Abstract
The Flt3-Flt3 ligand (Flt3L) pathway is critically involved in the differentiation and homeostasis of myeloid cells, including dendritic cells (DC); however, its role in the expansion and function of myeloid-derived suppressor cells (MDSC) has not been determined. Herein, we describe the ability of Flt3L to expand and activate murine MDSC capable of suppressing allograft rejection upon adoptive transfer. While Flt3L expands and augments the stimulatory capacity of myeloid DC, MDSC expanded by Flt3L have increased suppressive activity. Although STAT3 is considered the central transcription factor for MDSC expansion, inhibition and genetic ablation of STAT3 did not block, but augmented Flt3L-mediated MDSC expansion. MDSC suppressive function, preserved when STAT3 inhibition was removed, was reduced by genetic STAT3 deletion. Both STAT3 inhibition and deletion reduced Flt3L-mediated DC expansion, signifying that STAT3 had reciprocal effects on suppressive MDSC and immunostimulatory DC expansion. Together, these findings enhance understanding of the immunomodulatory properties of Flt3L.
Introduction
Myeloid-derived suppressor cells (MDSC) are recently-characterized innate immunoregulatory cells that expand under inflammatory conditions, such as cancer, sepsis, allograft rejection, and autoimmunity [reviewed (1, 2)]. Although mouse and human MDSC exhibit considerable heterogeneity, they share the ability to induce apoptosis or suppress T cell proliferation and secretion of cytokines (2, 3). In mice, MDSC are broadly identified as CD11b+Gr1+ cells, while cell morphology and differential surface expression of the Gr1 Ag Ly6G and Ly6C distinguish granulocytic (CD11b+Ly6G+) and monocytic (CD11b+Ly6C+) subsets (1). Expansion and activation of MDSC occurs through the action of growth factors that promote myelopoiesis (4, 5) and pro-inflammatory cytokines (1, 5).
Fms-like tyrosine kinase 3 [Flt3; CD135; fetal liver kinase-2 (Flk2)] is a receptor tyrosine kinase expressed on hematopoietic stem cells and early precursors (6). The Flt3-Flt3 ligand (Flt3L) pathway is critically involved in dendritic cell (DC) homeostasis (7–9). Flt3L activates the transcription factor STAT3 (10), that is strongly implicated in MDSC expansion and function (1). However, the potential of Flt3L to support MDSC expansion/activation is undefined. Due to the potent ability of Flt3L to increase myeloid precursors and activate STAT3, we hypothesized that Flt3L-driven myelopoiesis would not only promote DC, but also suppressive MDSC.
Herein, we report that Flt3L expands and activates Ly6G+ and Ly6C+ MDSC. In contrast, DC expanded by Flt3L are more stimulatory than steady-state DC. Although DC expansion by Flt3L is dependent on STAT3, surprisingly, conditional ablation of STAT3 enhances Flt3L-induced mobilization of MDSC. However, Flt3L-expanded MDSC depended on STAT3 for optimal suppressive function. Adoptive transfer of Flt3L-mobilized MDSC, but not steady-state CD11b+Gr1+ cells, prolongs fully MHC-mismatched cardiac allograft survival.
Materials and Methods
Animals and drug administration
All mice for breeding and experimentation were from The Jackson Laboratory. 8–12 week old male BALB/c (H2Kd) or C57BL/6 (B6; H2Kb) mice were given r human Flt3L (10 μg/d i.p.; Amgen) for 10 d. Mice with conditional STAT3 gene disruption were generated by interbreeding mice expressing Cre under the LysM promoter (B6.129P2-Lyz2tm1(cre)Ifo/J) and floxed STAT3 (B6.129S1-Stat3tm1Xyfu/J) genes. The genetic background of crossed mice was verified by PCR genotyping and littermates used as negative controls. The STAT3 inhibitor S31-201 (5 mg/kg; Selleck Chemicals) was administered i.p. as described (11). All studies were performed under an institutional animal care and use committee-approved protocol.
Flow cytometry
Cell surface and intracellular marker expression was analyzed as described (12, 13).
MLR and suppression assay
MDSC were isolated from splenocytes by positive selection with FITC anti-Ly6C, PE anti-Ly6G, or PE anti-Gr1 using anti-FITC or anti-PE microbeads (Miltenyi Biotec), as described (12, 14). DC were isolated by CD11c immunomagnetic bead selection and γ-irradiated (20 Gy). T cell proliferation was assessed at 72 h by [3H] TdR incorporation or CFSE dilution (Invitrogen).
