Abstract
Objective
To investigate the therapeutic effects of methanol extract of Citrus macroptera Montr.fruit in α-amylase inhibitory activity (in vitro) and hypoglycemic activity in normal and glucose induced hyperglycemic rats (in vivo).
Methods
Fruits of Citrus macroptera without rind was extracted with pure methanol following cold extraction and tested for presence of phytochemical constituents, α-amylase inhibitory activity, and hypoglycemic effect in normal rats and glucose induced hyperglycemic rats.
Results
Presence of saponin, steroid and terpenoid were identified in the extract. The results showed that fruit extract had moderate α-amylase inhibitory activity [IC50 value=(3.638±0.190) mg/mL] as compared to acarbose. Moreover at 500 mg/kg and 1 000 mg/kg doses fruit extract significantly (P<0.05 and P<0.01 respectively) reduced fasting blood glucose level in normal rats as compared to glibenclamide (5 mg/kg). In oral glucose tolerance test, 500 mg/kg dose significantly reduced blood glucose level (P<0.05) at 2 h but 1 000 mg/kg dose significantly reduced blood glucose level at 2 h and 3 h (P<0.05 and P<0.01 respectively) whereas glibenclamide (5 mg/kg) significantly reduced glucose level at every hour after administration. Overall time effect is also considered extremely significant with F value=23.83 and P value=0.0001 in oral glucose tolerance test.
Conclusion
These findings suggest that the plant may be a potential source for the development of new oral hypoglycemic agent.
Keywords: Diabetes mellitus, Hypoglycemic, Citrus macroptera, α-Amylase, OGTT, Glibenclamide
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by both postprandial and fasting hyperglycemia with disturbances in carbohydrate, fat and protein metabolism. Hyperglycemia in diabetes results either from an absolute deficiency in insulin secretion (type 1 DM) or insulin action (type 2 DM) or both. The incidence of diabetes has increased worldwide in recent years. The estimated number of people with diabetes was 30 million in 1985, 150 million in 2000 and then 246 million in 2007, according to the International Diabetes Federation. It expects this number to hit 380 million by 2025[1].
Treatment of diabetes include: enhancement of the action of insulin at the target tissues, with the use of sensitizers (biguanides, thiozolidinediones); stimulation of endogenous insulin secretion with the use of sulfonylureas (glibenclamide, glimepiride), and reduction of the demand for insulin using specific enzyme inhibitors (acarbose, miglitol)[2]. However, there is a burden of unwanted side effects like diarrhea, nausea, dyspepsia, myocardial infarction, peripheral edema and dizziness with the use of these drugs. Plants have been an exemplary source of drugs that have been derived directly or indirectly from them. It is reported that about 800 plants may possess anti-diabetic potential[3]. Hypoglycemic activity of medicinal plants is due to their ability to restore the function of pancreatic tissues by causing an increase in insulin output, inhibiting the intestinal absorption of glucose or facilitating metabolites in insulin dependent processes[4],[5].
Citrus macroptera Montr. (family-Rutaceae) (C. macroptera) commonly called Sat Kara (wild orange), is a semi-wild species of Citrus native in Malesia and Melanesia. The tree, which has thorns, can reach 5 m in height. Its fruit is about 6–7 cm in diameter, has a fairly smooth, moderately thick rind, and turns yellow when it is ripe. The pulp of the fruit is greenish yellow and dry (does not produce much juice). The juice is very sour, and somewhat bitter. In Bangladesh the rind of the C. macroptera is eaten as a vegetable. This plant is medicinally used locally in Assam. Physicians always suggest diabetic patient to eat Citrus fruits to control their blood glucose level. Researchers also concluded that Citrus fruit extracts represent an excellent alternative for nutraceuticals and functional foods geared towards the management of diabetes. Essential oil of fruit of Citrus maximally showed significant reduction of fasting blood glucose and hepatic glucose levels while hepatic glycogen significantly increased when compared to diabetic control animals[6]. According to a 2006 animal study, Citrus extracts not only slow glucose uptake, but also inhibit the movement or transport of glucose through the intestines and liver[7]. A 2009 study examining extracts from a Korean Citrus fruit called Dangyuja (Citrus grandis) found that it holds great potential for controlling blood glucose levels in diabetic patients[8]. Citrus limetta fruit peel demonstrated a potential anti-hyperglycemic effect in streptozotocin induced diabetic rats[9]. There are also many other research reports about this therapeutic effect of Citrus fruits. Investigation on C. macroptera for hypoglycemic property has not been performed yet. That's why we have designed our research work to explore possible mechanism of hypoglycemic activity of this fruit extract.
