Abstract
Gaze-following behaviour is considered crucial for social interactions which are influenced by social similarity. We investigated whether the degree of similarity, as indicated by the perceived age of another person, can modulate gaze following. Participants of three different age-groups (18–25; 35–45; over 65) performed an eye movement (a saccade) towards an instructed target while ignoring the gaze-shift of distracters of different age-ranges (6–10; 18–25; 35–45; over 70). The results show that gaze following was modulated by the distracter face age only for young adults. Particularly, the over 70 year-old distracters exerted the least interference effect. The distracters of a similar age-range as the young adults (18–25; 35–45) had the most effect, indicating a blurred own-age bias (OAB) only for the young age group. These findings suggest that face age can modulate gaze following, but this modulation could be due to factors other than just OAB (e.g., familiarity).
The gaze of other people allows the rapid extraction of socially relevant information (e.g., their focus of attention, their intentional and mental states) and triggers gaze-following behaviour (i.e., an automatic tendency to look and pay attention to where another person is looking). Gaze following behaviour provides an early1,2 and direct index of the automatic overt orienting of social attention3 and is thought to be a building block of social interactions4,5,6. The automatic nature of gaze-following behaviour has been demonstrated by the fact that seeing another's person gaze-shift can interfere with one's own execution of an oculomotor task7.
Despite the automatic-reflexive nature of gaze-following behaviour, recent studies have shown that the orienting of social attention is likely to be a product of both stimulus-driven and top-down attentional mechanisms8. With reference to top-down mechanisms, it has been demonstrated that the orienting of social attention can be influenced by high-order socio-cognitive variables, such as social status9, political affiliation10, context11,12, and familiarity13. Similar results have been reported by studies (e.g., Dalmaso et al.9) that investigated the impact of social status on covert orienting of social attention. For instance, Dalmaso and colleagues9, using fictional curriculum vitae conveying either high or low social status information, found greater gaze-cuing for faces associated with high social status than for faces associated with low social status, suggesting that the orienting of social attention is not immune to top-down influences and that it can be modulated by contextually relevant information.
In terms of stimulus-driven mechanisms, it has recently been shown that social attention is sensitive to facial cues other than gaze direction, such as facial physical self-similarity14, facial masculinity which provides cues of dominance15,16, and emotional expressions17,18. For example, Hungr and Hunt14 manipulated self-similarity by morphing the face of each participant with those of strangers and found a stronger gaze cueing effect when self-similar faces were created by morphing in equal proportion the participant's face with that of a stranger of the same gender (a manipulation that made explicit the recognition of the participants in the cueing face), suggesting that the perceived self-similarity of a distracting face influences the degree to which participants use gaze direction to orient their own attention14.
Interestingly, age acts as an automatic or stimulus-driven in-group categorization cue19, which is rapidly extracted from the visual features of the other's person's face and can give rise to an Own-Age Bias (OAB, i.e., the superiority of face processing skills - e.g., face recognition - for faces of one's own-age group compared to faces of another age group20).
Taken together, the aforementioned findings suggest that we do not orient our own attention in response to another person's gaze always in the same way. In other words, the orienting of social attention is not truly reflexive, but depends in part on who the person is that we are looking at. In fact, sometimes the gaze-shifts of some people seem to be more important or salient than others depending on facial features, familiarity, social and environmental relevance.
As far as face age is concerned, we hypothesized that perceived similarity, being either an OAB and/or in-group membership conveyed by face age may render the other person particularly relevant to the observer, since social interaction and communication are facilitated between similar individuals21,22,23,24. Thus, we expected that perceived similarity conveyed by face age may modulate gaze-following behaviour. The reason is that the other person's gaze direction communicates a point of interest in the environment for the observer. Orienting one's own attention in the same direction establishes a social relationship between the looker and the observer which would be enhanced between similar individuals and/or individuals of the same group (in-group members), particularly in young adults, because it is likely that they have similar interests or have similar aims.