Vascularized heart transplantation
Heterotopic intra-abdominal mouse heart transplantation was performed and graft survival monitored as described (14).
Statistics
Data are presented as means ± 1 standard deviation. Significant differences between means and survival curves were determined using a two-tailed, Student’s ‘t’ test and log-rank test, respectively.
Results and Discussion
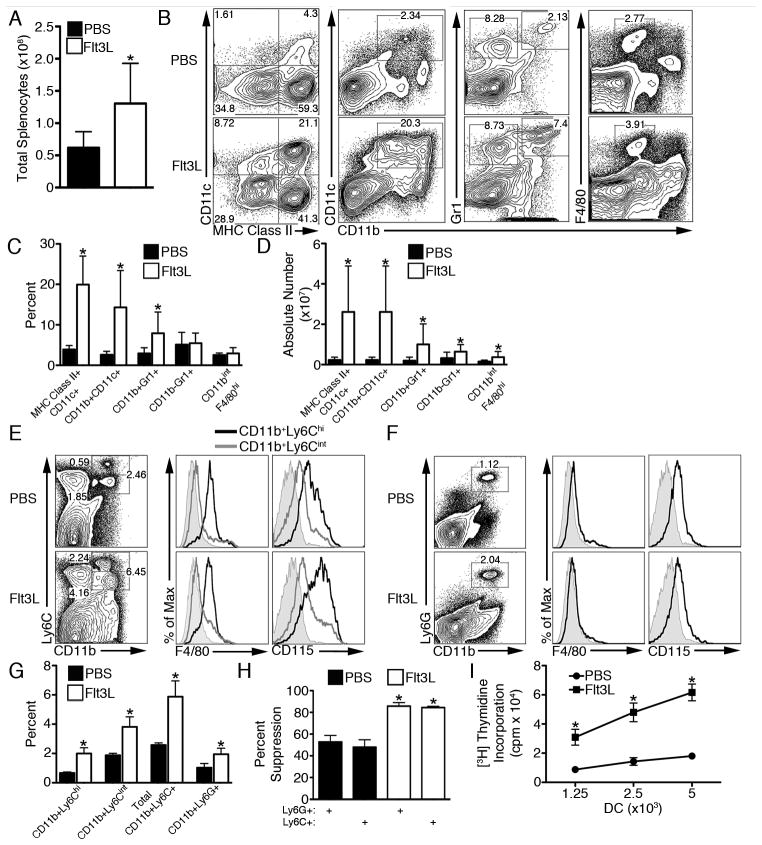
We first examined myeloid populations expanded by Flt3L. Total splenocyte number was increased (Fig. 1A) and, in agreement with previous studies (7, 9), Flt3L increased the frequency and absolute number of conventional myeloid DC (Fig. 1B–D). Splenic CD11b+Gr1+ cells were also increased in frequency and absolute number by Flt3L; however, macrophage (CD11bintF4/80hi) frequency was unchanged (Fig. 1B, 1C). Of the lymphoid populations examined, Flt3L only increased the frequency of Foxp3+ regulatory T cells (Treg); however, the absolute number of all T cell populations was augmented (Supplementary Fig. 1). This increase in naturally-occurring Treg is thought to be due to DC-mediated Treg expansion (15). Pro-inflammatory cytokines are not expected to play a significant role in modulating the incidence or frequency of cell populations, as IL-1β, IL-4, IL-6, IL-10, IL-12p40, TNFα, and IFNγ were all nor detected in the serum of both PBS- and Flt3L-treated mice (data not shown). Interestingly, circulating TGF-β1 levels were reduced by 3-fold in Flt3L-treated mice (1229±265.8 pg/ml in naïve mice versus 441.1±26.15 pg/ml in Flt3L-treated mice; p<0.05).
FIGURE 1.
Flt3L expands myeloid DC with augmented T cell stimulatory capacity but suppressive CD11b+Gr1+ cells. A, Total viable splenocytes from Flt3L-treated mice were enumerated using trypan blue exclusion. B–C, The frequency of splenic myeloid DC (CD11c+MHC class II+; CD11b+CD11c+), CD11b+Gr1+ cells, and macrophages (CD11bintF4/80hi) within CD45+-gated cells was determined and (D) absolute number quantified. F4/80 and CD115 expression was determined on (E) CD11b+Ly6Cint/hi and (F) CD11b+Ly6G+ splenocytes and (G) their frequency quantified. H, BALB/c Ly6C+ and Ly6G+ splenocytes (2×105) were used as suppressors of B6 CD3+ T cells (2×105) stimulated with Flt3L-mobilized BALB/c CD11c+ DC (2×104). I, BALB/c CD11c+ DC were used to stimulate B6 CD3+ T cells (1×105). Data are representative of n≥2 experiments with n≥3 mice per group. A–I, * p<0.05.