2. Materials and methods
2.1. Drugs, chemicals and apparatus
Methanol was bought from SIGMA® (Sigma-Aldrich®, St Louis, USA), while acarbose tablet was purchased from local market, manufactured by Pacific Pharmaceuticals Ltd., Bangladesh. Starch was purchased from local scientific market, Motijheel, Dhaka. Heparin injection was purchased from Rotex Medica, Germany. Amylase was obtained from Merck, Germany. All the chemicals and reagents were analytical grade. Match® glucometer with strips were purchased from Mohammadpur, Dhaka. Humalyzer 3500 was obtained from Human Inc., Germany.
2.2. Plant material
Fruits of C. macroptera were collected from Sylhet, Bangladesh and authenticated by Md. Abdur Rahim, Technical officer, Department of Botany, Jahangirnagar University. A voucher specimen (Acc. No. 38619) was deposited in the herbarium for future reference. The peels were removed and the fruits were dried and used for further processing.
2.3. Preparation of plant extract
Fruits without rind were treated with sufficient amount of pure methanol for one week at room temperature with occasional shaking. The extract was filtered through a cotton plug followed by Whatman No. 1 filter paper. The filtrate was then evaporated under reduced pressure to give a dark green viscous mass and stored at 4 °C until use.
2.4. Animals and experimental set-up
Sprague-Dawley female rats of 100-200 g were collected from Pharmacology Laboratory, Department of Pharmacy, Jahangirnagar University and were acclimatized to normal laboratory conditions for one week prior to study and were assessed to pellet diet and water ad libitum. Temperature of facility was (22±3) °C and light/darkness alternated 12 h apart. The animals were divided into four groups of five animals each.
2.5. Phytochemical screening
The crude methanol extract of C. macroptera fruits underwent phytochemical screening to detect presence of potential phytochemical constituents like alkaloid, flavonoid, saponin, tannin, carbohydrate, glycoside, glucoside, fat and fixed oil, steroid and terpenoid[10].
2.6. In vitro α-amylase inhibitory activity
This study was performed by a modified starch iodine protocol[11]. In short, 1 mL of plant extract or standard of different concentration (2, 1, 0.5 mg/mL) was taken in pre-labeled test tubes. A volume of 20 µL of α-amylase was added to each test tube and incubated for 10 min at 37 °C. After the incubation 200 µL of 1% starch solution was added to each test tube and the mixture was re-incubated for 1 h at 37 °C. Then 200 µL of 1% iodine solution was added to each test tube and after that, 10 mL distilled water was added. Absorbance of the mixture was taken at 565 nm. Sample, substrate and α-amylase blank were undertaken under the same conditions. Each experiment was done in triplicate. IC50 value was calculated by using regression analysis.
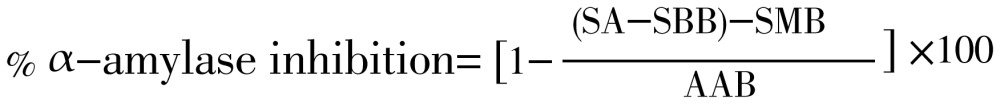 |
SA=Sample absorbance, SMB=Sample blank, SBB=Substrate blank, AAB=α-Amylase blank
2.7. Acute toxicity study
According to the method of Walum, et al. rats were divided into four groups of five animals each[12]. Different doses (1 000 mg/kg, 2 000 mg/kg, 3 000 mg/kg and 4 000 mg/kg) of methanol extracts were administered by stomach tube. Then the animals were observed for general toxicity signs.