Only two studies25,26 have investigated the effect of face age on covert orienting of social attention, and none have investigated its effect on overt orienting. Using a gaze-cueing task, Slessor and colleagues26 tested both young and old participants and reported a greater gaze-cueing effect only in younger adults for own-age distracters compared to elderly faces, suggesting a possible role of OAB in social attention orienting similar to that found for face recognition memory20,27,28. However, Slessor and colleagues25,26 limited their interest to only two ages ranges: young adults and elderly people. Age, however, is a continuous variable, and it appears important to examine effects of face age using stimuli with a fine-grained age range. A wider range of ages was used by Wolff and colleagues28 but to test the age effect on face recognition memory. To the best of our knowledge no studies have investigated the effect of face age on social attention and in particular on gaze-following behaviour, using more than two face-age categories. Similarly, although previous studies have investigated the effect of several facial cues, the effect of face age as an important similarity factor29 has not been tested before.
Our aim was to systematically investigate in young adults whether gaze-following behaviour can be modulated by the visual characteristics of a face, and in particular by face age that provides a similarity cue and which we are widely exposed to from a very young age (see30 for a review). Moreover, it leads to an automatic and spontaneous social categorization of unfamiliar individuals into in-group vs. out-group members19. To this end, we asked young participants (18–25 years old) to perform goal-directed saccades towards an instructed target while ignoring distracters of different ages whose gaze shifted towards or away from the instructed target (Main experiment). Two additional groups of participants (35–45 and over 65 years old) were also tested in a control experiment to check whether a similar effect could be found in middle-aged and elderly participants. We recorded participants' eye movements since they provide a more direct measure of the automaticity of the orienting of social attention than manual response times. We predicted that similarity conveyed by face age would increase the occurrence of gaze-following behaviour, leading to a higher interfering effect on oculomotor performance (e.g., higher percentage of saccadic errors in the direction of the distracter's gaze), specifically when the age of the distracting face matches that of the participants. In particular, we were expected to find it in young adults whereas, in line with previous gaze-cueing studies, this may not be the case with elderly participants26. Such a result would provide new evidence of the role of bottom-up manipulation in mediating the enhancement of gaze-following behaviour by implicitly changing the relevance of the gaze cue for the observer.
Results
For data correction and age manipulation check analysis see Supplementary information S1 and S2.
Antisaccadic error analysis
Antisaccadic errors were our first variable of interest since they provide a direct measure of the automatic tendency to follow somebody else's gaze7,10,11. Indeed, if people tend to automatically follow the gaze direction of other individuals, then one might predict a greater violation of the instruction cue (i.e., higher percentage of antisaccades) in the incongruent condition (where there is a mismatching between distracter's gaze direction and instructed direction) than in the congruent condition (where distracter's gaze direction and instructed direction match). The automaticity of gaze-following behaviour should also lead to a higher percentage of antisaccades when the observers see the distracters' gaze moving before the instruction cue onset than after it, indicating that an oculomotor programme to saccade in the same direction of the distracting gaze is initially and automatically induced by the observation of gaze direction7,11. In other words, by varying when the instruction cue onset occurs in relation to the distracter gaze shift (Stimulus Onset Asynchrony or SOA manipulation) it is possible to obtain a measure of the automaticity of the gaze-following behaviour. In particular, the distracting gaze onset could occur 100 ms after (−100-ms SOA) the instruction cue (the least distracting condition), or 100 before (100-ms SOA) the instruction cue (the most distracting condition, and thus the one which indicates that the gaze-following behavior is automatic). Moreover, a modulatory effect of age similarity on gaze-following behaviour should be revealed by a greater violation of the instruction when the age of the distracting face matches that of the participants.
