SYNOPSIS
Signaling through the receptor tyrosine kinase KIT mediates differentiation, proliferation and survival of hematopoietic precursor cells and mast cells. Constitutive KIT signaling due to somatic point mutations in c-Kit is an important occurrence in the development of mast cell proliferation disorders and other hematological malignancies. In this review, we discuss the common gain-of-function mutations found in these malignancies, particularly in mast cell proliferation disorders, and summarize the current understanding of the molecular mechanisms by which transforming point mutations in KIT may affect KIT structure and function and lead to altered downstream signaling and cellular transformation. Drugs targeting KIT have shown mixed success in the treatment of these diseases. A brief overview of the most common KIT inhibitors currently used, the reasons for the varied clinical results of such inhibitors and a discussion of potential new strategies are provided.
Keywords: Mastocytosis, KIT mutations, KIT signaling, KIT trafficking, KIT inhibitors
INTRODUCTION
The c-Kit proto-oncogene is the cellular, un-truncated counterpart of the gene in the Hardy-Zuckerman feline sarcoma virus genome (v-Kit) responsible for its transforming activity (1). Gain-of-function mutations in c-Kit promoting tumor formation and progression have been identified in certain human cancers, a knowledge that has boosted an interest in targeting the activity of this receptor.
c-Kit encodes for a protein, KIT (CD117), belonging to a family of transmembrane growth factor receptors with intrinsic tyrosine kinase activity (2). Its specific ligand is stem cell factor (SCF), also known as KIT ligand, mast cell growth factor, or steel factor (3, 4). SCF is primarily, but not exclusively, produced by stromal cells such as fibroblasts in two major forms, a soluble form and a membrane-bound form, which are present at varying ratios in different tissues (3, 5, 6). Both forms activate KIT but may mediate qualitatively and quantitatively different types of responses (7, 8), although the specific mechanisms remain largely unknown.
KIT is highly expressed in hematopoietic stem cells from the bone marrow and its activity is critical for constitutive hematopoiesis and for the proliferation, survival, differentiation and homing of these cells (8–10). Expression of KIT is generally lost during the differentiation process of most hematopoietic cells, with the exception of mast cells, which retain KIT through their lifespan. KIT thus plays an important role in mast cell proliferation, survival and function (11–15). KIT expression can be upregulated during an immune response in eosinophils (16) and dendritic cells (17), whereas in both human basophils and eosinophils KIT expression is generally found at low levels (18, 19). The expression of KIT is, however, not restricted to hematopoietic cells: it is expressed in melanocytes, interstitial cells of Cajal in the gastrointestinal tract (20) and other cell types (21–24). Accumulated evidence in rodent models with KIT alterations has provided insights on the cell populations that are most critically KIT-dependent. Thus, mice carrying mutations that impair KIT structure or expression (such as WBB6F1- KitW/W-v and C57BL/6-KitW-sh/W-sh mice) exhibit specific phenotypic abnormalities in their adulthood, including profound mast cell and melanocyte deficiency, macrocytic anemia, reduced fertility and a lack of gut interstitial cells of Cajal resulting in reduced pacemaker activity in the small intestine (4, 20, 24). Absence of KIT or its ligand in mice is embryonic or perinatal lethal, suggesting a critical, broader biological role of SCF/KIT signaling during embryogenesis (4). In humans, loss-of-function mutations in c-Kit associate with piebaldism, a rare, autosomal dominant disorder characterized by congenital white patches in the skin and hair caused by improper migration of melanoblasts in the embryo (25), while acquired gain-of–function mutations in c-Kit result in particular neoplastic diseases.
In this review, we will provide a general overview of the consequences of gain-of-function mutations in c-Kit, the structure and molecular mechanisms governing KIT signaling and describe how gain-of-function mutations in c-Kit result in its overactive function and lead to cellular transformation, with particular focus on mast cells and disorders of pathologic mast cell proliferation.
c-Kit MUTATIONS AND LINK TO MALIGNANCIES
Human malignancies associated with activating c-Kit mutations include mast cell proliferative disorders, gastrointestinal stromal tumors (GISTs) and less commonly, melanoma and acute myeloid leukemia. Increased expression of normal c-Kit may also contribute to tumorigenesis in solid lung cancers from small lung cells that do not normally express KIT and are exposed to environments rich in SCF. Activating mutations in small lung cancer cells, nonetheless, have rarely been found and their involvement in tumor progression is still unclear (26–28). Dysregulation of KIT activity plays a central role, however, in the pathogenesis of those malignancies originated from cells dependent on SCF for differentiation/survival such as mast cells and interstitial cells of Cajal (29–33). GISTs are believed to derive from interstitial cells of Cajal and in up to 80% of sporadic GISTs, at least 17 different activating mutations involving exons 8, 11, 13 or 17 of c-Kit have been reported (29, 34). Similarly, approximately 90% of adults with diseases of abnormal mast cell proliferation (mastocytosis), have at least a point mutation consisting of a substitution of Aspartic acid to Valine in the catalytic domain of c-Kit (D816V), rendering it constitutively active (35–37) and/or other mutations in c-Kit.
Although KIT is also critical for melanocyte physiology, transforming mutations in c-Kit appear only in about 3% of all melanomas, particularly in metastatic melanoma (reviewed in (38)). This is consistent with the observation that an activating c-Kit point mutation in genetically modified mouse melanocytes predominantly increased melanocyte migration over any effect in proliferation or pigment production (39). Because maturation of hematopoietic cells other than mast cells results in down-regulation of c-Kit expression, transforming mutations of this receptor rarely affect most hematopoietic lineages (31). However, mutations or internal tandem duplications in c-Kit that contribute to pathogenesis have been observed in approximately 17% of acute myeloid leukemias (AML) (31, 40, 41). These are acquired somatic mutations present in a clonal lineage population and it is thought that the ultimate phenotype of malignant hemopoietic cells of a specific lineage expressing mutant KIT is influenced by additional complementing co-oncogenic events or epigenetic modifications that affect their differentiation process, proliferation and survival (31, 42).
As it will be discussed, therapies blocking KIT activity have been somewhat successful in the treatment of some of these malignancies alone or in combination, but not in others where complete remissions or improved survival time is rare. The success of therapy is linked to the type of mutation and/or the presence of additional mutations in c-Kit or other proto-oncogenes.
c-Kit and neoplastic growth of mast cells
Mast cells are derived from CD34+ bone marrow hematopoietic pluripotent progenitors (43), but fully differentiate in tissues, where they establish residency (for review see (44)). Mast cells are usually located around blood vessels and nerves within the connective tissue, where SCF is abundant. The role of mast cells in the regulation of adaptive responses occurs primarily through cell surface Fc receptors that bind immunoglobulin antibodies, particularly FcεRI, the high receptor for immunoglobulin IgE (reviewed in (45)). Upon encountering a multivalent allergen (antigen, Ag), IgE receptors in the mast cell surface bound to Ag-specific IgE are aggregated and the pro-inflammatory mediators contained in mast cell granules are secreted by degranulation or are synthesized de novo, causing an immediate hypersensitivity allergic reaction. Mast cells also respond to a variety of stimuli in the tissue environment that can alter mast cell function, and thus the allergic response. Among those, SCF can synergistically enhance mast cell degranulation, cytokine production and chemotactic migration induced by other stimulants, particularly IgE/antigen (reviewed in (46)). Thus, changes in SCF concentrations in the tissue environment or dysregulated KIT activity may not only affect mast cell numbers and homeostasis (11) but also mast cell responsiveness.