We next sought to further characterize surface Ag expression on CD11b+Gr1+ cells expanded by Flt3L. Flt3L induced expansion of both CD11b+Ly6Cint/hi and CD11b+Ly6G+ cells (Fig. 1E–G). CD11b+Ly6Chi cells expressed an intermediate level of F4/80 and were CD115 (M-CSF receptor)+, consistent with surface Ag expression described for MDSC (Fig. 1E) (1). CD11b+Ly6Cint and CD11b+Ly6G+ cells were F4/80− and expressed only low levels of CD115 (Fig. 1E, 1F). Previously, Solheim et al (16) described an increase in splenic CD11b+Gr1+ cells following adenoviral delivery of Flt3L to tumor-bearing mice; however, the suppressive function of these cells was not assessed. We now show that both Ly6C+ monocytic and Ly6G+ granulocytic MDSC from Flt3L-treated mice are suppressive in MLR (Fig. 1H). Moreover, both Flt3L-expanded Ly6C+ and Ly6G+ MDSC were significantly more potent suppressors than their counterparts from steady-state control mice (Fig. 1H). By contrast, CD11c+ DC isolated from Flt3L-treated mice demonstrated increased allogeneic T cell stimulatory capacity (Fig. 1I). Thus, these data reveal that Flt3L has reciprocal capacities to expand functionally distinct populations of stimulatory DC and suppressive MDSC.
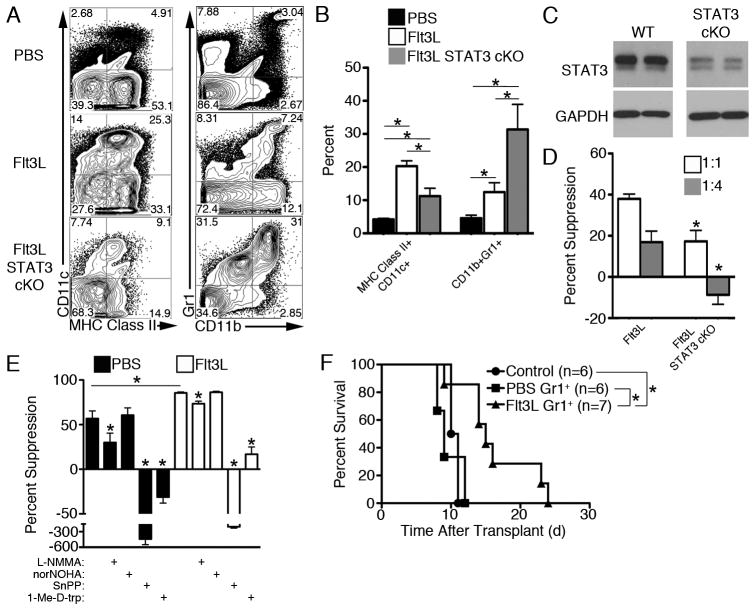
STAT3 is considered the key regulator of MDSC expansion and suppressive function, and Flt3L is a potent activator of STAT3 (10). Therefore, we next ascertained whether STAT3 is required for Flt3L-mediated MDSC expansion. Genetic ablation of STAT3 prior to Flt3L administration reduced the frequency of myeloid DC (Fig. 2A–B), consistent with earlier reports using conditional STAT3 knockout mice (10). By contrast, expansion of CD11b+Gr1+ cells by Flt3L was augmented by STAT3 deletion (Fig. 2A–C). Chemical inhibition of STAT3 in vivo during Flt3L administration generated similar results (Supplementary Fig. 2). Flt3L causes an accumulation of common myeloid progenitors in conditional STAT3 knockout mice (10), which may serve as an important source of immunosuppressive MDSC. Consistent with the importance of STAT3 in GM-CSF-mediated activation (17), STAT3 deletion reduced Flt3L-expanded MDSC suppressive function (Fig. 2D). However, this did not occur when MDSC were isolated from mice treated with a STAT3 inhibitor and Flt3L (Supplementary Fig. 2F) potentially due to reversibility of STAT3 inhibition during ex vivo suppression. MDSC suppress T cell proliferation through several immunosuppressive enzymes, including arginase-1, inducible nitric oxide synthase, heme oxygenase-1 (HO-1), and IDO (1, 18, 19). Both steady-state control and Flt3L-mobilized Gr1+ cells independently required HO-1 and IDO for suppression of T cell proliferation (Fig. 2E) and predominantly suppressed CD4+ T cells without affecting Treg frequency (Supplementary Fig. 2G–J).
