Abstract
Head restraining is an experimental technique that firmly secures the animal's head to a fixation apparatus for the precise control and sensing of behaviors. However, procedural and surgical difficulties and limitations have been obstructing the use of the technique in neurophysiological and behavioral experiments. Here, we propose a novel design of the head-restraining apparatus which is easy to develop and convenient for practical use. Head restraining procedure can be completed by sliding the head mounter, which is molded by dental cement during implantation surgery, into the port, which serves as matching guide rails for the mounter, of the fixation bar. So neither skull-attached plates nor screws for fixation are needed. We performed intracranial self stimulation experiment in rats using the newly designed device. Rats were habituated to acclimatize the head-restraint environment and trained to discriminate two spatially distinguished cues using a customized push-pull lever as an operandum. Direct electrical stimulation into the medial forebrain bundle served as reward. We confirmed that head restraining was stable throughout experiments and rats were able to learn to manipulate the lever after successful habituation. Our experimental framework might help precise control or sensing of behavior under head fixed rats using direct electrical brain stimulation as a reward.
Keywords: Apparatus, Head fixation, Intra-cranial self-stimulation, Operant conditioning, Rat
INTRODUCTION
Head restraining (or head fixation) is an experimental technique that firmly secures the animal's head (or skull) to a fixing apparatus not to move freely. This technique has been used for experimentation on precise control and sensing of behavior [1,2,3,4] and brain imaging [5] in awake behaving animals. The experimental procedure incorporating head restraining basically begins with exposing the animals repeatedly to head restraining situation. Throughout several sessions of habituation, animals are getting accustomed to the unfamiliar and stressful environment. Skillful handling and not to make them annoying seem to be essential for the successful head restraining. More details about head restraining, particularly in rodents, are well documented by Schwarz [6].
Most of the fixation has attained by anchoring plates that is attached on the animal's skull to the fixation apparatus [7,8]. Under such designs, additional plates or pieces should be surgically glued on top of the animal's skull [1,5,9,10] and the plates are fixed to the fixation apparatus by screws. If the electrode implantation for recording and/or stimulation needs to be concurrent with the head restraining, for example, to examine neuronal activity while whisker movements and intracranial self stimulation (ICSS) task under the head restraining, the glued plates could spatially restrict the implantation of electrodes. Another minor problem is the perturbation of animals' natural behavior when they are not engaged in experiments, for example, when they are in the home cage. Moreover, inconvenience is originated from screw-tightening. During the habituation period of the head restraint, animals show struggling behavior at the moment of which the head is about to be fixed. This indicates that experimenters who have little experience of handling animals could be somewhat uncomfortable with tightening screws.
To overcome the incompatibility of the fixation apparatus and electrodes implantation and its inconvenience, a novel design for head fixation apparatus is suggested and developed. Two design criteria were considered for the fixation apparatus used in this study. First, there should be no glued attachment of metal and/or plastic plates on the skull except dental cement which is generally used for implantation surgery. Second, head fixation should be implemented without any screw-like stuff.
The capacity of the devised apparatus was tested using head restraint rats performing ICSS tasks. ICSS is an operant conditioning using electrical stimulation delivered directly into the brain reward circuitry such as medial forebrain bundle (MFB) passing lateral hypothalamus area [11,12]. To our knowledge, no study has been reported about performing ICSS tasks under head restraining condition in rats. Though a whole-body movement is a major movement component of rats, there, recently, has been a success in training forepaw movements in head-restrained rats [6]. Therefore, we decided to make a small lever to serve as an operandum for head-restrained rats.
Using the general levers installed on the commercial operant chambers is virtually undesirable for both its size and ergonomic problems. It appeared to be natural to push and pull the lever rather than depressing a lever from top to bottom using a unilateral forelimb (or forepaw). This idea led to the design of the bi-directional push-pull lever. Operant behaviors could be successfully conditioned by ICSS paradigm in head restrained rats.