Mast cells are found in excessive numbers in tissues in a heterogeneous group of disorders collectively known as mastocytosis. Diseases of pathologic mast cell proliferation are classified into disease variants based on clinical presentation, pathologic findings, and prognosis. There are excellent reviews covering the criteria and symptoms of these variants and thus they will not be detailed here (33, 37, 47). In general, mastocytosis is classified under cutaneous (the most benign form and best prognosis with mostly skin involvement) and systemic mastocytosis (with mast cell infiltrates and effects in the bone marrow and extracutaneous organs such as liver, spleen or lymph nodes). The occurrence of somatic activating mutations in the c-Kit gene in a hematopoietic progenitor cell is considered an important early event in the progression of mastocytosis. Particularly, a mutation in position 816 of KIT from Aspartate to Valine that results in constitutive ligand-independent tyrosine phosphorylation of KIT and tumorigenecity in mice (48), is highly associated with mastocytosis in adult patients, but is less frequently found in cases of children with mastocytosis, which is usually cutaneous and often resolves before puberty (37, 49–51). Although this is suggestive of a different basis for the children’s form of this disease, a recent study in a larger cohort of pediatric mastocytosis found that indeed 86% of these patients had activating mutations in c-Kit, with the D816V mutation present in 35% of cases (52). The reasons for the frequent recession rate in children are, however, still unclear. It appears that the presence of gain-of-function mutations of KIT is a necessary prerequisite for mastocytosis in the majority of cases, but the phenotypic diversity in mastocytosis may arise from a combination of additional acquired mutations or other inherited genetic polymorphisms. For example, recent studies in our lab identified activating mutations in N-RAS in 2 out of 8 patients with advanced mastocytosis that seemed to precede the D816V c-Kit mutation because they were present in the CD34+ progenitors while the D816V mutation was not (53). In addition to N-RAS mutations that may further promote the clonal expansion of mast cells and disease severity in some patients, other epigenetic changes or alterations in RNA splicing can also influence the pathogenesis of mastocytosis. Along these lines, a splice variant of KIT missing 4 amino acids in the extracellular domain (GNNK-) (8) is preferentially expressed in severe mastocytosis patients with the D816V mutation as compared to normal individuals (54, 55), a finding that correlates with the ability of this isoform to enhance KIT signaling and the maturation of mast cells (55). In agreement with a participation of splice variant expression in the overall disease severity, no differences in the relative expression of the GNNK− and GNNK+ isoforms were found in the pediatric, less severe form of the disease (52). Other alterations in c-Kit gene transcripts in patients with systemic mast cell activation disorders have been described (54), however the significance of these alternative splice forms is unclear.
In addition to promoting mast cell proliferation and survival, persistent activation of KIT may reduce the threshold of mast cell activation to other stimuli. Thus it is not unexpected that patients suffer recurrent spontaneous episodes of flushing, shortness of breath, palpitations, nausea, diarrhea, abdominal pain, hypotension or a combination of these symptoms (37) as a consequence of increased mast cell mediator release. In fact, the prevalence of anaphylaxis in adults diagnosed with mastocytosis is considerably higher than expected in the general population (56). Despite the progress in the understanding of KIT signaling in mast cells the exact triggers and specific signaling mechanisms involved in these episodes remain poorly understood (37, 56).
In the next section we will describe the structural and mechanistic characteristics of KIT in normal conditions and how the structure/function interrelationship of the SCF receptor can be altered by mutation in neoplastic diseases, particularly in mastocytosis.
MECHANISMS OF KIT ACTIVATION
Structure of KIT
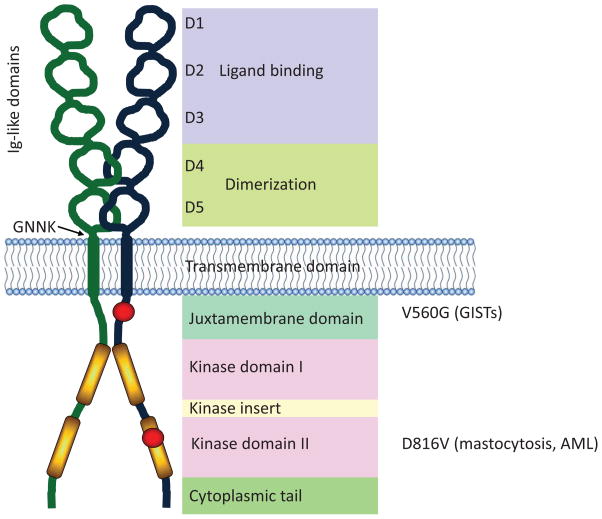
KIT is a type III receptor tyrosine kinase (RTK) that exists as multiple splice variants and in various states of glycosylation, which may represent the maturity of the receptor. Therefore, the molecular weight of KIT ranges between 120–150 kDa depending upon post-translational modifications. KIT and other type III RTKs contain five extracellular immunoglobulin-like domains (D1 distal to D5 juxta-membrane) (Figure 1) that form an elongated serpentine shape (57). The distal D1, D2 and D3 domains constitute the SCF binding portion of KIT with SCF and KIT forming a 2:2 stoichiometry (57), supporting suggestions that KIT dimerization is a consequence of bivalent binding to SCF homodimers (32, 58). The intracellular juxtamembrane domain (JM) of KIT, in the inactive state, interacts with the KD preventing its catalytic function and providing a negative switch regulatory mechanism (59, 60). KIT signaling in response to SCF is triggered by the intrinsic kinase activity of the receptor by way of two catalytic kinase domains (KD) separated by a kinase insert domain (Figure 1). Trans-phosphorylation of tyrosine residues in the JM, kinase insert domain and cytoplasmic tail participate in recruitment of signaling molecules to the receptor complex, initiating signaling cascades through several divergent pathways (Figure 2).
Figure 1.
Structure of KIT. KIT comprises five extracellular immunoglobulin-like domains, a membrane spanning domain, and two catalytic kinase domains. The first three immunoglobulin-like domains are responsible for binding to the KIT ligand, SCF. The two immunoglobulin-like domains proximal to the plasma membrane interact and facilitate dimerization of KIT. A region of four amino acids (GNNK) lies adjacent to the plasma membrane region, and alternative splicing of KIT results in GNNK+ and GNNK− isoforms. The juxtamembrane (JM) domain of KIT contains the Tyr residues Y568 and Y570, which become phosphorylated upon activation releasing its auto-inhibitory function. Point mutations in the JM domain change its conformation and prevent its regulatory function. The V560G mutation is an example of an activating mutation in the JM domain, particularly in association with GISTs. There are two catalytic kinase domains (KD) separated by a kinase insert domain. Several activating mutations have been reported in the KD of KIT. The D816V mutation is a common mutation and is associated with mastocytosis.
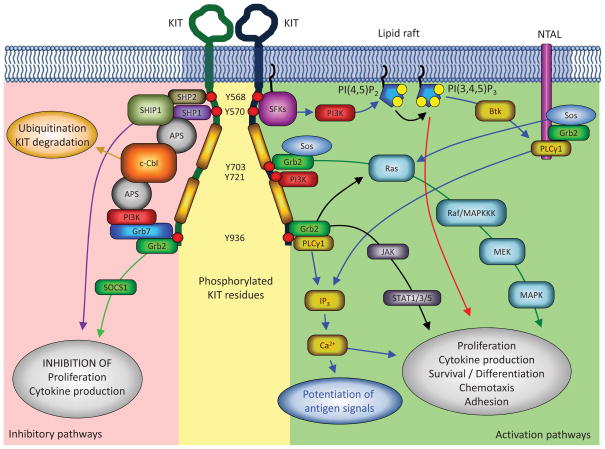
Figure 2.