FIGURE 2.
Flt3L-mobilized MDSC are expanded in a STAT3-independent manner and prolong cardiac allograft survival. A, STAT3 conditional KO (cKO) mice were generated using LysM-Cre and floxed STAT3 mice and administered Flt3L. Splenic DC and MDSC were identified by flow cytometry and (B) quantified. C, STAT3 deletion in isolated splenic Flt3L-expanded Gr-1+ cells was verified by Western blot. D, Suppressive capacity of Flt3L-expanded MDSC from STAT3+ and STAT3 cKO were compared in suppression assays when splenic B6 Gr1+ cells (1×105 or 0.25×105) were used as suppressors of B6 CD3+ T cells (1×105) stimulated with BALB/c Flt3L-mobilized CD11c+ DC (1.25×104). E, Splenic BALB/c Gr1+ cells (1×105) were used as suppressors of BALB/c CD3+ T cells (1×105) stimulated with B6 Flt3L-mobilized CD11c+ DC (1.25×104). Inhibitors of nitric oxide synthase (NG-Methyl-L-arginine; L-NMMA; 0.5 mM), arginase-1 (Nω-Hydroxy-nor-L-arginine; norNOHA; 0.5 mM), HO-1 (tin protoporphyrin; SnPP; 0.15 mM), or IDO (1-methyl-D-tryptophan; 1-Me-D-trp; 0.2 mM) were added at the start of co-culture where indicated. F, 5×106 BALB/c Gr1+ cells were administered to BALB/c recipients i.v., 1 d before B6 heart transplant, and allograft survival was monitored. Data are representative of n=2 independent experiments with 6–7 total mice per group. D, * p<0.05 compared to Flt3L. E, * p<0.05 compared to PBS or Flt3L Gr1+ cells in the absence of inhibitor, unless otherwise indicated.
Adoptively-transferred bone marrow-derived MDSC inhibit graft-versus-host disease (20), and allogeneic skin transplant-activated MDSC transferred to skin graft recipients prolong survival (21). Furthermore, MDSC are required for the induction of organ transplant tolerance by costimulation blockade (22). In the present study, Gr1+ cells isolated from splenocytes of Flt3L-treated mice, but not control mice, prolonged fully MHC-mismatched cardiac allograft survival significantly in the absence of additional immunosuppression (Fig. 2F), thus demonstrating their in vivo suppressive function. Flt3L has been reported to have both pro- and anti-inflammatory effects in disease models (23–25). Thus, the varying impact of Flt3L on immune responses in vivo remains poorly understood, and the role of MDSC in these models has not been explored. Our data show that Flt3L mediates STAT3-independent expansion of suppressive MDSC but STAT3-dependent expansion of stimulatory CD11c+ DC. These data also add further support for the importance of the STAT3 pathway for suppressive activity of cytokine-expanded MDSC.
These findings have significant clinical relevance for the use of Flt3L as an immune modulating agent. Combination of Flt3L administration with STAT3 inhibition could promote effective immune regulation, given the expectation that STAT3 inhibition will counter Flt3L-driven DC generation, but allow MDSC expansion. Plus, our data suggest that upon clearance of STAT3 inhibition, augmented MDSC will be functionally suppressive. Conversely, delivery of Flt3L with inhibitors of IDO or HO-1 would be expected to augment previously demonstrated immune adjuvant properties of Flt3L.
Supplementary Material
Acknowledgments
BRR was supported by an American Heart Association Pre-Doctoral Fellowship (11PRE7070020), and DRR was supported by a European Society for Organ Transplantation/American Society of Transplantation Grant. BMM was supported by National Institutes of Health (NIH) institutional training grant T32AI74490 (AWT). This work was also supported by NIH grants R00HL097155 (HRT) and R01AI67541 (AWT).