METHODS
Design of head restraining apparatus
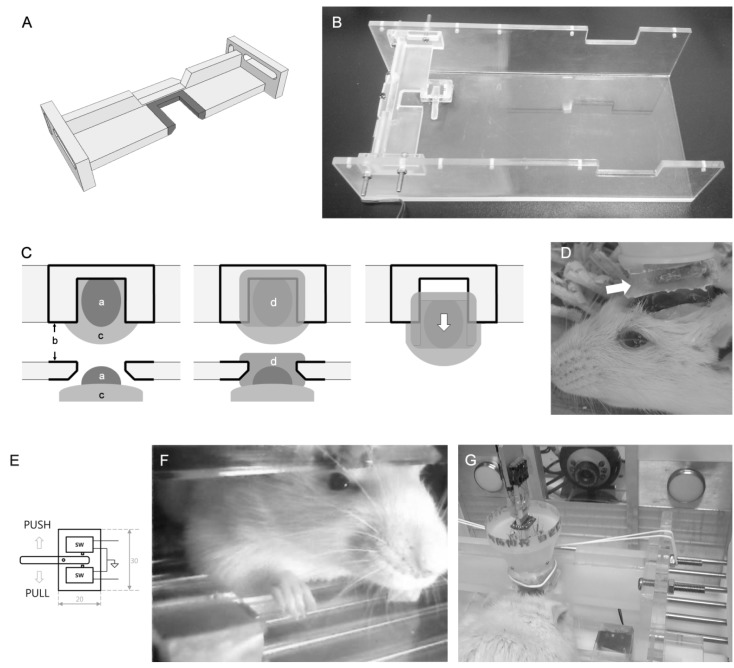
The drawing and implementation of the fixation apparatus are shown in Fig. 1A and B. The apparatus is composed of the fixation bar and box, both made of acrylic panel (6 mm thickness). The bar is the key component for a convenient fixation procedure and can be installed into the box by tightening screws through the two elongated holes at each side. Those holes allowed the bar to be installed with a tilt. A port is prepared in the middle of the bar (Fig. 1A, shaded area) and it fits the overhead mounter molded in dental cement applied during implantation surgery (See surgery for details). The box serves as an operant chamber which is equipped with modules, such as levers, for operant behaviors.
Fig. 1.
Head restraining apparatus. (A) Drawing of the head-restraining bar. Shaded area indicates the rim of the port. (B) Practical implementation of the head-restraining bar and the push-pull lever. (C) The mounter is implementated during surgery by applying additional dental cement between the bar and rat skull. a: dental cement applied for electrode implantation, b: Parafilm (surrounded by black solid-lines), c: rat skull, d: mounter. After the mounter is harden, it can be pulled off (white arrow) since Parafile separates the restraining bar and mounter. (D) Head restraining is simply achieved by the guide rails in the mounter (white arrow). (E) The drawing of the push-pull lever. (F) An example of a rat performing ICSS tasks. The push-pull lever is ergonomically designed for head-restrained rats. (G) The molded overhead mounter is inserted into the port, at the middle of the fixation bar and simply tightened by a rubber band.
The push-pull lever was basically designed to be small enough to be installed in the fixation box and could indicate at least two states using a single arm operation of the animal. The lever was assembled with a stick and two micro-switches disjoined from the computer mouse, and cased with acrylic plate of 1 mm thickness. Those two micro-switches were oppositely placed adjacent to the lever stick as shown in Fig. 1B and E. Operational principles are simple. If the lever stick was pushed, it triggered the micro-switch of the pull position and vice versa. The on and off signal was then delivered to the digital IO device (National Instruments) and detected by computer programs. Since about 70 to 80% of rats are right-pawed [7,8], the lever was attached at the right side of the floor where rat's forepaw was naturally placed upon the lever stick when head was fixed (Fig. 1F).