KIT signaling pathways. KIT signaling occurs through several pathways. Phosphorylation of the juxta-membrane Tyr residues recruits Src family kinases (SFKs), in particular Lyn, initiating PI3K signaling and the phosphorylation of other signaling and adaptor proteins. These events most likely occur within lipid rafts where the PI3K substrate, PI(4,5)P2, is converted to PI(3,4,5)P3, a signaling lipid that contributes to the activation of other enzymes. Adaptor proteins (NTAL and Grb2) are phosphorylated by the receptor or by Src kinases and recruit other signaling proteins, forming signaling complexes. PLCγ1, for example is recruited by the adaptor protein NTAL to lipid rafts, activated by phosphorylation and by PI(3,4,5)P3, cleaving PI(3,4)P2 to form IP3, which induces the release of Ca2+ from intracellular stores; and DAG, together with Ca2+ activates PKC. Tyr residues within the kinase insert domain of KIT interact with PI3K and Grb2, leading to the activation of the Ras/Raf/MAPK pathway. Another pathway critical for KIT mediated proliferation, particularly in gain-of-function KIT mutants, is the activation of the transcription factors STAT1/3/5 (signal transducers and activators of transcription). Janus kinase (JAK) is phosphorylated after KIT activation, and in turn phosphorylates STAT allowing its translocation to the nucleus, where it exerts its function. Inhibitory pathways of KIT also regulate responsiveness to SCF. The juxtamembrane Tyr residues of KIT can recruit the phosphatases, SHP1, SHP2 and SHIP1, which may contribute to negative regulation of KIT signaling. In addition, recruitment of the ubiquitin E3 ligase, c-Cbl, via the dimeric adaptor protein APS, may be a critical determinant for ubiquitination and degradation of KIT. Both Tyr 936 and Tyr 570 appear to regulate the recruitment of c-Cbl and binding of APS. APS exists as a dimer and thus may require two simultaneously phosphorylated KIT residues to recruit c-Cbl. For simplification, only one extracellular domain of KIT is represented and bound ligand is not depicted.
Signaling pathways of KIT in mast cells
As we have discussed, signaling through KIT is critical for human mast cell survival, growth, differentiation and proliferation. In addition, SCF affects secretion, adhesion and migration, and therefore regulates most normal mast cell functions. Although the signaling pathways that are activated downstream of KIT are well established, mechanisms that regulate specific responses to SCF signals are not well understood. One potential mechanism for directing specific mast cell responses to SCF stimulation is differential phosphorylation of residues within the cytoplasmic domains of KIT. Activation of KIT by SCF induces KIT dimerization and initiation of intrinsic tyrosine kinase activity, resulting in autophosphorylation of tyrosine residues that act as docking sites for signaling proteins containing either Src homology 2 (SH2) or phospho-tyrosine binding (PTB) domains (61–63). Thus, phosphorylation of specific KIT tyrosine residues triggers signal transduction through divergent pathways that may drive distinct mast cell responses.
One of the early signaling events after activation of KIT is recruitment of Src family kinases (SFKs) to phosphorylated tyrosines (Y568 and Y570) in the JM domain of KIT (64, 65). SFK signaling may regulate several critical SCF-dependent mast cell functions. For example, transfection of a dominant negative form of the SFK, Lyn, inhibits mast cell proliferation and chemotaxis in response to SCF (66). SFKs, such as Lyn, contain N-terminal myristoylation sites, and in some cases a palmitoylation site, which are critical for anchoring the kinases, respectively, to the plasma membrane and to specialized membrane microdomains (lipid rafts) (67). Therefore, SFK signaling most likely occurs at the plasma membrane where the JM domain of KIT is easily accessible for binding membrane anchored SFKs. Indeed, SFK signaling may actually be required for KIT internalization as well as migration (68). Further indication that plasma membrane localization is key for KIT signaling through SFKs comes from the observation that KIT associates with lipid rafts after stimulation with SCF, and that lipid rafts are essential for activation of SFKs in response to SCF (69, 70).
Lipid rafts may also be important for signal transduction through the phosphatidylinositol-3-kinase (PI3K) pathway since disruption of lipid rafts specifically diminishes phosphorylation of the PI3K surrogate, AKT, in response to SCF stimulation (69, 70). PI3K phosphorylates the plasma membrane-associated phosphatidylinositol-4,5-biphosphate (PI(4,5)P2) to form phosphatidylinositol-3,4,5,-triphosphate (PI(3,4,5)P3), which in turn recruits pleckstrin homology (PH) domain-containing signaling proteins to the plasma membrane (reviewed by (71)). The classical class I PI3K complex consists of a regulatory p85 subunit and a catalytic p110 subunit. Bone marrow derived mast cells (BMMCs) from mice with targeted deletion of PI3K exhibit reduced survival and proliferate less than wild-type BMMC in response to SCF, which is associated with a reduction in the phosphorylation status of the mitogen-activated protein kinase (MAPK) JNK (Jun-amino-terminal kinase) (72). In addition, PI3K also appears to play an important role in mast cell chemotaxis (73) suggesting that PI3K and SFKs regulate similar functional responses to SCF.
Activation of KIT by SCF also triggers activation of the MAPKs, extracellular-signal-regulated kinase 1 and 2 (ERK1 and ERK2), JNK and p38. Phosphorylated tyrosine residues in activated KIT (Figure 2) are recognized by the SH2 domain of the adaptor protein Grb2 (growth factor receptor-bound protein 2), which then forms a complex with the guanine exchange factor (GEF) Sos (son-of sevenless). Sos activates the G protein Ras by promoting the exchange of GDP by GTP (74) (Figure 2). GTP-bound, active Ras initiates a cascade of Serine/Threonine kinases (Raf, MEK) that lead to the activation of the ERK1 and ERK2 (75). The pathway leading to p38 and JNK activation by KIT in mast cells is not well established (46). The Ras-Raf-Mek-Erk pathway regulates many cellular processes, particularly survival, proliferation and cytokine production in mast cells. Therefore, targeting this pathway is attractive for cancer treatment (76) and potentially mastocytosis.
Gain-of-function c-Kit mutations and mechanisms for constitutive activation
The oncogenic c-Kit mutations found in neoplasms are gain-of-function mutations that result in ligand-independent tyrosine kinase activity and lead to ligand-independent proliferation, differentiation and/or survival of the affected cells (31, 32, 77). Despite the large number of individual oncogenic mutations identified, most are grouped within mutational hot-spots in exons 11 encoding for the JM domain and 17 encoding for the second kinase domain (KD) of KIT. JM mutations appear more frequently in GISTs, while 90% of patients with systemic mastocytosis, most cases of AML and other germ cell cancers have the D816V c-Kit point mutation in the KD. The reasons for the preference for exon 17 mutations in hematological malignancies as compared to exon 11 in GISTs are not known. Other exons, such as 8, 9 and 10, coding for the extracellular domain (8 and 9) and transmembrane domain of the receptor have been described in AML (40), GISTs (30, 32) and in childhood mastocytosis patients (52), but the incidence of these mutations is much lower (32). We refer the reader to other reviews or reports for more detailed listings of the specific oncogenic mutations (33, 37, 47, 52, 78, 79). In this section, we will briefly review the current understanding on how these hot-spot point mutations mechanistically affect receptor function.
Activating mutations in JM domains mainly relieve the suppressive effect of the JM region on the activity of the receptor and are mechanistically regarded as regulatory, while those in the KD are catalytic in nature (77, 80). Less common mutations in the extracellular and transmembrane domains (ectodomains) may also lead to KIT hyperactivity, probably by stabilizing receptor dimers and facilitating the activation of the RTK (57, 81). Despite the distinct nature of the mechanisms by which different mutations lead to KIT hyperactivation, they are not completely dissociated due to the interconnected nature of the secondary-tertiary structures of KIT. The JM domain in resting conditions forms a hairpin loop that inserts into the active site of KIT, maintaining the activation loop (A-loop) inactive (59, 82). Upon binding to SCF and consequent dimerization, the primary sites in the JM domain (Y568 and Y570) are transphosphorylated, lifting the auto-inhibition and allowing further activation (83). This activation process also involves a large rearrangement of the A-loop (from folded to extended) that facilitates access to Mg2+-ATP and protein substrate(s) to the kinase catalytic site (83). Mutations in the JM region lead to release of this regulatory suppression, permitting the extended, active conformation (83) and promoting ligand-independent dimerization (77, 84), favoring further signaling. Mutations in KD, particularly the D816V mutation, not only cause a local structural unfolding in the A-loop (directly effecting the enzymatic site configuration) (60, 85–87) but also a long-range structural re-arrangement of the JM region that in turn weakens its interaction with the KD, relieving the regulatory inhibition. The greater freedom of movement of the JM domain in D816V mutants also allows for interactions with another KIT receptor and promotes dimerization (80, 82, 84, 86). Moreover, due to increased catalytic activity in KD mutants, tyrosines Y568 and Y570 in the JM domain are phosphorylated, and thus their inhibition of the kinase is further disrupted (32, 80, 88). Thus, the negative switch mechanism by the JM region may be suppressed not only by direct mutations in this region but also indirectly by mutations in the catalytic domain; furthermore, increased dimerization may occur in all mutated receptors. Structure-function studies on KIT have been instrumental in understanding why, in some instances, differences in the type of mutation lead to similar disease phenotypes and why drug sensitivity differs depending on the mutation site, as discussed below.