Main Experiment
The mean percentages of antisaccadic errors were computed across participants for each combination of the experimental variables. These data were then submitted to a three-way repeated measures analysis of variance (ANOVA), with Distracter Age [6–10 years (children), vs. 18–25 years (young adults), vs. 35–45 years (adults), vs. over 70 years (elderly adults)], SOA [−100 ms (distracting gaze onset after the instruction cue) vs. 100 ms (distracting gaze onset before the instruction cue)], and Congruence between the distracting gaze direction and the instructed direction (congruent vs. incongruent) as the within-subjects factors. Post-hoc comparisons were performed using the Duncan's test with an alpha level of .05. We also reported the partial eta squared values (ηp2) as an additional metric of effect size for all significant or marginally significant ANOVA contrasts.
The ANOVA confirmed an automatic tendency to follow the distracting gaze, as it revealed a significant main effect of Congruence [F(1,22) = 37.72, MSE = 6965.50, p < .0001, ηp2 = .63], reflecting a higher percentage of antisaccades in the incongruent trials (12.76 ± 8.87%) compared to the congruent trials (4.06 ± 3.39%), and a significant main effect of SOA [F(1,22) = 23.02, MSE = 4342.28, p < .0001, ηp2 = .51], reflecting a higher percentage of antisaccades when the distracting gaze preceded (11.84 ± 7.71%) the instruction cue onset, rather than followed it (4.97 ± 3.13%). The automaticity of gaze-following behaviour was further confirmed by the significance of the interaction between Congruence and SOA [F(1,22) = 8.96, MSE = 990.57, p < .01, ηp2 = .29], indicating that the difference in percentage of antisaccades between incongruent and congruent trials was significantly higher when the distracting gaze was presented at the 100-ms SOA (incongruent trials = 17.84 ± 13.26%, congruent trials = 5.85 ± 3.67%, Δ = 11.99%, p < .05) than at the −100-ms SOA (incongruent trials = 7.68 ± 5.09%, congruent trials = 2.26 ± 1.82%, Δ = 5.42%, p < .01).
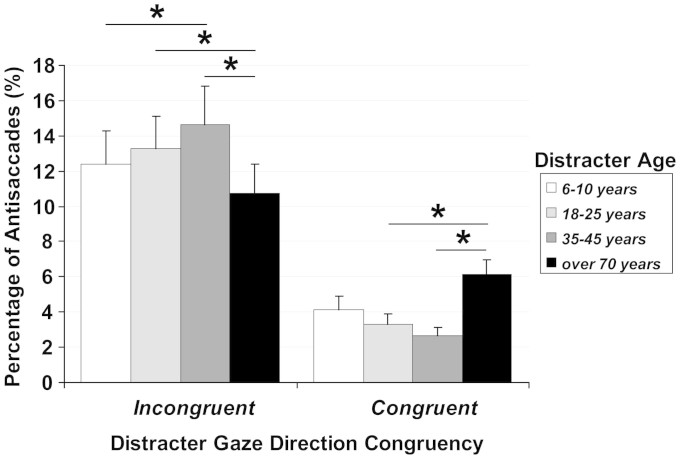
More importantly, the analysis also revealed an effect of the perceived age of the distracting face on gaze-following behaviour, as indicated by the significant interaction between Congruence and Distracter Age [F(3,66) = 9.42, MSE = 229.78, p < .0001, ηp2 = .30; see Fig. 1]. Post-hoc comparisons showed that when the distracters were of the same age as the participants (i.e., young adults with an age ranging between 18 and 25 years), the percentage of antisaccades in the incongruent trials was significantly higher than for elderly distracters (18–25 years: 13.27 ± 8.70% vs. over-70 years: 10.71 ± 7.83%, p < .03). No difference in the percentage of antisaccades between young adult and child distracters (6–10 years: 12.39 ± 8.90%, p = .40), and between young adult and adult distracters (35–45 years: 14.66 ± 10.04%, p = .18) was found. Interestingly, the percentage of antisaccades measured for adult distracters (35–45 years: 14.66%) was significantly higher than for child distracters (6–10 years: 12.39%, p < .05) and elderly distracters (over-70 years: 10.71%, p < .001). Post-hoc comparisons showed a reversed pattern of results in congruent trials (Fig. 1). Specifically, the percentage of antisaccades when the distracters were of the same age as the participants was significantly lower than for elderly distracters (3.31 ± 2.86% vs. 6.15 ± 3.79%, p < .02). Again, there was no difference in percentage of antisaccades between young adult and child distracters (4.15 ± 3.53%), and between young adult and adult distracters (2.63 ± 2.31%). No other main effects or interaction in the analysis were significant (ps > .25).