2.8. Experimental protocol for in vivo hypoglycemic activity
2.8.1. Hypoglycemic effect in normal rats
Rats were kept fasting overnight with free access to water. Group I was treated as control group, Group II was treated with glibenclamide (5 mg/kg body weight), Group III and IV was treated with 500 mg/kg and 1 000 mg/kg body weight respectively. Before administration of drug and extract solutions fasting blood glucose levels were estimated by glucose oxidase method[13]. Then blood glucose levels were again estimated after 2 h of administration of drug and extract solutions. Glucose levels were measured by blood Humalyzer instrument. The maximum hypoglycemic effect of glibenclamide was found after 2 h of its administration.
2.8.2. Hypoglycemic effect in glucose induced hyperglycemic rats (OGTT)
Oral glucose tolerance test (OGTT) was performed according to the standard method[14]. Group I was treated as normal control group, Group II treated with glibenclamide (5 mg/kg body weight), Group III and IV treated with 500 mg/kg and 1 000 mg/kg body weight respectively. Glucose solution (1 g/kg body weight) was administered at first. Then drug and extract solutions were administered to the glucose fed. Serum glucose level of blood sample from tail vein was estimated by using glucometer at 0, 1, 2 and 3 h.
2.9. Statistical analysis
The results were expressed as the mean±SEM. The results were statistically analyzed using repeated measures analysis of variance with Dunnett's multiple comparison when compared against control in OGTT. Paired t test was performed to show significant variation between before and after blood glucose level. Student's t test was performed between IC50 values. Regression analysis was performed to calculate IC50 values. P<0.05, P<0.01 and P<0.001 were considered as statistically significant. Statistical programs used were GRAPHPAD PRISM® (version 6.00; GraphPad Software Inc., San Diego, CA, USA), SIGMAPLOT (version 12.0, Systat Software Inc., San Jose, California, USA), and Microsoft Excel, 2007.
3. Results
3.1. Phytochemical screening
The active components found in the extract include; saponin, steroid and terpenoid. Results are shown in Table 1.
Table 1. Phytochemical constituents identified in methanol extract of C. macroptera fruit.
| Phytochemical constituents | Specific tests | Result |
| Carbohydrate | Molisch 's test | - |
| Barfoed's test | - | |
| Benedict's test | - | |
| Fehling test | - | |
| Glycoside | General test | - |
| Bromine water test | - | |
| Glucoside | General test | - |
| Fat and fixed oils | Stain test | - |
| Saponification test | - | |
| Alkaloid | Mayer's test | - |
| Hager's test | - | |
| Dragendorff's test | - | |
| Wagner test | - | |
| Tannic acid test | - | |
| Tannin | Lead acetate test | - |
| Ferric chloride test | - | |
| Alkaline reagent test | - | |
| Steroid | Liebermann-Burchard's test | + |
| Terpenoid | Salkowski test | + |
| Saponin | Frothing test | + + |
| Flavonoid | General test | - |
| Shinoda test | +/- |
+: Presence, -: Absence, +/-: Presence/absence not ascertained.
3.2. In vitro α-amylase inhibitory activity
Methanol extract showed IC50 value (3.638±0.190) mg/mL whereas standard acarbose showed (0.912±0.015) mg/mL. Methanol extract significantly inhibited α-amylase activity in a dose dependent manner like acarbose. Therefore we can conclude that this fruit extract have moderate α-amylase inhibitory activity. All results are shown in Table 2.
Table 2. IC50 values (mg/mL) for C. macroptera methanolic fruit extract and acarbose in α-amylase inhibitory assay.
| Extract/Standard | Concentrations in mg/mL with ( % Inhibition ) | IC50 value ( mg/mL) | ||
| Methanol extract | 0.50 (8.974±0.256) | 1.00 (13.846±0.444) | 2.00 (28.718±1.282) | 3.638±0.190b |
| Acarbose | 0.25 (15.897±1.117) | 0.50 (26.051±0.438) | 1.00 (55.385±0.888) | 0.912± 0.015a |
Values are the mean of triplicate experiments and represented as mean±SEM (n=3). Values in the same column with different superscripts are significantly different (P<0.05). Student's t test was performed to analyze this data set.