Nonstandard abbreviations used in this paper
- DC
dendritic cell
- Flt3L
Flt3 ligand
- HO-1
heme oxygenase-1
- MDSC
myeloid-derived suppressor cell
- Treg
regulatory T cell
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lees JR, Azimzadeh AM, Bromberg JS. Myeloid derived suppressor cells in transplantation. Curr Opin Immunol. 2011;23:692–697. doi: 10.1016/j.coi.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 5.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin−Sca1+c-kit+ stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 7.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 9.O’Keeffe M, Hochrein H, Vremec D, Pooley J, Evans R, Woulfe S, Shortman K. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–2130. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 10.Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity. 2003;19:903–912. doi: 10.1016/s1074-7613(03)00332-7. [DOI] [PubMed] [Google Scholar]
- 11.Mir SA, Chatterjee A, Mitra A, Pathak K, Mahata SK, Sarkar S. Inhibition of signal transducer and activator of transcription 3 (STAT3) attenuates interleukin-6 (IL-6)-induced collagen synthesis and resultant hypertrophy in rat heart. J Biol Chem. 2012;287:2666–2677. doi: 10.1074/jbc.M111.246173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosborough BR, Castellaneta A, Natarajan S, Thomson AW, Turnquist HR. Histone deacetylase inhibition facilitates GM-CSF-mediated expansion of myeloid-derived suppressor cells in vitro and in vivo. J Leuk Biol. 2012;91:701–709. doi: 10.1189/jlb.0311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnquist HR, Cardinal J, Macedo C, Rosborough BR, Sumpter TL, Geller DA, Metes D, Thomson AW. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood. 2010;115:4758–4769. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Stolz DB, Zheng XX, Demetris AJ, Liew FY, Wood KJ, Thomson AW. IL-33 expands suppressive CD11b+Gr-1int and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol. 2011;187:4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solheim JC, Reber AJ, Ashour AE, Robinson S, Futakuchi M, Kurz SG, Hood K, Fields RR, Shafer LR, Cornell D, Sutjipto S, Zurawski S, LaFace DM, Singh RK, Talmadge JE. Spleen but not tumor infiltration by dendritic and T cells is increased by intravenous adenovirus-Flt3 ligand injection. Cancer Gene Ther. 2007;14:364–371. doi: 10.1038/sj.cgt.7701018. [DOI] [PubMed] [Google Scholar]
- 17.Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K, Abrams SI. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123:4464–4478. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Wilde V, Van Rompaey N, Hill M, Lebrun JF, Lemaitre P, Lhomme F, Kubjak C, Vokaer B, Oldenhove G, Charbonnier LM, Cuturi MC, Goldman M, Le Moine A. Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant. 2009;9:2034–2047. doi: 10.1111/j.1600-6143.2009.02757.x. [DOI] [PubMed] [Google Scholar]
- 19.Mougiakakos D, Jitschin R, von Bahr L, Poschke I, Gary R, Sundberg B, Gerbitz A, Ljungman P, Le Blanc K. Immunosuppressive CD14+HLA-DRlow/neg IDO+ myeloid cells in patients following allogeneic hematopoietic stem cell transplantation. Leukemia. 2013;27:377–388. doi: 10.1038/leu.2012.215. [DOI] [PubMed] [Google Scholar]
- 20.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, Tolar J, Ochoa AC, Blazar BR. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Liang S, Wu J, Horuzsko A. Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation. 2008;86:1125–1134. doi: 10.1097/TP.0b013e318186fccd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, Ma G, Hashimoto D, Li Y, Boros P, Grisotto M, van Rooijen N, Matesanz R, Tacke F, Ginhoux F, Ding Y, Chen SH, Randolph G, Merad M, Bromberg JS, Ochando JC. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest. 2010;120:2486–2496. doi: 10.1172/JCI41628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eto M, Hackstein H, Kaneko K, Nomoto K, Thomson AW. Promotion of skin graft tolerance across MHC barriers by mobilization of dendritic cells in donor hemopoietic cell infusions. J Immunol. 2002;169:2390–2396. doi: 10.4049/jimmunol.169.5.2390. [DOI] [PubMed] [Google Scholar]
- 24.Blazar BR, McKenna HJ, Panoskaltsis-Mortari A, Taylor PA. Flt3 ligand (FL) treatment of murine donors does not modify graft-versus-host disease (GVHD) but FL treatment of recipients post-bone marrow transplantation accelerates GVHD lethality. Biol Blood Marrow Transplant. 2001;7:197–207. doi: 10.1053/bbmt.2001.v7.pm11349806. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Taner T, Morelli AE, Thomson AW. Hosts lacking fms-like tyrosine kinase 3 ligand exhibit marked reductions in transplant vascular sclerosis. Transplantation. 2005;79:869–875. doi: 10.1097/01.tp.0000157120.43052.3a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.