Figure 1. Mean percentage of antisaccades (erroneous saccades) as a function of congruence between the distracter gaze, the instructed saccade, and the distracter age range for young adult participants.
Error bars represent the standard errors of means across participants. Asterisks mark significant differences between means.
Control Experiment
The mean percentages of antisaccadic errors were computed as before and were then submitted to a four-way mixed ANOVA, with Subject Age (Middle-aged group vs., Elderly Group) as a between-subjects factor, and Distracter Age (6–10 years, vs. 18–25 years, vs. 35–45 years, vs. over 70 years), SOA (−100 ms vs. 100 ms), and Congruence between the distracting gaze direction and the instructed direction (congruent vs. incongruent) as within-subjects factors. As in the main Experiment, post-hoc comparisons were performed using the Duncan's test (alpha level = .05) and the partial eta squared values (ηp2) were reported.
As in the main experiment, the ANOVA revealed a significant main effect of Congruence [F(1,20) = 20.09, MSE = 1792.32, p < .0003, ηp2 = .50], reflecting a higher percentage of antisaccades in the incongruent trials (10.56 ± 1.64%) compared to the congruent trials (6.03 ± 1.26%), and a nearly significant effect of SOA [F(1,20) = 20.09, MSE = 513.23, p = .063, ηp2 = .16], reflecting a higher percentage of antisaccades when the distracting gaze preceded (9.51 ± 1.81%) the instruction cue onset, rather than followed it (7.08 ± 1.13%). However, there was no effect of the perceived age of the distracting face on gaze-following behaviour as neither the interaction between Congruence and Distracter Age (p = .35) nor the interaction among Subject Age, Congruence and Distracter Ager was significant (p = .30). No other effects reach significance (all ps > .1).
Saccadic reaction times analysis
The reaction times of correct saccadic movements were our second variable of interest since they could provide an indirect measure of the automaticity of gaze-following behaviour. Indeed, although people may be able to suppress the tendency to automatically make saccades in the direction of the distracting gaze, one might predict that this suppression process would be more demanding in the incongruent trials compared to the congruent trials, even more so when the observers see the distracters' gaze moving before the instruction cue onset. As a consequence, higher reaction times should be observed under these conditions. Moreover, if age similarity modulates gaze-following behaviour, then the reaction times in incongruent trials should also increase when the age of the distracter matches that of the participants compared to conditions where no such matching occurs.
Main Experiment
We computed the mean values of SRTs across participants for each combination of the experimental variables. These data were submitted to a three-way repeated measures ANOVA, with the same within-subjects factors as in the previous analysis of errors. Post-hoc comparisons were performed as before.
The ANOVA showed a significant main effect of SOA [F (1, 22) = 75.97, MSE = 45338, p < .0001, ηp2 = .78]. More importantly, the automaticity of gaze-following behaviour was confirmed as the analysis revealed a significant main effect of Congruence [F(1,22) = 60.89, MSE = 16055, p < .0001, ηp2 = .73], indicating higher SRTs in the incongruent trials (311.63 ± 35.41 ms) than in the congruent trials (298.42 ± 35.95 ms), and a significant interaction between Congruency and SOA [F(1,22) = 4.75, MSE = 958, p < .05, ηp2 = .18], showing that the increase of SRTs in the incongruent trials was higher at 100-ms SOA (incongruent trials = 302.15 ± 33.71 ms, congruent trials = 285.71 ± 38.51 ms, Δ = 16.44 ms, p < .0001) than at −100-ms SOA (incongruent trials = 317.91 ± 36.82 ms, congruent trials = 311.14 ± 34.37 ms, Δ = 6.77 ms, p < .001).