3.3. Acute toxicity study
The extract administered up to high dose (4 000 mg/kg) produced no mortality. The animals did not manifest any sign of restlessness, respiratory distress, general irritation, coma or convulsion. Hence this extract was considered safe for rats.
3.4. In vivo hypoglycemic activity
3.4.1. Hypoglycemic effect in normal rats
Both doses of methanol extract and glibenclamide significantly reduced fasting blood glucose level. Glibenclamide showed significant reduction at level of P<0.01. Dose of 500 mg/kg and 1 000mg/kg methanolic fruit extract showed significant reduction at level of P<0.05 and P<0.01 respectively. These results suggest that hypoglycemic activity of 1 000 mg/kg dose and glibenclamide has similar significance level. All results are presented in Table 3.
Table 3. Effect of C. macroptera methanolic fruit extract on fasting blood glucose level (mmol/L) in normal rats.
| Group | Dose (oral) | Before administration | After administration |
| Control | 10 mL/kg | 4.851±1.188a | 5.764±0.668a |
| Glibenclamide | 5 mg/kg | 3.952±0.334a | 2.016±0.113b |
| 500 mg/kg | 500 mg/kg | 4.280±0.195a | 3.473±0.102b |
| 1000 mg/kg | 1000 mg/kg | 6.089±0.397a | 4.055±0.317b |
Values are presented in mean±SEM (n=4). Values in the same row with different superscripts are significantly different. For control (a, b) P>0.05, for glibenclamide (a, b) P<0.01, for Fruit-500 mg/kg (a, b) P<0.05 and for Fruit-1 000 mg/kg (a, b) P<0.01. Paired t test was performed to analyze this data set.
3.4.2. Hypoglycemic effect in glucose induced hyperglycemic rats (OGTT)
Experimental induction of hyperglycemia resulted in increased glucose level in blood (comparing the bar diagram of control of 0 h and 1 hour, Figure 1). Both dose of fruit extract did not manifest any significant reduction in 1st hour after administration. Most significant reduction (P<0.05) was observed for 500 mg/kg dose of methanolic fruit extract at 2 h but maximum reduction of 1 000 mg/kg dose occurred at 3 h showing a significance level of P<0.01. At 2 h this dose also showed significant reduction (P<0.05). Standard glibenclamide (5 mg/kg) showed significant reduction in 1, 2 and 3 h. These findings suggest that evidently 1 000 mg/kg dose is more potent than 500 mg/kg dose. Time interaction with each specific hour in this experiment was also found extremely significant (P<0.000 1) with an F value 23.83 (Table 4).
Figure 1. Effect of different doses of fruit extract of C. macroptera and glibenclamide on oral glucose tolerance test. Results are expressed as mean±SEM. Values with different superscripts are significantly different from control.
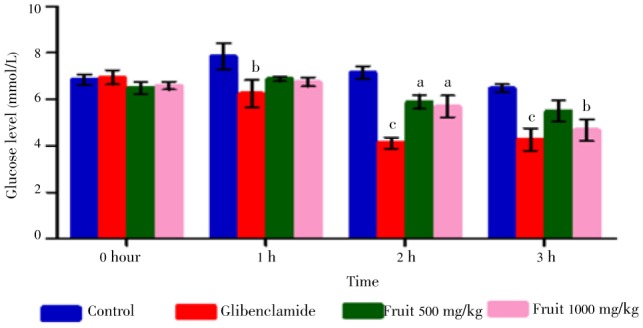
Table 4. Effect of C. macroptera methanolic fruit extract on glucose induced hyperglycemia (mmol/L) in normal rats.