Animals and surgery
Male Sprague-Dawley rats (n=4) weighing 300~350 g were housed in home cages with ad libitum access to food and water. The light cycle begins 7 AM and lasts for 12 hours and room temperature was maintained as 20 to 25℃. After surgery, rats were housed individually in separate cages. All experiments were performed according to Guide for the Care and Use of Laboratory Animals (National Institute of Health, 1996).
Rats were anesthetized with the cocktail mixture of ketamine 10 ml (50 mg/ml), xylazine hydrochloride 1.5 ml (mg/ml), and saline 2.5 ml. Anesthesia was confirmed by no responses to tail and toe pinches. Animals were then fixed in a stereotaxic apparatus (David Kopf Instruments, USA). The position of rat skull was set to be flatted. After 2% lidocaine s.c. injection and midline incision, the periosteum was completely scraped off the skull surface. Eight holes were drilled and screws (1mm diameter) were implanted into the holes of the skull (frontal AP5.0, ML±2.0 and AP0, ML±5.0, parietal AP-9.0, ML±5.0, occipital AP-3.0 from Lambda, ML±2.0) according to Paxinos and Watson coordinates [13]. Then super bond (Super-Bond C&B, Sun medical, Japan) was carefully applied around the screws over the skull. Two holes for stimulation electrodes (insect pins - stainless steel, No. 00 - coated with formvar except the tip) were drilled after the super bond became hard. The stimulation electrodes were then implanted into the bilateral MFB area of coordinate AP-2.3 mm, ML1.8 mm, DV-8.2 (from the dura). Dental cement was applied to fix the electrodes firmly.
After the implantation surgery, dental cement is applied around the edge of the port of the fixation bar (Fig. 1A) which is wrapped with Parafilm® (Pechiney Plastic Packaging, Chicago Il, USA) (Fig. 1C). Care was taken not to cover the bare acrylic part of the fixation bar with dental cement. When it hardened enough, the bar was fixed on the stereotaxic frame by aligning the port of the bar with the center of implantation cement (Fig. 1C, left). Additional application of dental cement into the gap between the port and cement applied during surgery adjoins dental cement of both permanently (Fig. 1C, center). When cement hardened, the mounter and fixation bar was separated by pulling the overhead mounting out of the port and this naturally formed the overhead mounter (Fig. 1C, right). Parafilm® covering the port enables this procedure to be simply achieved. After surgery, it is natural that the head mounter serves guide rails for head restraint as shown in Fig. 1D.
Behavioral tasks
After recovery, rats were first tested whether they respond to the brain stimulation reward by introducing them to a general operant chamber containing a lever. Whenever rats press the lever, they could receive an electrical stimulation directly into their MFB. During the initial shaping stage, stimulation intensity was gradually increased. Reached at a specific intensity, rats kept on pressing the lever and this was regarded as a successful demonstration of ICSS. For that case, it deemed that stimulation electrode was precisely located at the MFB area.
Next, rats showing operant behavior were introduced to initial head restraining training using the devised head fixation apparatus. The first step of head fixation was simply pushing the overhead mounter into the port. Then the overhead mounter was tightly secured to the bar using a general rubber band (the wrist rings of latex gloves were preferred) (Fig. 1G). No more cumbersome procedure was needed. Whenever rats were removed from the fixation apparatus and back into their cages, a sweet-flavored food was given for a reward.