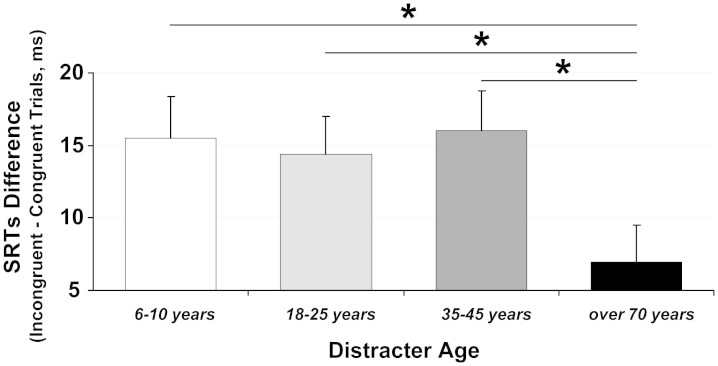
Interestingly, there was an effect of the perceived age of the distracting face on gaze-following behaviour as the interaction between Congruence and Distracter Age was significant [F(3,66) = 3.23, MSE = 413, p < .03, ηp2 = .13]. To better explore this two-way interaction, we calculated for each distracter age range the interference index by subtracting the SRTs' of congruent trials from those of the incongruent trials. T-tests for repeated measures were performed. They showed that the interference index for elderly distracters was lower (6.93 ± 12.20 ms; incongruent trials = 308.91 ± 34.95 ms, congruent trials = 301.98 ± 38 ms) than that for child distracters [15.48 ± 15.59 ms, t(22) = 2.24, p < .04; incongruent trials = 312.71 ± 36.73 ms, congruent trials = 297.23 ± 33.93 ms], young adult distracters [14.42 ± 12.00 ms, t(22) = 2.52, p < .02; incongruent trials = 310.65 ± 35.97 ms, congruent trials = 296.23 ± 36.09] and adult distracters [16.01 ± 13.03 ms, t(22) = 2.28, p < .04, see Fig. 2; incongruent trials = 314.27 ± 36.09 ms, congruent trials = 298.26 ± 38.63 ms], indicating that elderly distracters exerted the least interfering/cueing effect on gaze-following behaviour.
Figure 2. Mean Saccadic Reaction Times' difference of correct trials between incongruent and congruent trials as a function of the Distracter Age for young adult participants.
Error bars represent the standard errors of means across participants. Asterisks mark significant differences between means.
Control Experiment
We computed the mean values of SRTs as before and ran a four-way mixed ANOVA, with Subject Age (Middle-aged group vs. Elderly group) as a between-subjects factor and the same within-subjects factors as in the previous analysis. Post-hoc comparisons were performed as before.
The analysis revealed a significant main effect of Subject Age [F (1, 20) = 5.18, MSE = 286126, p < .04, ηp2 = .21], reflecting higher SRTs for elderly participants (419.53 ± 18.57 ms) than middle-aged participants (362.27 ± 16.96 ms). The interaction between Subject Age and Congruence was also significant [F(1, 20) = 5.46, MSE = 2230, p < .04, ηp2 = .21], indicating an automaticity of gaze-following behaviour in middle-aged participants (incongruent trials = 369.04 ± 16.81 ms, congruent trials = 355.50 ± 17.23 ms; p < .0005) but not in elderly participants (incongruent trials = 421.24 ± 18.41 ms, congruent trials = 417.82 ± 18.87 ms; p = .26). There was no effect of the perceived age of the distracting face as neither the interaction between Congruence and Distracter Age (p = .22) nor the interaction between Subject Age, Congruence and Distracter Age was significant (p = .40). No other effects reached significance (all ps> .1).
Discussion
We investigated whether in young adults the overt orienting of social attention triggered by seeing another person's gaze-shift is shaped by face age as an important similarity factor. To this end, we asked young, middle-aged and elderly adults to perform goal-directed saccades towards instructed peripheral stationary targets, while a distracting face of different ages (i.e., child, young adult, adult and elderly distracters) with averted gaze was presented.