| Treatment groups | 0 hour | 1 hour | 2 hour | 3 hour | Time effect (F value) | P value for time effect |
| Control | 6.850±0.222 | 7.850±0.569 | 7.150±0.272 | 6.475±0.175 | F(3, 36)=23.83 | P<0.000 1 |
| Glibenclamide | 6.950±0.301 | 6.250±0.589b | 4.125±0.239c | 4.275±0.476c | ||
| 500 mg/kg | 6.500±0.267 | 6.875±0.095 | 5.900±0.303a | 5.500±0.450 | ||
| 1000 mg/kg | 6.600±0.168 | 6.750±0.189 | 5.700±0.470a | 4.675±0.459b |
Values are presented in mean±SEM (n=4). Values with different superscripts in the same column are significantly different from control at each specific hour. a: P<0.05, b: P<0.01 and c: P<0.001. Overall time effect is considered extremely significant and its F and P value are given in the table. Repeated measures Anova with Dunnett's multiple comparison were performed to analyze this data set.
4. Discussion
4.1. α-Amylase inhibitory activity
α-Amylase is one of the main enzymes in human body that is responsible for the breakdown of starch to more simple sugars. α-Amylases hydrolyze complex polysaccharides to produce oligosaccharides and disaccharides which are then hydrolyzed by α-glycosidase to monosaccharide which are absorbed through the small intestines into the hepatic portal vein and increase postprandial glucose levels[15],[16]. Amylase inhibitors are also known as starch blockers because they prevent dietary starch from being absorbed by the body and thereby lower postprandial glucose levels. Slowing the digestion and breakdown of starch may have beneficial effects on insulin resistance and glycemic index control in people with diabetes[17]–[19]. In our investigation we found that methanolic fruit extract moderately inhibited α-amylase. From phytochemical screening we can see that presence of saponin, steroid and terpenoid which may be responsible for this therapeutic activity. Natural polyphenols have been reported to inhibit the activity of carbohydrate hydrolyzing enzymes like α-amylase, α-glucosidase[18]. Terpenoids represent a promising source for biologically active natural compounds which have potential for research and development of new substances with pharmacologic activity. α-Amylase inhibitory activity was related only for oleanane, ursane and lupane type terpenoids[20]. In a previous study saponins have also been found to be a probable α-amylase inhibitor[21],[22]. Lupeol, a terpenoid compound has been isolated from stem bark of C. macroptera which could be α-amylase inhibitor[23]. There are previous findings about the potentiality of this compound. Lupeol have been shown to inhibit α-amylase reported by Hasenah Ali, et al[24]. The mechanism by which this fruit extract exerted this effect may be due to its action on carbohydrate binding regions of α-amylase enzymes that catalyze hydrolysis of the internal α-1,4 glucosidic linkages in starch and other related polysaccharides have also been targeted for the suppression of postprandial hyperglycemia. Therefore, this study buttress the claim that natural inhibitors from dietary plants have α-amylase inhibitory activity and could be used as effective therapy for the management of postprandial hyperglycemia with minimal side effects.
4.2. In vivo hypoglycemic activity
In this study methanolic fruit extract of C. macroptera exerted significant hypoglycemic activity in both fasting glucose level reduction in normal rats and oral glucose tolerance test in glucose induced hyperglycemic rats. To our best knowledge this is the first study about hypoglycemic activity of C. macroptera. That's why the precise mode of action is not determined yet. However this fruit extract contains some potent phytochemical constituents like saponin, steroid and terpenoid which may be responsible for this action. Earlier investigations found saponins to be bioactive against diabetes[25],[26]. Saponins can influence transport systems that are situated in the brush-border. For example, Sidhu, et al. gave rats soyabean saponins (2 g/L) directly into the small intestine and this led to a reduced glucose uptake[27]. Glycemic decrease that may also occur due to the stimulatory effect on beta cells to promote insulin release and intracellular glycogen deposition. Total saponins identified from traditional medicinal plant in China dramatically reduced fasted blood glucose and serum insulin levels and alleviated hyperglycemia associated oxidative stress in experimental type 2 DM rats[28]. Terpenoids identified in primary screening may contribute to this property. Terpenoids isolated from the some anti-diabetic medicinal plants has been found to stimulate secretion or possess an insulin like-effect[29]. Glycogen concentration is directly proportional to insulin level[30]. Insulin promotes