The structural changes in the receptor induced by these mutations also quantitatively and qualitatively affect normal KIT signaling and cell function. For example, p38-MAPK pathways are preferentially activated over ERK-MAPK pathways in JM deletion mutants (87). Similarly, other important mitogenic pathways such as AKT- as well as STAT-dependent pathways are also constitutively activated in JM and D816V mutants (87, 89, 90). Interestingly, mutations in the extracellular domain of the receptor, like those found in some pediatric mastocytosis patients, when introduced in rodent cells resulted in preferential AKT activation as compared to D816V mutations (91). On the other hand, interaction of JM docking sites with phosphatases such as SHP1, SHP2 and SHIP, are disrupted in JM KIT mutants. These phosphatases normally dephosphorylate the receptor, tyrosine kinases associated with the receptor or, in the case of SHIP, cleave signaling lipids such as PIP3, thus downregulating KIT activity (87, 92) (Figure 2). Disruption of their suppression results in enhanced KIT activity. Overall, the altered signaling in KIT mutants may be a consequence of preferential phosphorylation of certain tyrosine sites and/or alterations in the trafficking of the receptor (as discussed in the next section).
Trafficking of KIT and its impact on signaling
There is growing evidence that trafficking and localization of RTKs modulate signal transduction and alter functional outcomes in response to ligand (for reviews see (93, 94)). RTKs, including KIT, are activated by ligand binding at the plasma membrane where rapid signaling is initiated. Signaling at the plasma membrane is a key process for many receptors where interactions with plasma membrane-associated adaptor proteins such as linker of activated T cells (LAT) and non-T cell activation linker (NTAL) are critical for propagation of signaling (reviewed by (46)). In addition, signaling through both PLCγ1 and PI3K most likely require plasma membrane localization since their lipid substrate, PI(4,5)P2, is plasma membrane-associated and access to PI(4,5)P2 is limited in endosomes (95). Therefore, receptor internalization and degradation attenuates the strength and duration of plasma membrane-associated signaling and thus has been considered as a negative regulator of receptor signal transduction. However for some receptors such as the RTK, epidermal growth factor receptor (EGFR), internalization into endosomal compartments is required for full activation of MAPK (96, 97). Receptor endocytosis may therefore alter signal transduction pathways and signaling at the plasma membrane may result in distinct responses compared to signaling within the endosomal compartments.
Much of the work performed on the effects of RTK trafficking on signal transduction thus far has been carried out with the EGFR. It has been demonstrated that the EGFR and downstream signaling pathways such as MAPKs signal in early endosomes (98–100) and that EGFR can continue to signal in late endosomes and multi-vesicular bodies until passage into the intraluminal vesicles of late endosomes and lysosomes halts signaling (96, 97, 101). One reason for the requirement of internalization for RTK signal transduction appears to be the composition of signaling scaffold complexes within distinct endocytic compartments that facilitate certain signal transduction pathways (Figure 3) (102–104). For example, signal transduction from EGFR to the ERK pathway in late endosomes is dependent upon localization of MEK1 kinase partner protein (MP1) and the adaptor protein p14, which are specific to late endosomes (103) and disruption of p14-MP1-MEK1 endosomal signaling complex inhibits proliferation (105).
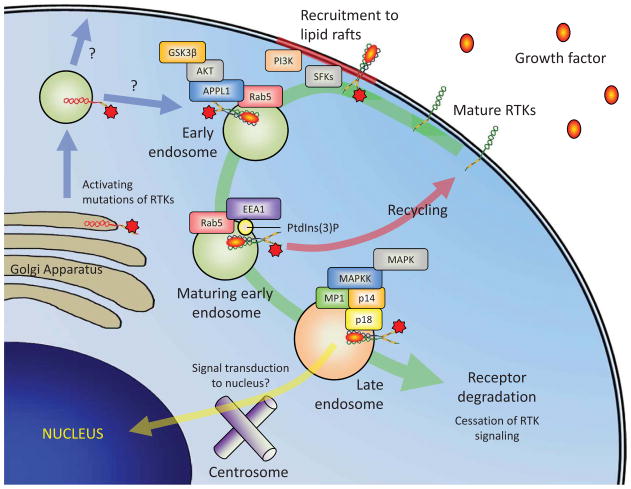
Figure 3.
Receptor tyrosine kinase trafficking may affect signal transduction. Trafficking of receptor tyrosine kinases (RTKs), such as KIT, may regulate signal transduction pathways by binding to adaptor proteins specific to endosomal compartments and altering the type of signaling complexes recruited. Initiation of RTK signaling triggers recruitment to lipid rafts where interactions with plasma membrane anchored signaling molecules is facilitated. In particular, lipid rafts may be critical for signaling through the Src family kinases, PI3K and PLCγ1. Internalization of RTKs can induce the activation of additional signaling pathways by recruitment of adaptor proteins such as adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 1 (APPL1) in very early endosomes, which can bind AKT and direct signaling through GSK3β. As the early endosome matures it becomes positive for PtdIns(3)P which recruits the early early endosomal antigen 1 (EEA1) protein. At this point, the early endosome may sort receptors for recycling back to the plasma membrane. Receptors that are not recycled remain in the endosome as it matures to a late endosome and control switches from Rab5 to Rab7. Signaling still occurs within the limiting membrane of late endosomes where adaptor protein complexes of p14, p18 and MP1, which are specific to the late endosomal compartments, may promote unique signaling to the MAPK pathway. The localization of late endosomes may also promote signal transduction to the nucleus because their close proximity to the centrosome allows weak signals to travel shorter distances and reach the nucleus. Eventually, receptors passage into intraluminal vesicles of multi-vesicular bodies and lysosomes, which results in cessation of signaling and receptor degradation. The schematic is an oversimplification, as multiple signaling pathways may well occur at all stages of receptor trafficking, but enrichment of particular adaptor proteins may promote a particular pathway by facilitating specific interactions. Activating mutations in RTKS favor their intracellular localization and trigger signaling from intracellular compartments in the absence of ligand. Further studies are needed to determine how the constitutively active RTKs traffic and signal in cells. Normal RTKs are depicted in green and mutated RTKs in red.
These observations suggest that signaling in the endocytic system and the formation of signaling complexes specific to late endosomes could be critical for determining a proliferative response to growth factors. Interestingly, RTKs with activating mutations that drive hematopoietic cell transformation exhibit abnormal cellular localization, localizing mainly within the ER and Golgi instead of in the plasma membrane, and this intracellular localization appears critical for the proliferative response (reviewed by (106)). Specifically, intracellular peri-nuclear signaling of KIT with the activating D816V mutation is sufficient to drive neoplasia, whilst plasma membrane signaling is dispensable in a mouse model of myelomonocytic neoplasia (107). Prolonged endosomal signaling may thus be important for neoplastic transformation and factors that reduce receptor degradation pathways could promote this response. For example, altered trafficking of KIT in bone marrow-derived MC (BMMC) by targeting small ARF GTPase-activating protein 1 (SMAP1) led to intracellular retention of KIT and hyper-phosphorylation of ERK by reducing KIT trafficking to lysosomes and thus degradation, which may predispose mice to myelodysplasia and acute myeloid leukemia (108).