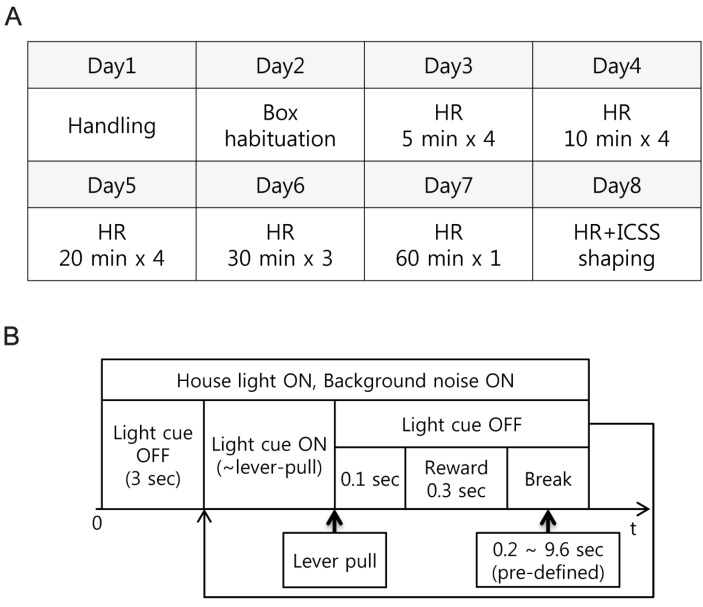
Previously suggested protocols [1,9] were selectively modified and combined for the habituation of head restraints. The detailed protocol for head restraining used in this study is described in Fig. 2A. Basic idea is that rats became exposed to head restraining condition with short time duration and this duration is getting longer across sessions (from 5 min to 60 min across 5 sessions). Rats that did not show any struggling and/or awkward behavior at least 1 hour of head restraining at the final session proceeded to the ICSS tasks under the head restraining (ICSS-HR) condition. For ICSS-HR training, the push-pull lever was installed on the box floor.
Fig. 2.
Procedures of habituation and experiment. (A) Habituation procedures for desensitizing rats to head-restraint constitutes repetitive and longer exposure to head-restraining condition. HR: head-restraining habituation only HR+ICSS: ICSS was allowed (B) The basic structure of the rat conditioning.
In general, reward stimulation into the MFB elicits hyperactivity including exploratory and sniffing actions in the freely moving condition. In the head restraining condition, such hyperactivity could result in the separation of the overhead mounter from the rat's skull. To prevent this tragic accident, stimulation intensity was lowered about 10 to 50 µA from the previous phase at the initial stage of ICSS-HR phase.
The experimental design of the initial ICSS-HR training was simple as shown in Fig. 2B. In a dark room, the house light and white noise were turned on, indicating the beginning of training. After three sec of delay, the light cue was turned on, indicating that rats could be rewarded when they pull the lever. If rats pull the lever by their right forelimb, the light cue immediately turned off, and after 100 msec delays, contingent reward stimulation (biphasic, train duration 300 msec, pulse duration 0.2 msec, pulse width 4 msec) was delivered. The light cue was turned on again following a break period (0.2 to 9.6 sec, pre-defined) and next trial was begun. This procedure lasts for about an hour for each of the individual rat.
Next, the design was slightly modified to see whether rats could learn the association between the cue and lever-pull. The duration of the light cue was fixed to 3 sec, and the break period after reward was randomly set for each trial (1.6 to 4.6 sec). Correct lever-pulls resulted in immediate cue-off and reward delivery. If rats pull the lever during the break, the period was reset from that moment indicating a longer break period.
Lastly, rats were introduced to spatial cue discrimination tasks after several sessions of left cue and lever- push training. In this task, rats were rewarded by pushing or pulling the lever when the left or right cue had been presented, respectively. The left or right cue was randomly presented at each trial. The duration of both cues was fixed to 5 sec but correct lever manipulation shorten the duration (that is, cue-off). The break between trials was set to 2.6 sec.
All training and task was under control of customized software implemented by Labview 8.5 (National Instruments) and behavioral data was collected using data acquisition device (National Instruments). Data was analyzed using Matlab software (Mathworks) off-line. The number of lever pull or push was converted "events per min" on a 5 min bin basis. The performance of lever-pull task was defined as the number of reward divided by the number of cue presented. In the spatial cue discrimination task, performance of the left and right cue was individually analyzed and response latency (time difference from cue on to lever push or pull) was computed. The performance of correct response was finally computed and analyzed. For example, if the left cue was on then the rat could push the lever to get reward. This reward was given by a correct response. However, when the left cue was on and the rat quickly pulled and then pushed the lever, the rat could receive reward but it was regarded as an error response.