intracellular glycogen deposition by stimulating glycogen synthesis and inhibiting glycogen phosphorylase[30]. Such as insulin, terpenoids type component or monoterpenes may cause a restoration to normal glycogen metabolism when hepatic glycogen concentration is decreased[31]. Lupeol and stigmasterol isolated from stem bark of C. macroptera may have hypoglycemic potential[23]. Lupeol is a pharmacologically active terpenoid. One study has also found some activity as a dipeptidyl peptidase-4 inhibitor. Dipeptidyl peptidase-4 plays a major role in glucose metabolism. It is responsible for the degradation of incretins such as glucagon-like peptide-1[32]. Lupeol may also have amylase inhibition property which we told in previous section. We found the presence of steroid in phytochemical screening test which could also contribute to hypoglycemic action. In an earlier investigation Daisy, et al. isolated a novel steroid demonstrated a significant anti-diabetic activity by reducing the elevated blood glucose levels and restoring the insulin levels in streptozotocin-induced diabetic rats[33]. Stigmasterol, an steroid molecule, also possess hypoglycemic property[34]. Lemonene isolated from peel extract of C. macroptera may be a potential alternative[35]. In a previous study its anti-hyperglycemic activity has been confirmed in streptozotocin-induced diabetic rats by increasing glucokinase activity along with liver glycogen synthesis and decreasing plasma glucose and glycosylated hemoglobin levels[36]. Besides phytochemical constituents found in this extract may also have anti-α-amylase activity (discussed in previous section) and contribute to this in vivo hypoglycemic action.
We are still not sure about how this fruit extract can exert potent hypoglycemic activity. It is a logical inference that this extract may decrease the activity of α-amylase in the digestive canal, improve the metabolism of glucose and increase insulin secretion by stimulating beta cells. It is possibe to suggest that the bioactive compounds present in the fruit extract may be responsible for multifaceted effects. However further co-ordinated and well-structured studies would be required to isolate the bioactive compounds and determine their underlying molecular mechanism of action on diabetes induced rat model.
Acknowledgments
The authors are thankful to Pharmacology Laboratory, Department of Pharmacy, Jahangirnagar University for providing sufficient number of rats and wish to thank Laboratory of Natural Products Research, Jahangirnagar University for providing financial support and necessary reagents to conduct this research project.
Comments
Background
C. macroptera is commonly called ‘wild orange’. It is so-called name because of the large ‘wings’ (-ptera) on the petiole, which is as large as the blade of the leaf. The juice is very sour, and somewhat bitter. It has a unique taste and aroma. According to the literature search, there is no work that has been done on the fruit of this plant for hypoglycemic activity.
Research frontiers
The hypoglycemic effect in OGTT and α-amylase inhibitory activity was evaluated. The results support significant activity of this fruit extract which was the cutting age in the field of the research in this paper.
Related reports
Lupeol and stigmasterol were isolated from the crude extracts of the stem bark of C. macroptera (family: Rutaceae) by Chowdhury, et al (2008). The essential oil of C. macroptera Montr. contained limonene, beta-caryophyllene and geranial as main compounds reported by Rana, et al (2012). Traditionally this fruit is used as vegetable and curries cooked with Satkara and beef or mutton is now served in many restaurants in Bangladesh.
Innovations and breakthroughs
This is the first research work on C. macroptera fruit for hypoglycemic effect. In future these findings will help researchers to find out potential antidiabetic agents in this plant.
Applications
C. macroptera fruit is safe to eat. This study supports the claim that diabetes patients can eat this fruit because there is no restriction from physicians to take Citrus fruits. So this plant could be further studied for both drug development and establishment of the ethno medicinal use from this.
Peer review
This research is an excellent piece of work where hypoglycemic effect of a fruit extract from C. macroptera observed and this is a unique work of this fruit in the world as far as I know. Author coherently discussed therapeutic properties of phytochemical constituents to support the hypoglycemic potential of the fruit extract. α-Amylase inhibition property and OGTT was performed for this research and the result was significant; also there was a set up of standard methodology.