Our results confirmed the automatic nature of gaze-following behaviour7,31,32 as indicated by both antisaccadic errors and correct saccadic latencies. Specifically, our results showed that, regardless of the age of participants, antisaccadic errors increased for averted-gaze that was mismatched with the instructed saccade (incongruent trials) when the distracting gaze-shift preceded the instruction7,10. In a similar vein, we found faster correct saccadic latencies for congruent trials than incongruent trials, especially when the distracting gaze-shift occurred before the instruction to make the saccade in young and middle-aged adults. On the contrary, for elderly adults the execution of correct saccades were not facilitated by congruent distracting gaze-shift; this could be attributed to the overall slowdown of saccade latencies observed for elderly compared to middle-aged adults, and is in line with a reduced gaze cueing effect found with manual RTs for old adults by Slessor and colleagues26.
Interestingly however, despite the automatic nature of gaze following, the age of the distracters affected both the accuracy and the latency of the instructed saccades only in young adults participants. In particular for this age category on the one hand, the results show that the effect of the distracter age on antisaccadic errors in incongruent trials was stronger for adult distracters than for child distracters and elderly distracters. On the other hand, elderly distracters exerted less interference effect than young adult and adult distracters in incongruent trials (i.e., lower percentage of antisaccadic errors) and less facilitation in congruent trials (i.e., higher percentage of antisaccadic errors than young adult and adult distracters, and the lowest difference of the correct saccadic latencies between incongruent and congruent trials). However, this was not the case for the middle-aged and elderly participants whose results showed that the distracter face age did not affect gaze-following behaviour.
These findings are consistent with previous studies that investigated the impact of face age on covert orienting of social attention25,26 which showed a reduced gaze-cueing effect in young participants for elderly distracters than for young face distracters25 and did not report age-related differences in the gaze-cueing effect in elderly participants26. Since all these previous studies used faces of only two age ranges (young vs. elderly faces) and participants from a wider range of ages (e.g., 17 to 41) were classified as young, the observed difference in gaze-cueing effect was interpreted by the authors as evidence of an OAB in covert orienting of attention in young adults. Here, the results obtained by using a more fine-grained manipulation of the distracter's age, advocate a more cautious interpretation of the difference in the gaze-interfering/cueing effect exerted by distracters of different ages. Specifically, in young adults although the effect of elderly distracters was less strong than young adult and adult distracters (both in term of interfering and facilitating effects), our findings do not show a clear-cut OAB, as the interfering effect exerted by the distracters of the same age of the participants (i.e., young distracter) did not significantly differ from that of adult and child distracters. Rather, our results as well as showing no OAB for middle-aged and elderly participants, suggest the presence of a blurred OAB in overt orienting of social attention in young adults. A possible explanation for this undefined border could be due to the influence on gaze following of familiarity with these particular face age categories (for example as a result of perceptual learning processes33), and/or social-cognitive mechanisms, which may shift the boundary of the categories, so as to include a wider age range of adult individuals. For example, this may depend on the fact that in our society, due to a longer life expectancy and social economical factors (such as the age of entry in the Italian job market and later retirement age) the border that defines a person as an adult, and in particular as a young adult or a middle-aged adults is rapidly changing and is not so well defined34,35. Interestingly, our findings are in good keeping with those reported by Wolff and colleagues28 in which young adults showed similar recognition memory for young faces (18–29 years) and young middle-aged (30–44 years) faces. In particular, they found that the OAB in adults is not exclusively directed toward age-congruent in-group faces (see also36). The fact that we did not find the OAB in middle-aged and elderly people is not surprising given that the evidence concerning its presence across the lifespan is mixed20,37,38. Such inconsistency may be due to the fact that an individual's specific living conditions and social experience affect an individual's face representation so as to better represent, and be more sensitive to, the most predominant age traits of the faces present in his/her environment. In other words, visual experience with faces of a wider age range, which is likely to characterize both elderly and middle-aged individuals (but less so young adults) can tune their perceptual face processing to the point of abolishing the disadvantage for other-age face processing in comparison to young adults. Note that our elderly participants were also grandparents (except for one) of children of broadly of the same age of our distracters who interact regularly with their grandchildren and the parents of their grandchildren (whose ages fall in the middle-age range). Similarly, our middle-aged participants were colleagues of some of the authors, who lecture to young adults, and have elderly parents with whom they interact actively.