RESULTS
During the initial habituation in the head restraining apparatus, rats showed struggling behavior, usually running in place and squeaked when touched their back or trunk by an experimenter accidentally. However, those stressful responses were diminishing across the habituation session. The overhead mounter inserted into the port was firmly secured by general rubber band and rats could never fall off by their muscle force. The rats sit in the rear part of the box but nestled down somewhat forward when they were fully habituated. Respiration rate slowed down across sessions was observed by visual inspection. Food reward which was given to the rats after daily habituation was consumed by both rat RS007 and RS008 but the rat RS009 was not.
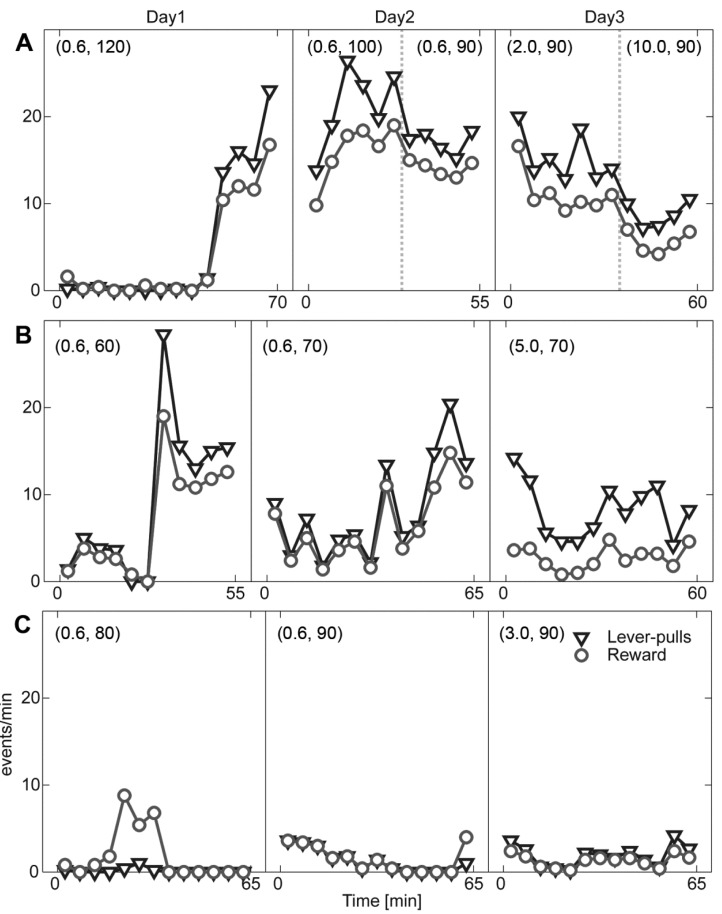
In the ICSS check phase, three rats showed repeated lever presses at the stimulation intensity of 90 to 150 µA, thus those rats underwent subsequent ICSS-HR training. Rats readily pulled the lever even though their head was fixed. Their locomotion activity was increased by stimulation reward at first but decreased across session. Shaping of two rats (lever-pull task only) was successful in the ICSS-HR paradigm as shown in Fig. 3. In the first session (Day0), no rat manipulated the lever. In the second session (Day1), the rat (RS007) rarely pulled the lever until 50 min (Fig. 3A). However, the rat began to pull the lever from 45 min and the frequency tended to increase until the end of the session. The more number of rewards than lever-pulls in the first bin indicates that free reward was delivered by the experimenter for shaping purposes. At Day2, the rat pulled the lever from the beginning of the session. The higher number of lever-pulls than rewards implies that the rat pulled the lever during the break period. The slight reduction in stimulation intensity (100 to 90 µA; separated by a dotted bar, Day2 in Fig. 3A) resulted in less lever-pulls (30 to 55 min). The longer inter-trial-interval was associated with the decrease in the number of lever-pulls in the later part (2 to 10 sec; separated by a dotted bar, Day3 in Fig. 3A). Another rat (RS008) showed an abrupt increase in lever pulls around 30 min of Day1 (Fig. 3B). The number of lever-pulls increased in Day2. Increased inter-trial interval resulted in the decreased number of rewards (Day3). The other rat (RS009) showed no interest in pulling the lever even though extensive free reward in Day1 (Fig. 3C). The intermittent lever-pulls were highly associated with struggling behavior.