Footnotes
Foundation Project: Supported by Laboratory of Natural Products Research, Jahangirnagar University, Dhaka, Bangladesh
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Riaz S. Diabetes mellitus. Sci Res Essay. 2009;4(5):367–373. [Google Scholar]
- 2.Groop L, Forsblom C, Lehtovirta M. Characterization of the prediabetic state. Am J Hypertens. 1997;10:172S–180S. doi: 10.1016/s0895-7061(97)00149-0. [DOI] [PubMed] [Google Scholar]
- 3.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetes potential. J Ethnopharmacol. 2002;81(1):81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 4.Algariri K, Meng KY, Atangwho IJ, Asmawi MZ, Sadikun A, Murugaiyah V, et al. Hypoglycemic and anti-hyperglycemic study of Gynura procumbens leaf extracts. Asian Pac J Trop Biomed. 2013;3(5):358–366. doi: 10.1016/S2221-1691(13)60077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malviya N, Jain S, Malviya S. Antidiabetic potential of medicinal plants. Acta Pol Pharm. 2010;67(2):113–118. [PubMed] [Google Scholar]
- 6.Peng CH, Ker YB, Weng CF, Peng CC, Huang CN, Lin LY, et al. Insulin secretagogue bioactivity of finger citron fruit (Citrus medica L. var. Sarcodactylis Hort, Rutaceae) J Agric Food Chem. 2009;57(19):8812–8819. doi: 10.1021/jf902143x. [DOI] [PubMed] [Google Scholar]
- 7.Li JM, Che CT, Lau CBS, Leung PS, Cheng CHK. Inhibition of intestinal and renal Na+-glucose cotransporter by naringenin. Int J Biochem Cell Biol. 2006;38:985–995. doi: 10.1016/j.biocel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Kim GS, Shin JG, Jang HD. Antioxidant and antidiabetic activity of Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoi. Food Chem. 2009;117(1):35–41. [Google Scholar]
- 9.Kundusen S, Haldar PK, Gupta M, Mazumder UK, Saha P, Bala A, et al. Evaluation of antihyperglycemic activity of Citrus limetta fruit peel in streptozotocin-induced diabetic rats. ISRN Endocrinol 2011; doi. 2011 doi: 10.5402/2011/869273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kujur RS, Singh V, Ram M, Yadava HN, Singh KK, Kumari S, et al. Antidiabetic activity and phytochemical screening of crude extract of Stevia rebaudiana in alloxan-induced diabetic rats. Pharmacogn Res. 2010;2(4):258–263. doi: 10.4103/0974-8490.69128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hossan SJ, El-Sayed M, Aoshima H. Antioxidative and anti α-amylase activities of four wild plants consumed by nomads in Egypt. Orient Pharm Exp Med. 2009;9(3):217–224. [Google Scholar]
- 12.Walum E. Acute oral toxicity. Environ Health Perspect. 1998;106(Suppl 2):497–503. doi: 10.1289/ehp.98106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barham D, Trinder P. An improved color reagent for the determination of blood glucose by oxidase system. Analyst. 1972;97:142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 14.Vigneaud DU, Karr WG. Carbohydrate utilization: I. rate of disappearance of d-glucose from the blood. J Biol Chem. 1925;66:281–300. [Google Scholar]
- 15.Ranilla LG, Kwon YI, Apostolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour Technol. 2010;101(12):4676–4689. doi: 10.1016/j.biortech.2010.01.093. [DOI] [PubMed] [Google Scholar]
- 16.El-Kaissi S, Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: an update. Curr Diabetes Rev. 2011;7(6):392–405. doi: 10.2174/157339911797579160. [DOI] [PubMed] [Google Scholar]
- 17.Barrett ML, Udani JK. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): a review of clinical studies on weight loss and glycemic control. Nutr J. 2011;10:24. doi: 10.1186/1475-2891-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tundis R, Loizzo MR, Menichini F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycemic potential in the treatment of diabetes: an update. Mini Rev Med Chem. 2010;10(4):315–331. doi: 10.2174/138955710791331007. [DOI] [PubMed] [Google Scholar]