The regulation of KIT and RTK trafficking and signal transduction appears complex. However, understanding the interplay between these processes may prove critical for our understanding of how KIT regulates many distinct cellular functions, which could aid in the design of more effective inhibitors of KIT-mediated proliferative diseases.
KIT INHIBITORS AND TREATMENT OF MUTATED KIT-DRIVEN PROLIFERATIVE DISEASES
Pharmacological targeting of KIT catalytic activity has been a major strategy for blocking KIT-mediated responses. This is because KIT with oncogenic c-Kit mutations are mostly expressed inside organelles, precluding the potential use of neutralizing antibodies. Another limiting factor in the treatment of KIT-related malignancies has been that inhibitors targeting the inactive ATP-binding site conformation of KIT, such as imatinib mesylate (imatinib, STI571, Gleevec or Glivec) and its derivatives (nilotinib (AMN107) and PD180970), are unsuccessful in inhibiting the active, extended conformation of KIT. Therefore, mutations that stabilize the active conformation (mostly KD mutations, including the common D816V mutation), as those found in most aggressive mastocytosis patients, are resistant to this drug. Nevertheless, imatinib has been an efficient drug with relatively few side effects (109) for the treatment of patients with GIST who harbor c-Kit mutations in exon 11 (corresponding to the JM domain) and some mastocytosis patients with a V560G mutation in the JM domain, a novel mutation in the transmembrane region (F522C) and other mutations (33). An additional drawback of imatinib has been the incidence in some of these patients of secondary resistance to the drug, apparently due in part to the acquisition (or enrichment) of other mutations in the KD of the receptor that are insensitive to imatinib (32, 41).
Other compounds, including dasatinib (BMS-354825), have been found to target the catalytic activity associated with D816V and other KD mutations. Dasatinib was reported to inhibit KIT autophosphorylation and the growth of both human mast cell line (HMC)-1.1 and HMC-1.2 human mast cell lines, which express the V560G mutation or the V560G and D816V mutations, respectively (12). Dasatinib was approved in 2010 as a first-line treatment of chronic myeloid leukemia (CML) in chronic phase and for the treatment of any-phase CML resistant or intolerant to a previous treatment (110). The current data on early response to treatment to dasatinib is predictive of improved long-term outcomes in CML (110). Dasatinib and derivatives, however, have wider specificity and affect multiple kinases such as Src Kinases, Tec kinases, Bruton’s tyrosine kinase (Btk), mitogen-activated protein kinases and AKT as well as other receptor kinases (12) that are important for growth and other functions in mast cells and other cell types. Thus, while being potentially more effective by acting on various pathways, their distinct side effects should also be taken into consideration depending on the patient (110–112). Another second generation tyrosine kinase inhibitor is midostaurin (PKC412), a N-benzoyl derivative of staurosporine that inhibits PKC as well as KIT, FLT-3, and PDGFR. Midostaurin has activity in vitro against the D816 KIT mutants by itself and shows synergistic effects in combination with other KIT inhibitors (reviewed in (79)). In a phase-2 study, midostaurin was orally administered to patients with various forms of advanced mastocytosis and showed a major response rate of 38%, which included improvement of hemoglobin and platelet counts, improvement of liver function abnormalities and improvement of ascites (113). The observation of a major response rate (as compared to any other type of clinical response) was significantly associated with the presence of a D816V mutation. Side effects were also present in these patients and treatment was discontinued in 69% of the patients. Additional studies are needed to clarify the advantage of midostaurine vs. other treatments.
The better understanding of the KIT structure, domains and mechanisms of activation has allowed for rational drug design approaches to intercept the activity of KIT mutants, although this is at its early stages and there is need for further testing and clinical development. An example is the development of compounds (DP-2976 and DP-4851) designed to target access to the KIT switch pocket. The switch pocket can bind either the JM region maintaining the inactive conformation, or the KD activation loop maintaining the active conformation. Both inhibitors potently and selectively inhibit the activity of normal KIT and KIT harboring the D816V mutation, proliferation of neoplastic and non-neoplastic mast cells, as well as the ability of KIT to enhance mast cell activation (114).
CONCLUDING REMARKS AND FUTURE DIRECTIONS
Transforming point mutations in KIT, which are critical for the manifestation of neoplastic diseases such as mastocytosis and GISTs, appear primarily in regions of the receptor involved in maintaining the normal structural, regulatory and activation dynamics of KIT, thus rendering a constitutively active receptor. Despite the progress identifying specific transforming mutations, the increased understanding of how these mutations affect KIT signaling and function, and the boosted interest in targeting KIT, important challenges in the treatment of these diseases still lie ahead. Both the heterogeneity in the phenotype of mastocytosis and success of therapy may be in part related to the presence of a combination of KIT mutations and mutations in other proto-oncogenes. Even though joint drug treatments targeting KIT and other oncogenes could be successful, this will entail tailoring the type of therapy, if available, to the genotype of each patient (41). Despite the interest and benefits of individualized treatments, more general pharmacological approaches should not be overlooked. Studies on gene expression profiling in bone marrow mast cells (115) and whole peripheral blood (116) from cohorts of mastocytosis patients suggest that a number of general biological processes and pathways are commonly altered in these patients. For example, not surprisingly, metabolic pathways and pathways involved in proliferation and apoptosis are significantly changed in mastocytosis patients (115, 116). One could speculate that drugs that reprogram metabolic pathways and restore normal energy and growth homeostasis of transformed cells, such as metformin (a drug approved for treatment of type II diabetic patients that has shown promising effects in treating human cancers (117, 118)), may revert, at least in part, the observed pathology. Although the importance of such pathways in the development or symptomatology of mastocytosis is presently unknown, a further understanding may provide the grounds for novel therapeutic approaches for mastocytosis treatment in combination with specific KIT inhibitors. Other novel therapeutic approaches of increasing appeal and promise for cancer treatment include miRNA therapies, although adjustments in this technology are still needed to achieve full potential (119). The increased interest in miRNA technology is in part based on the finding that miRNA profiles can accurately predict the origin of the cancer tissue (120) and that manipulation of these molecules may affect the behavior of neoplastic cells (119, 121). No miRNA profiling has been so far strongly linked to specific patient populations with mastocytosis; however, the evidence of specific miRNAs involved in the regulation of the cell cycle in mast cells (122), degranulation, cytokine production and migration of mast cells (123) and in the expression of transcription factors mediating proliferation of primary and KIT-transformed mast cells (124), makes this approach one of promising potential (125).
In summary, although complete remission for some of the proliferative diseases harboring KIT mutations has not yet been fully achieved, new drug designs of KIT inhibitors together with the advances in individual genotyping and in identifying the molecular mechanisms that underlie these diseases, may afford new possibilities and potential breakthroughs in treatment.
KEY POINTS.
KIT, a growth factor tyrosine kinase receptor, is critical for the proliferation, survival, differentiation and homing of hematopoietic bone marrow stem cells, and particularly mast cells, which retain KIT expression and are dependent on KIT activity during their lifespan.
Gain-of-function mutations in c-Kit resulting in ligand-independent receptor activity associate with hyperproliferative diseases, especially mast cell proliferation disorders (mastocytosis), gastrointestinal stromal tumors (GISTs) and other hematological neoplasms.
Despite the large number of individual somatic oncogenic mutations identified, most are grouped within mutational hot-spots in exon 11 (more frequent in GISTs), encoding for the regulatory juxtamembrane domain, and exon 17 (more frequent in mastocytosis and other germ line malignancies), encoding for the catalytic kinase domain of KIT.