Fig. 3.
Behavioral data for the initial ICSS shaping phases. The number of lever-pulls (triangle) and consequent rewards (circle) are depicted for the rat RS007 (A), RS008 (B), and RS009 (C) for consecutive three sessions (Day1 to 3). Numbers in the parenthesis indicates predetermined inter-trial intervals and stimulation current intensity. When the parameters are modified, it is denoted by the dotted vertical line. The number of rewards that is larger than the number of lever-pulls indicates the delivery of free-reward by the experimenter.
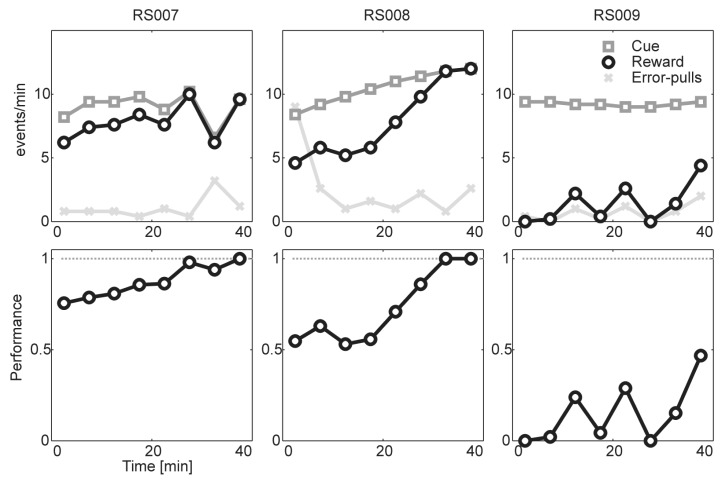
The performance of lever-pull training is shown in Fig. 4. The rat RS007 showed overall 87.94% of success rate and the rat RS008 showed overall 74.94% of success rate. The performance of rats during the final 10 to 15 min of training was nearly perfect in both of the rats, indicating that rats learned how to behave (pull the lever) to receive reward. The rat RS009 did not show any capacity of task learning, so it was excluded in the following cue discrimination task.
Fig. 4.
Behavioral performance session. Given light cues (rectangular), the rats pulled the lever to get stimulation reward (circle). Lever-pulls during the break period (error-pulls, x-mark) increase the duration of the break period, indicating a longer inter-trial interval. All event occurrences and their performance (reward/cue) are depicted for each rat at the top and bottom, respectively. While the rat RS007 and RS008 shows an improvement within the session, the rat RS009 shows no improvement.