- 19.Nair SS, Kavrekar V, Mishra A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur J Exp Biol. 2013;3(1):128–132. [Google Scholar]
- 20.Sales PM, Souza PM, Simeoni LA, Silveira D. α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharm Sci. 2012;15(1):141–183. doi: 10.18433/j35s3k. [DOI] [PubMed] [Google Scholar]
- 21.Ponnusamy S, Ravindran R, Zinjarde S, Bhargava S, Ravi Kumar A. Evaluation of traditional Indian antidiabetic medicinal plants for human pancreatic amylase inhibitory effect in vitro. Evid Based Complement Alternat Med. 2011 doi: 10.1155/2011/515647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudha P, Zinjarde SS, Bhargava SY, Kumar AR. Potent α-amylase inhibitory activity of Indian ayurvedic medicinal plants. BMC Complement Altern Med. 2011 doi: 10.1155/2011/515647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhury SA, Sohrab MH, Datta BK, Hasan CM. Chemical and antioxidant studies of Citrus macroptera. Bangladesh J Sci Ind Res. 2008;43(4):449–454. [Google Scholar]
- 24.Ali H, Houghton PJ, Soumyanath A. Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006;107(3):449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Hamden K, Jaouadi B, Salami T, Carreau S, Bejar S, Elfeki A. Modulatory effect of fenugreek saponins on the activities of intestinal and hepatic disaccharidase and glycogen and liver function of diabetic rats. Biotechnol Bioprocess Eng. 2010;15(5):745–753. [Google Scholar]
- 26.Chen ZH, Li J, Liu J, Zhao Y, Zhang P, Zhang MX, et al. Saponins isolated from the root of Panax notoginseng showed significant anti-diabetic effects in KK-Ay mice. Am J Chin Med. 2008;36(5):939–951. doi: 10.1142/S0192415X08006363. [DOI] [PubMed] [Google Scholar]
- 27.Sidhu GS, Upson B, Malinow MR. Effects of soy saponins and tigogenin cellobioside on intestinal uptake of cholesterol, cholate and glucose. Nutr Rep Int. 1987;35(3):615–623. [Google Scholar]
- 28.Zheng T, Shu G, Yang Z, Mo S, Zhao Y, Mei Z. Antidiabetic effect of total saponins from Entada phaseoloides (L.) Merr. in type 2 diabetic rats. J Ethnopharmacol. 2012;139(3):814–821. doi: 10.1016/j.jep.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Goto T, Takahashi N, Hirai S, Kawada T. Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res. 2010 doi: 10.1155/2010/483958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen J, Lai YC. Regulation of muscle glycogen synthase phosphorylation and kinetic properties by insulin, exercise, adrenaline and role in insulin resistance. Arch Physiol Biochem. 2009;115(1):13–21. doi: 10.1080/13813450902778171. [DOI] [PubMed] [Google Scholar]
- 31.Muhammad NO, Soji-Omoniwa O, Usman LA, Omoniwa BP. Antihyperglycemic activity of leaf essential oil of Citrus sinensis (L.) Osbeck on alloxan-induced diabetic rats. Ann Rev Res Biol. 2013;3(4):825–334. [Google Scholar]
- 32.Marques MR, Stüker C, Kichik N, Tarragó T, Giralt E, Morel AF, et al. Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers. Fitoterapia. 2010;81(6):552–556. doi: 10.1016/j.fitote.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Daisy P, Jasmine R, Ignacimuthu S, Murugan E. A novel steroid from Elephantopus scaber L. an ethnomedicinal plant with antidiabetic activity. Phytomedicine. 2009;16:252–257. doi: 10.1016/j.phymed.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Panda S, Jafri M, Kar A, Meheta BK. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia. 2009;80(2):123–126. doi: 10.1016/j.fitote.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Rana VS, Blazquez MA. Compositions of the volatile oils of Citrus macroptera and C. maxima. Nat Prod Commun. 2012;7(10):1371–1372. [PubMed] [Google Scholar]
- 36.Murali R, Saravanan R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed Prev Nutri. 2012;2(4):269–275. [Google Scholar]