Structural changes in the receptor induced by these mutations affect the intracellular trafficking of KIT and quantitatively and qualitatively alter normal KIT signaling leading to enhanced proliferation.
Challenges in the treatment of these diseases include the differential sensitivity to known KIT inhibitors depending on the type of mutation and their relatively low selectivity to KIT, the development of drug resistance, and the presence of other complementing co-oncogenic events or epigenetic modifications contributing to the pathology.
Acknowledgments
Financial support was provided by the Division of Intramural Research of NIAID within the National Institutes of Health.
Footnotes
Disclaimers: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yarden Y, Kuang WJ, Yang-Feng T, et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu FH, Ray P, Brown K, et al. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family--oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988;7:1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flanagan JG, Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990;63:185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- 4.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 5.Toksoz D, Zsebo KM, Smith KA, et al. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci U S A. 1992;89:7350–7354. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 2006;533:327–340. doi: 10.1016/j.ejphar.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 7.Miyazawa K, Williams DA, Gotoh A, et al. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–649. [PubMed] [Google Scholar]
- 8.Ashman LK. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. 1999;31:1037–1051. doi: 10.1016/s1357-2725(99)00076-x. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa M, Matsuzaki Y, Nishikawa S, et al. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowie MB, Kent DG, Copley MR, et al. Steel factor responsiveness regulates the high self-renewal phenotype of fetal hematopoietic stem cells. Blood. 2007;109:5043–5048. doi: 10.1182/blood-2006-08-037770. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura Y, Oboki K, Ito A. Molecular mechanisms of mast cell development. Immunol Allergy Clin North Am. 2006;26:387–405. doi: 10.1016/j.iac.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Jensen BM, Akin C, Gilfillan AM. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. Br J Pharmacol. 2008;154:1572–1582. doi: 10.1038/bjp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lennartsson J, Jelacic T, Linnekin D, et al. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells. 2005;23:16–43. doi: 10.1634/stemcells.2004-0117. [DOI] [PubMed] [Google Scholar]
- 14.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 15.Galli SJ, Tsai M, Wershil BK, et al. Regulation of mouse and human mast cell development, survival and function by stem cell factor, the ligand for the c-kit receptor. Int Arch Allergy Immunol. 1995;107:51–53. doi: 10.1159/000236928. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira SH, Taub DD, Nagel J, et al. Stem cell factor induces eosinophil activation and degranulation: mediator release and gene array analysis. Blood. 2002;100:4291–4297. doi: 10.1182/blood.V100.13.4291. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamoorthy N, Oriss TB, Paglia M, et al. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med. 2008;14:565–573. doi: 10.1038/nm1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan Q, Austen KF, Friend DS, et al. Human peripheral blood eosinophils express a functional c-kit receptor for stem cell factor that stimulates very late antigen 4 (VLA-4)-mediated cell adhesion to fibronectin and vascular cell adhesion molecule 1 (VCAM-1) J Exp Med. 1997;186:313–323. doi: 10.1084/jem.186.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinemann A, Sturm GJ, Ofner M, et al. Stem cell factor stimulates the chemotaxis, integrin upregulation, and survival of human basophils. J Allergy Clin Immunol. 2005;116:820–826. doi: 10.1016/j.jaci.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Huizinga JD, Thuneberg L, Kluppel M, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 21.Al-Muhsen SZ, Shablovsky G, Olivenstein R, et al. The expression of stem cell factor and c-kit receptor in human asthmatic airways. Clin Exp Allergy. 2004;34:911–916. doi: 10.1111/j.1365-2222.2004.01975.x. [DOI] [PubMed] [Google Scholar]
- 22.Hollenbeck ST, Sakakibara K, Faries PL, et al. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res. 2004;120:288–294. doi: 10.1016/j.jss.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Hirata T, Kasugai T, Morii E, et al. Characterization of c-kit-positive neurons in the dorsal root ganglion of mouse. Brain Res Dev Brain Res. 1995;85:201–211. doi: 10.1016/0165-3806(94)00205-e. [DOI] [PubMed] [Google Scholar]
- 24.Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–625. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330–335. doi: 10.1111/j.1346-8138.2012.01583.x. [DOI] [PubMed] [Google Scholar]
- 26.Sekido Y, Takahashi T, Ueda R, et al. Recombinant human stem cell factor mediates chemotaxis of small-cell lung cancer cell lines aberrantly expressing the c-kit protooncogene. Cancer Res. 1993;53:1709–1714. [PubMed] [Google Scholar]
- 27.Sihto H, Sarlomo-Rikala M, Tynninen O, et al. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23:49–57. doi: 10.1200/JCO.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 28.Lu HY, Zhang G, Cheng QY, et al. Expression and mutation of the c-kit gene and correlation with prognosis of small cell lung cancer. Oncol Lett. 2012;4:89–93. doi: 10.3892/ol.2012.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachet JB, Landi B, Laurent-Puig P, et al. Diagnosis, prognosis and treatment of patients with gastrointestinal stromal tumour (GIST) and germline mutation of KIT exon 13. Eur J Cancer. 2013;49:2531–2541. doi: 10.1016/j.ejca.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 31.Ashman LK, Ferrao P, Cole SR, et al. Effects of mutant c-Kit in early myeloid cells. Leuk Lymphoma. 1999;34:451–461. doi: 10.3109/10428199909058472. [DOI] [PubMed] [Google Scholar]
- 32.Lennartsson J, Ronnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92:1619–1649. doi: 10.1152/physrev.00046.2011. [DOI] [PubMed] [Google Scholar]
- 33.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rammohan A, Sathyanesan J, Rajendran K, et al. A gist of gastrointestinal stromal tumors: A review. World J Gastrointest Oncol. 2013;5:102–112. doi: 10.4251/wjgo.v5.i6.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagata H, Worobec AS, Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–2372. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 37.Brockow K, Metcalfe DD. Mastocytosis. Chem Immunol Allergy. 2010;95:110–124. doi: 10.1159/000315946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran A, Tawbi HA. A potential role for nilotinib in KIT-mutated melanoma. Expert Opin Investig Drugs. 2012;21:861–869. doi: 10.1517/13543784.2012.679341. [DOI] [PubMed] [Google Scholar]
- 39.Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol. 2006;126:1102–1110. doi: 10.1038/sj.jid.5700125. [DOI] [PubMed] [Google Scholar]
- 40.Malaise M, Steinbach D, Corbacioglu S. Clinical implications of c-Kit mutations in acute myelogenous leukemia. Curr Hematol Malig Rep. 2009;4:77–82. doi: 10.1007/s11899-009-0011-8. [DOI] [PubMed] [Google Scholar]
- 41.Ashman LK, Griffith R. Therapeutic targeting of c-KIT in cancer. Expert Opin Investig Drugs. 2013;22:103–115. doi: 10.1517/13543784.2013.740010. [DOI] [PubMed] [Google Scholar]
- 42.Odenike O, Thirman MJ, Artz AS, et al. Gene mutations, epigenetic dysregulation, and personalized therapy in myeloid neoplasia: are we there yet? Semin Oncol. 2011;38:196–214. doi: 10.1053/j.seminoncol.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Kirshenbaum AS, Kessler SW, Goff JP, et al. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol. 1991;146:1410–1415. [PubMed] [Google Scholar]