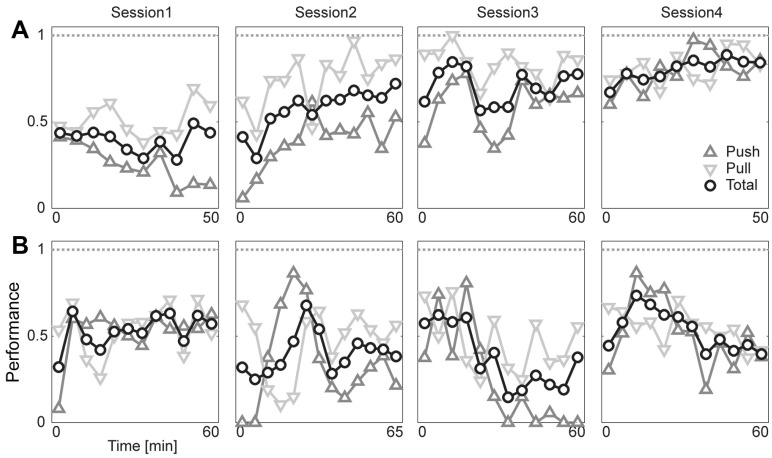
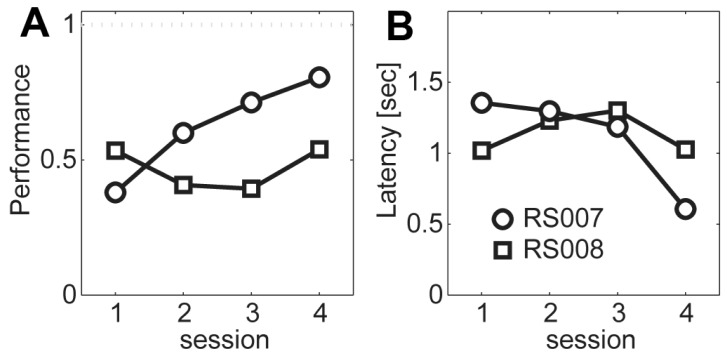
Lastly, the result of spatial cue discrimination in the ICSS-HR condition is shown in Fig. 5. During the consecutive four sessions of training, the rat RS007 showed growing performance (Fig. 5A), while the other rat RS008 showed nebulous performance (Fig. 5B). Those results are summarized in Fig. 6. Overall performance tends to increase (Fig. 6A) and the response latency tends to decrease (Fig. 6B) across session in the RS007 rat.
Fig. 5.
Performance of the cue discrimination tasks. (A) The rat, RS007, shows an improvement in the cue-discrimination tasks across session. Performances of both push (triangle) and pull (inverted triangle) were separately analyzed, and total (circle) indicates their averages. (B) RS008 shows no improvement.
Fig. 6.
Summary of the cue discrimination tasks. (A) Average performance across session for the two rats (RS007; circle, RS008; rectangle). (B) Response latency.
DISCUSSION
In this study, a novel head-restraining apparatus was developed and tested for its feasibility of instrumental conditioning using brain stimulation reward.
Head-restraining using the devised apparatus had been stable enough throughout experiments. The overhead mounter guaranteed a firm fixation. It was designed to use no additional attachment such as plates and pieces and be compatible with brain recording and stimulation electrodes by providing sufficient space above the skull. The experimenter can easily and firmly fix a rat's head to the apparatus without any screw-like fastener.
The design of the push-pull lever was also adequate to manipulate by rats. The stick of the lever could be grabbed by the right forepaw and manipulated within the moving range of the lever, indicating ergonomically suitable for rats. The lever position that should be placed beneath the right forepaw might be important for successful behavioral tasks in head-restrained rats.
In the following behavioral tasks, successful operant conditioning was not observed for all rats. Only the rat, RS007, operantly conditioned and performed discrimination tasks successfully under head-restraining using the push-pull lever. The behavioral performance was better than other rats from the initial shaping phases. Another rat, RS008, showed an imperfect task performance. Though it showed good performance during initial shaping phases which used lever-pull task, but it did not learned to manipulate the push-pull lever during the cue discrimination task. It seems that the rat was not exposed enough to precedent push training to learn the association between the left cues and push behavior. Another possibility might be an improper conditioning. The rat showed excessive running behavior during shaping. This resulted in unintended lever-pulls and reward delivery. Admitting the individual differences, RS007 showed better leaning capability compared with RS008. The other rat, RS009, showed no interest in manipulating the lever. The rat did not show intake of food reward after head-restraining habituation, which might be unable to overcome the stress. During the initial shaping phases, the rat received many free rewards without any voluntary or even unintended lever manipulation, indicating an improper conditioning. So it is possible that the rat was highly stressful during the habituation and was not properly habituated and trained.
