TG, Hong HR, Lee J, Kang HS. Lifestyle plus exercise intervention improves metabolic syndrome markers without change in adiponectin in obese girls. Ann Nutr Metab. 2007;51(3):197–203. doi: 10.1159/000104137. [DOI] [PubMed] [Google Scholar]
- Ryo M, Maeda K, Onda T, Katashima M, Okumiya A, Nishida M, Yamaguchi T, Funahashi T, Matsuzawa Y, Nakamura T, Shimomura I. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care. 2005;28(2):451–453. doi: 10.2337/diacare.28.2.451. [DOI] [PubMed] [Google Scholar]
- Scharfetter H, Schlager T, Stollberger R, Felsberger R, Hutten H, Hinghofer-Szalkay H. Assessing abdominal fatness with local bioimpedance analysis: basics and experimental findings. Int J Obes Relat Metab Disord. 2001;25(4):502–511. doi: 10.1038/sj.ijo.0801556. [DOI] [PubMed] [Google Scholar]
- Choi KM. Sarcopenia and sarcopenic obesity. Endocrinol Metab (Seoul) 2013;28(2):86–89. doi: 10.3803/EnM.2013.28.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Choi HY, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Association of adiponectin, resistin, and vascular inflammation: analysis with 18 F-fluorodeoxyglucose positron emission tomography. Arterioscler Thromb Vasc Biol. 2011;31(4):944–949. doi: 10.1161/ATVBAHA.110.220673. [DOI] [PubMed] [Google Scholar]
- Bucerius J, Mani V, Moncrieff C, Rudd JH, Machac J, Fuster V, Farkouh ME, Fayad ZA. Impact of noninsulin-dependent type 2 diabetes on carotid wall 18 F-fluorodeoxyglucose positron emission tomography uptake. J Am Coll Cardiol. 2012;59(23):2080–2088. doi: 10.1016/j.jacc.2011.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Kao HL, Huang CL, Chen MF, Lin LY, Wang YC, Lin YH, Lin HJ, Tzen KY, Yen RF, Chi YC, Huang PJ, Yang WS. The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. Eur J Nucl Med Mol Imaging. 2012;39(3):399–407. doi: 10.1007/s00259-011-1994-7. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89(6):2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- Reape TJ, Groot PH. Chemokines and atherosclerosis. Atherosclerosis. 1999;147(2):213–225. doi: 10.1016/S0021-9150(99)00346-9. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Kim S, Park MS, Choi HY, Yang SJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Serum adipocyte fatty acid-binding protein is associated independently with vascular inflammation: analysis with (18)F-fluorodeoxyglucose positron emission tomography. J Clin Endocrinol Metab. 2011;96(3):E488–492. doi: 10.1210/jc.2010-1473. [DOI] [PubMed] [Google Scholar]
- Di Renzo L, Bertoli A, Bigioni M, Del Gobbo V, Premrov MG, Calabrese V, Di Daniele N, De Lorenzo A. Body composition and -174G/C interleukin-6 promoter gene polymorphism: association with progression of insulin resistance in normal weight obese syndrome. Curr Pharm Des. 2008;14(26):2699–2706. doi: 10.2174/138161208786264061. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Johnson WD, Johannsen D, Ravussin E. Cardiovascular risk escalation with caloric excess: a prospective demonstration of the mechanics in healthy adults. Cardiovasc Diabetol. 2013;12:23. doi: 10.1186/1475-2840-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo S, Peritore A, Trovato RL, Guarneri FP, Di Lisi D, Muratori I, Novo G. Preclinical atherosclerosis and metabolic syndrome increase cardio- and cerebrovascular events rate: a 20-year follow up. Cardiovasc Diabetol. 2013;12(1):155. doi: 10.1186/1475-2840-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnstrom P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL. Imaging atherosclerotic plaque inflammation with [18 F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–2711. doi: 10.1161/01.CIR.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- Kai H. Novel non-invasive approach for visualizing inflamed atherosclerotic plaques using fluorodeoxyglucose-positron emission tomography. Geriatr Gerontol Int. 2010;10(1):1–8. doi: 10.1111/j.1447-0594.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- Degnan AJ, Young VE, Gillard JH. Advances in noninvasive imaging for evaluating clinical risk and guiding therapy in carotid atherosclerosis. Expert Rev Cardiovasc Ther. 2012;10(1):37–53. doi: 10.1586/erc.11.168. [DOI] [PubMed] [Google Scholar]
- Chen W, Dilsizian V. Targeted PET/CT imaging of vulnerable atherosclerotic plaques: microcalcification with sodium fluoride and inflammation with fluorodeoxyglucose. Curr Cardiol Rep. 2013;15(6):364. doi: 10.1007/s11886-013-0364-4. [DOI] [PubMed] [Google Scholar]
- Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, Nikolaou K, Reiser MF, Bartenstein P, Hacker M. 18 F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50(10):1611–1620. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18 F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008;49(8):1277–1282. doi: 10.2967/jnumed.108.052233. [DOI] [PubMed] [Google Scholar]
- Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T, Mawatari K, Yasukawa H, Kaida H, Ishibashi M, Hayabuchi N, Harada H, Ikeda H, Yamagishi S, Imaizumi T. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging. 2011;4(10):1110–1118. doi: 10.1016/j.jcmg.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Magata Y, Kato T, Hatano K, Ishino S, Mukai T, Shiomi M, Ito K, Saji H. Application of 18 F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. J Nucl Med. 2006;47(11):1845–1850. [PubMed] [Google Scholar]
- Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48(9):1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- Snijder MB, Kuyf BE, Deurenberg P. Effect of body build on the validity of predicted body fat from body mass index and bioelectrical impedance. Ann Nutr Metab. 1999;43(5):277–285. doi: 10.1159/000012795. [DOI] [PubMed] [Google Scholar]
- Fogelholm M, van Marken LW. Comparison of body composition methods: a literature analysis. Eur J Clin Nutr. 1997;51(8):495–503. doi: 10.1038/sj.ejcn.1600448. [DOI] [PubMed] [Google Scholar]