Both Wolff et al.'s results and our results are consistent with the idea that in young adults the higher number of daily contacts with own-age and middle-aged faces could explain the absence of differences between young adult and adult distracters on gaze-following behaviour. Indeed, several studies have shown that younger adults report having more contact with young people and adults than the elderly28,39. Therefore, in the present study participants might have found it more difficult to ignore the gaze cues of adults (but also of children) because they are more familiar13 with the faces of individuals belonging to these two age groups. Thus, a greater and early experience with these face age categories could have affected perceptual and social categorization processes38. Both the degree of familiarity or experience with a given age category, and in young adults the social categorization into in-group based on the perceived age, may enhance the relevance (or salience) of the face we are looking at, thus making it more difficult to ignore the direction of its gaze.
It could also be argued that our pattern of results could be determined by age-related physical differences of the distracter faces (i.e., winkles, skin excess or the width of gaze), however results from an additional control experiment (see Supplementary information S3) exclude this possibility. No differences in discriminating the direction of gaze shifts related to the age of the distracter face, in fact, were found across the three age groups of participants.
In summary, our findings indicate that face age modulates gaze-following behaviour in young adults, extending previous evidence of the role of bottom-up visual facial cues in shaping social attention14. Moreover, in line with the previous study which investigated the OAB in face recognition28, our results indicate, for the first time, the presence in young adults of a broader OAB in the orienting of social attention.
Future research is required to evaluate whether in young adults the amount of contact that people of different ages have with other age groups modulates the own-age bias found in gaze following.
Our study also suggests a complex interplay of face age, familiarity, or experience with different age groups40, and social categorization on gaze-following behaviour. Future studies are needed to clarify the role of implicit cues of similarity on social attention. In particular on the one hand, they should systematically investigate participants' expertise and the level of daily contact with people of different age groups. On the other hand, they should test whether the manipulation of cues which communicate similarity (e.g., age, ethnic group, gender, personal familiarity, religion, and political affiliation) have a different effect depending on whether they are conveyed in a bottom-up or top-down manner.
In conclusion, our findings provide new evidence regarding the role of implicit cues such as face age in shaping social attention, and suggest that the social and environmental salience of the seen face by the observer affect the mechanisms underlying gaze-following behaviour, and influence how (and with whom) we orient our attention.
Method
Main Experiment
Participants
Twenty-seven right-handed undergraduate students (19 females and 8 male; mean age = 22.4 ± 2.4 years) from the University of Milano-Bicocca received course credits for their participation in the study. All participants had normal or corrected-to normal vision and were unaware of the purpose of the experiment. All gave their informed consent. The study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and fulfilled the ethical standard procedure recommended by the Italian Association of Psychology (AIP). All experimental protocols were also approved by the ethical committee of the University of Milano-Bicocca.