Overall, rats showing a poor performance were not trained with additional sessions, so if they had been habituated and trained more sessions they might have learned and performed the task better under minimized stress and aversion. While other study had spent two or more weeks [6,10], only minimum time (five days) was spent for habituating head-restrained rats. For a better behavioral performance under head-restraint condition, sufficient habituation period seems to be crucial.
This study demonstrated a successful operant conditioning in head-restrained rats using brain stimulation reward for the first time. ICSS paradigm was compatible with head-restraining condition, thus, our experimental framework might contribute to examine not only the precise control or sensing of behavior but also behavioral operant conditioning in head fixed rats. Though this study focused on the feasibility of operant conditioning using brain stimulation reward (ICSS) under head restraining, the apparatus can be used in combination with electrophysiological techniques that are extensively employed such as single or multi unit recording and in vivo patch clamping.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the NRF funded by the Ministry of Education, Science and Technology (2010-0022362) and by Kyungpook National University Research Fund 2012.
ABBREVIATIONS
- ICSS
intracranial self stimulation
- ICSS-HR
intracranial self stimulation under head restraining
- MFB
medial forebrain bundle
References
- 1.Isomura Y, Harukuni R, Takekawa T, Aizawa H, Fukai T. Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat Neurosci. 2009;12:1586–1593. doi: 10.1038/nn.2431. [DOI] [PubMed] [Google Scholar]
- 2.Topchiy IA, Wood RM, Peterson B, Navas JA, Rojas MJ, Rector DM. Conditioned lick behavior and evoked responses using whisker twitches in head restrained rats. Behav Brain Res. 2009;197:16–23. doi: 10.1016/j.bbr.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Connor DH, Clack NG, Huber D, Komiyama T, Myers EW, Svoboda K. Vibrissa-based object localization in head-fixed mice. J Neurosci. 2010;30:1947–1967. doi: 10.1523/JNEUROSCI.3762-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayrhofer JM, Skreb V, von der, Musall S, Weber B, Haiss F. Novel two-alternative forced choice paradigm for bilateral vibrotactile whisker frequency discrimination in head-fixed mice and rats. J Neurophysiol. 2013;109:273–284. doi: 10.1152/jn.00488.2012. [DOI] [PubMed] [Google Scholar]
- 5.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz C, Hentschke H, Butovas S, Haiss F, Stüttgen MC, Gerdjikov TV, Bergner CG, Waiblinger C. The head-fixed behaving rat--procedures and pitfalls. Somatosens Mot Res. 2010;27:131–148. doi: 10.3109/08990220.2010.513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MG, Manns ID, Alonso A, Jones BE. Sleep-wake related discharge properties of basal forebrain neurons recorded with micropipettes in head-fixed rats. J Neurophysiol. 2004;92:1182–1198. doi: 10.1152/jn.01003.2003. [DOI] [PubMed] [Google Scholar]
- 8.Parry TJ, McElligott JG. A method for restraining awake rats using head immobilization. Physiol Behav. 1993;53:1011–1015. doi: 10.1016/0031-9384(93)90283-l. [DOI] [PubMed] [Google Scholar]
- 9.Bryant JL, Roy S, Heck DH. A technique for stereotaxic recordings of neuronal activity in awake, head-restrained mice. J Neurosci Methods. 2009;178:75–79. doi: 10.1016/j.jneumeth.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haiss F, Butovas S, Schwarz C. A miniaturized chronic microelectrode drive for awake behaving head restrained mice and rats. J Neurosci Methods. 2010;187:67–72. doi: 10.1016/j.jneumeth.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 12.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. 4th ed. New York: Academic Press; 1998. [Google Scholar]