- 44.Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25–33. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 46.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 47.Verstovsek S. Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression. Eur J Haematol. 2013;90:89–98. doi: 10.1111/ejh.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piao X, Bernstein A. A point mutation in the catalytic domain of c-kit induces growth factor independence, tumorigenicity, and differentiation of mast cells. Blood. 1996;87:3117–3123. [PubMed] [Google Scholar]
- 49.Nagata H, Okada T, Worobec AS, et al. c-kit mutation in a population of patients with mastocytosis. Int Arch Allergy Immunol. 1997;113:184–186. doi: 10.1159/000237541. [DOI] [PubMed] [Google Scholar]
- 50.Longley BJ, Tyrrell L, Lu SZ, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312–314. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 51.Buttner C, Henz BM, Welker P, et al. Identification of activating c-kit mutations in adult-, but not in childhood-onset indolent mastocytosis: a possible explanation for divergent clinical behavior. J Invest Dermatol. 1998;111:1227–1231. doi: 10.1046/j.1523-1747.1998.00414.x. [DOI] [PubMed] [Google Scholar]
- 52.Bodemer C, Hermine O, Palmerini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804–815. doi: 10.1038/jid.2009.281. [DOI] [PubMed] [Google Scholar]
- 53.Wilson TM, Maric I, Simakova O, et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011;96:459–463. doi: 10.3324/haematol.2010.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molderings GJ, Kolck UW, Scheurlen C, et al. Multiple novel alterations in Kit tyrosine kinase in patients with gastrointestinally pronounced systemic mast cell activation disorder. Scand J Gastroenterol. 2007;42:1045–1053. doi: 10.1080/00365520701245744. [DOI] [PubMed] [Google Scholar]
- 55.Chan EC, Bai Y, Bandara G, et al. KIT GNNK splice variants: Expression in systemic mastocytosis and influence on the activating potential of the D816V mutation in mast cells. Exp Hematol. 2013;41:870–881. e872. doi: 10.1016/j.exphem.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brockow K, Jofer C, Behrendt H, et al. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–232. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 57.Yuzawa S, Opatowsky Y, Zhang Z, et al. Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell. 2007;130:323–334. doi: 10.1016/j.cell.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 58.Lemmon MA, Pinchasi D, Zhou M, et al. Kit receptor dimerization is driven by bivalent binding of stem cell factor. J Biol Chem. 1997;272:6311–6317. doi: 10.1074/jbc.272.10.6311. [DOI] [PubMed] [Google Scholar]
- 59.Mol CD, Dougan DR, Schneider TR, et al. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem. 2004;279:31655–31663. doi: 10.1074/jbc.M403319200. [DOI] [PubMed] [Google Scholar]
- 60.Mol CD, Lim KB, Sridhar V, et al. Structure of a c-kit product complex reveals the basis for kinase transactivation. J Biol Chem. 2003;278:31461–31464. doi: 10.1074/jbc.C300186200. [DOI] [PubMed] [Google Scholar]
- 61.Blume-Jensen P, Claesson-Welsh L, Siegbahn A, et al. Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J. 1991;10:4121–4128. doi: 10.1002/j.1460-2075.1991.tb04989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pawson T. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell. 2004;116:191–203. doi: 10.1016/s0092-8674(03)01077-8. [DOI] [PubMed] [Google Scholar]
- 63.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–2548. doi: 10.1007/s00018-004-4189-6. [DOI] [PubMed] [Google Scholar]
- 64.Linnekin D, DeBerry CS, Mou S. Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. J Biol Chem. 1997;272:27450–27455. doi: 10.1074/jbc.272.43.27450. [DOI] [PubMed] [Google Scholar]
- 65.Abram CL, Courtneidge SA. Src family tyrosine kinases and growth factor signaling. Exp Cell Res. 2000;254:1–13. doi: 10.1006/excr.1999.4732. [DOI] [PubMed] [Google Scholar]
- 66.O’Laughlin-Bunner B, Radosevic N, Taylor ML, et al. Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood. 2001;98:343–350. doi: 10.1182/blood.v98.2.343. [DOI] [PubMed] [Google Scholar]
- 67.Kovarova M, Tolar P, Arudchandran R, et al. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcepsilon receptor I aggregation. Mol Cell Biol. 2001;21:8318–8328. doi: 10.1128/MCB.21.24.8318-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broudy VC, Lin NL, Liles WC, et al. Signaling via Src family kinases is required for normal internalization of the receptor c-Kit. Blood. 1999;94:1979–1986. [PubMed] [Google Scholar]
- 69.Jahn T, Leifheit E, Gooch S, et al. Lipid rafts are required for Kit survival and proliferation signals. Blood. 2007;110:1739–1747. doi: 10.1182/blood-2006-05-020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arcaro A, Aubert M, Espinosa del Hierro ME, et al. Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signalling. Cell Signal. 2007;19:1081–1092. doi: 10.1016/j.cellsig.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Foster FM, Traer CJ, Abraham SM, et al. The phosphoinositide (PI) 3-kinase family. J Cell Sci. 2003;116:3037–3040. doi: 10.1242/jcs.00609. [DOI] [PubMed] [Google Scholar]
- 72.Fukao T, Yamada T, Tanabe M, et al. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 73.Tan BL, Yazicioglu MN, Ingram D, et al. Genetic evidence for convergence of c-Kit- and alpha4 integrin-mediated signals on class IA PI-3kinase and the Rac pathway in regulating integrin-directed migration in mast cells. Blood. 2003;101:4725–4732. doi: 10.1182/blood-2002-08-2521. [DOI] [PubMed] [Google Scholar]
- 74.Chardin P, Camonis JH, Gale NW, et al. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993;260:1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- 75.Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–134. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 77.Kitayama H, Kanakura Y, Furitsu T, et al. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood. 1995;85:790–798. [PubMed] [Google Scholar]
- 78.Boissan M, Feger F, Guillosson JJ, et al. c-Kit and c-kit mutations in mastocytosis and other hematological diseases. J Leukoc Biol. 2000;67:135–148. doi: 10.1002/jlb.67.2.135. [DOI] [PubMed] [Google Scholar]
- 79.Ustun C, DeRemer DL, Akin C. Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk Res. 2011;35:1143–1152. doi: 10.1016/j.leukres.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Laine E, Chauvot de Beauchene I, Perahia D, et al. Mutation D816V alters the internal structure and dynamics of c-KIT receptor cytoplasmic region: implications for dimerization and activation mechanisms. PLoS Comput Biol. 2011;7:e1002068. doi: 10.1371/journal.pcbi.1002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kohl TM, Schnittger S, Ellwart JW, et al. KIT exon 8 mutations associated with core-binding factor (CBF)-acute myeloid leukemia (AML) cause hyperactivation of the receptor in response to stem cell factor. Blood. 2005;105:3319–3321. doi: 10.1182/blood-2004-06-2068. [DOI] [PubMed] [Google Scholar]
- 82.Laine E, Auclair C, Tchertanov L. Allosteric communication across the native and mutated KIT receptor tyrosine kinase. PLoS Comput Biol. 2012;8:e1002661. doi: 10.1371/journal.pcbi.1002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zou J, Wang YD, Ma FX, et al. Detailed conformational dynamics of juxtamembrane region and activation loop in c-Kit kinase activation process. Proteins. 2008;72:323–332. doi: 10.1002/prot.21928. [DOI] [PubMed] [Google Scholar]
- 84.Tsujimura T, Hashimoto K, Kitayama H, et al. Activating mutation in the catalytic domain of c-kit elicits hematopoietic transformation by receptor self-association not at the ligand-induced dimerization site. Blood. 1999;93:1319–1329. [PubMed] [Google Scholar]
- 85.Vendome J, Letard S, Martin F, et al. Molecular modeling of wild-type and D816V c-Kit inhibition based on ATP-competitive binding of ellipticine derivatives to tyrosine kinases. J Med Chem. 2005;48:6194–6201. doi: 10.1021/jm050231m. [DOI] [PubMed] [Google Scholar]
- 86.Lam LP, Chow RY, Berger SA. A transforming mutation enhances the activity of the c-Kit soluble tyrosine kinase domain. Biochem J. 1999;338 (Pt 1):131–138. [PMC free article] [PubMed] [Google Scholar]
- 87.Casteran N, De Sepulveda P, Beslu N, et al. Signal transduction by several KIT juxtamembrane domain mutations. Oncogene. 2003;22:4710–4722. doi: 10.1038/sj.onc.1206587. [DOI] [PubMed] [Google Scholar]
- 88.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 89.Chian R, Young S, Danilkovitch-Miagkova A, et al. Phosphatidylinositol 3 kinase contributes to the transformation of hematopoietic cells by the D816V c-Kit mutant. Blood. 2001;98:1365–1373. doi: 10.1182/blood.v98.5.1365. [DOI] [PubMed] [Google Scholar]
- 90.Ning ZQ, Li J, Arceci RJ. Signal transducer and activator of transcription 3 activation is required for Asp(816) mutant c-Kit-mediated cytokine-independent survival and proliferation in human leukemia cells. Blood. 2001;97:3559–3567. doi: 10.1182/blood.v97.11.3559. [DOI] [PubMed] [Google Scholar]
- 91.Yang Y, Letard S, Borge L, et al. Pediatric mastocytosis-associated KIT extracellular domain mutations exhibit different functional and signaling properties compared with KIT-phosphotransferase domain mutations. Blood. 2010;116:1114–1123. doi: 10.1182/blood-2009-06-226027. [DOI] [PubMed] [Google Scholar]
- 92.Kozlowski M, Larose L, Lee F, et al. SHP-1 binds and negatively modulates the c-Kit receptor by interaction with tyrosine 569 in the c-Kit juxtamembrane domain. Mol Cell Biol. 1998;18:2089–2099. doi: 10.1128/mcb.18.4.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teis D, Huber LA. The odd couple: signal transduction and endocytosis. Cell Mol Life Sci. 2003;60:2020–2033. doi: 10.1007/s00018-003-3010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haugh JM, Meyer T. Active EGF receptors have limited access to PtdIns(4,5)P(2) in endosomes: implications for phospholipase C and PI 3-kinase signaling. J Cell Sci. 2002;115:303–310. doi: 10.1242/jcs.115.2.303. [DOI] [PubMed] [Google Scholar]
- 96.Kranenburg O, Verlaan I, Moolenaar WH. Dynamin is required for the activation of mitogen-activated protein (MAP) kinase by MAP kinase kinase. J Biol Chem. 1999;274:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- 97.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 98.Wu P, Wee P, Jiang J, et al. Differential regulation of transcription factors by location-specific EGF receptor signaling via a spatio-temporal interplay of ERK activation. PLoS One. 2012;7:e41354. doi: 10.1371/journal.pone.0041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu J, Troglio F, Mukhopadhyay A, et al. F-BAR-containing adaptor CIP4 localizes to early endosomes and regulates Epidermal Growth Factor Receptor trafficking and downregulation. Cell Signal. 2009;21:1686–1697. doi: 10.1016/j.cellsig.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 100.Brankatschk B, Wichert SP, Johnson SD, et al. Regulation of the EGF transcriptional response by endocytic sorting. Sci Signal. 2012;5:ra21. doi: 10.1126/scisignal.2002351. [DOI] [PubMed] [Google Scholar]
- 101.Burke P, Schooler K, Wiley HS. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol Biol Cell. 2001;12:1897–1910. doi: 10.1091/mbc.12.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 103.Teis D, Wunderlich W, Huber LA. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev Cell. 2002;3:803–814. doi: 10.1016/s1534-5807(02)00364-7. [DOI] [PubMed] [Google Scholar]
- 104.Taub N, Teis D, Ebner HL, et al. Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol Biol Cell. 2007;18:4698–4710. doi: 10.1091/mbc.E07-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Teis D, Taub N, Kurzbauer R, et al. p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J Cell Biol. 2006;175:861–868. doi: 10.1083/jcb.200607025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Toffalini F, Demoulin JB. New insights into the mechanisms of hematopoietic cell transformation by activated receptor tyrosine kinases. Blood. 2010;116:2429–2437. doi: 10.1182/blood-2010-04-279752. [DOI] [PubMed] [Google Scholar]
- 107.Xiang Z, Kreisel F, Cain J, et al. Neoplasia driven by mutant c-KIT is mediated by intracellular, not plasma membrane, receptor signaling. Mol Cell Biol. 2007;27:267–282. doi: 10.1128/MCB.01153-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kon S, Minegishi N, Tanabe K, et al. Smap1 deficiency perturbs receptor trafficking and predisposes mice to myelodysplasia. J Clin Invest. 2013;123:1123–1137. doi: 10.1172/JCI63711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levitzki A, Mishani E. Tyrphostins and other tyrosine kinase inhibitors. Annu Rev Biochem. 2006;75:93–109. doi: 10.1146/annurev.biochem.75.103004.142657. [DOI] [PubMed] [Google Scholar]
- 110.Jabbour E, Lipton JH. A Critical Review of Trials of First-Line BCR-ABL Inhibitor Treatment in Patients With Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase. Clin Lymphoma Myeloma Leuk. 2013 doi: 10.1016/j.clml.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delgado L, Giraudier S, Ortonne N, et al. Adverse cutaneous reactions to the new second-generation tyrosine kinase inhibitors (dasatinib, nilotinib) in chronic myeloid leukemia. J Am Acad Dermatol. 2013;69:839–840. doi: 10.1016/j.jaad.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 112.Saglio G, Baccarani M. First-line therapy for chronic myeloid leukemia: new horizons and an update. Clin Lymphoma Myeloma Leuk. 2010;10:169–176. doi: 10.3816/CLML.2010.n.026. [DOI] [PubMed] [Google Scholar]
- 113.Gotlib J, DeAngelo DJ, George TI, et al. KIT Inhibitor Midostaurin Exhibits a High Rate of Clinically Meaningful and Durable Responses in Advanced Systemic Mastocytosis: Report of a Fully Accrued Phase II Trial. Blood. ASH Annual Meeting Abstracts. 2010;116:316. [Google Scholar]
- 114.Bai Y, Bandara G, Ching Chan E, et al. Targeting the KIT activating switch control pocket: a novel mechanism to inhibit neoplastic mast cell proliferation and mast cell activation. Leukemia. 2013;27:278–285. doi: 10.1038/leu.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Teodosio C, Garcia-Montero AC, Jara-Acevedo M, et al. Gene expression profile of highly purified bone marrow mast cells in systemic mastocytosis. J Allergy Clin Immunol. 2013;131:1213–1224. 1224 e1211–1214. doi: 10.1016/j.jaci.2012.12.674. [DOI] [PubMed] [Google Scholar]
- 116.Niedoszytko M, Oude Elberink JN, Bruinenberg M, et al. Gene expression profile, pathways, and transcriptional system regulation in indolent systemic mastocytosis. Allergy. 2011;66:229–237. doi: 10.1111/j.1398-9995.2010.02477.x. [DOI] [PubMed] [Google Scholar]
- 117.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, et al. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 118.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 119.Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosenfeld N, Aharonov R, Meiri E, et al. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26:462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 121.Sethi A, Sholl LM. Emerging Evidence for MicroRNAs as Regulators of Cancer Stem Cells. Cancers (Basel) 2011;3:3957–3971. doi: 10.3390/cancers3043957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mayoral RJ, Pipkin ME, Pachkov M, et al. MicroRNA-221-222 regulate the cell cycle in mast cells. J Immunol. 2009;182:433–445. doi: 10.4049/jimmunol.182.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mayoral RJ, Deho L, Rusca N, et al. MiR-221 influences effector functions and actin cytoskeleton in mast cells. PLoS One. 2011;6:e26133. doi: 10.1371/journal.pone.0026133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee YN, Brandal S, Noel P, et al. KIT signaling regulates MITF expression through miRNAs in normal and malignant mast cell proliferation. Blood. 2011;117:3629–3640. doi: 10.1182/blood-2010-07-293548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Deho L, Monticelli S. Human mast cells and mastocytosis: harnessing microRNA expression as a new approach to therapy? Arch Immunol Ther Exp (Warsz) 2010;58:279–286. doi: 10.1007/s00005-010-0086-x. [DOI] [PubMed] [Google Scholar]