Apparatus and Stimuli
For all experiments the apparatus and stimuli were identical. The participants sat about 50 cm from a 19-inch LCD monitor (Samsung SyncMaster 943; resolution: 1280 × 1024 pixels; refresh rate: 60 Hz) with their head placed on a chinrest in order to maintain a stable eye-to-screen distance. Eye position and eye movements were measured monocularly in real-time by means of an infrared video-based system (EyeLink II, SR Research Ltd., Mississauga, Ontario, Canada). For all the participants, the movements of their right eye were recorded. Stimulus presentation and response collection were controlled by the SR Research Experiment Builder software (version 1.10.56). Since previous studies showed gaze cueing to be relatively unaffected by changes of facial identity41,42 we did not include as experimental stimuli a wide range of face identity but we selected 16 grayscale photographs (7.98 × 15.76 degrees of visual angle) depicting faces of 4 children (age range: 6–10 years), 4 young adults (age range: 18–25 years), 4 adults (age range: 35–45 years), and 4 elderly adults (age range: over 70 years); all bearing a neutral expression and a straight gaze. For each age range, two photographs were of female faces and the other two were of male faces. The photographs of younger adult, adult and elderly adult faces were extracted from the Productive Aging Lab Face Database43. The photographs of the children were provided by their parents, who were colleagues of some of the authors and gave their written consent to use the photographs for scientific purposes. The selection of each stimulus face and its assignment to one of the four age ranges was performed on the basis of the perceived age rating score provided by 12 undergraduate students (manipulation check control group: 8 females and 4 males; mean age = 25.1 ± 3.9 years), who did not take part in any of the experiments. The straight gaze of each stimulus face was averted 0.75 degrees of visual angle both to the left and to the right using Adobe Photoshop.
Procedure
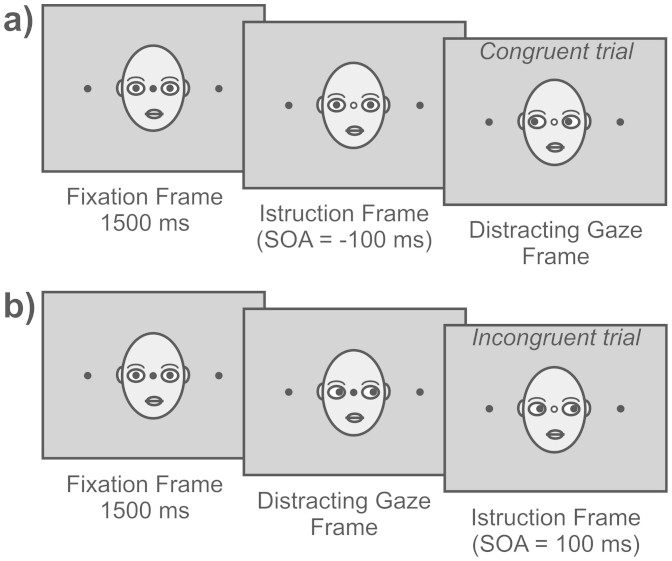
Young adult participants were tested in one experimental session, lasting about 70 minutes. The session comprised 640 trials, divided into four blocks of 160 trials each, with the 16 conditions (2 SOAs × 4 Distracter Age × 2 Distracter Congruence) being equally probable, and repeated randomly 20 times. A practice session of 16 trials (i.e., one trial per condition) was given prior to the beginning of the first block. The practice trials were discarded from subsequent analyses. Each trial started with the appearance of the black central fixation circle (diameter: 0.51 degrees of visual angle) presented on the nose (centre of the picture) of the displayed face with straight gaze. The face was flanked by two black target circles (diameter: 0.89 degrees of visual angle), one to the left and the other to the right of the horizontally aligned fixation circle (eccentricity: 10.66 degrees of visual angle). After 1500 ms the colour of the fixation turned to either green or red. The participants were required to perform a fast and accurate saccade toward the left or the right peripheral target depending on the colour (red or green) of the fixation circle. The colour instruction and saccades direction were counter-balanced across the participants. 100 ms before or after the colour change (stimulus onset asynchrony, SOA), the distracting face shifted his/her gaze toward the left or the right target. Targets remained visible throughout the duration of the trial (Fig. 3).
Figure 3.
Schematic illustration of the sequence of events for (a) Congruent Distracter (Instructed Direction: Left) at −100-ms SOA and (b) Incongruent Distracter (Instructed Direction: Left) at +100-ms SOA. B.F.M.M. drew this figure.
The distracting gaze could be congruent or incongruent with the instructed direction. Since it was task irrelevant, participants were explicitly instructed to ignore the distracting face. The eye-movement accuracy of the participants was measured by recording the first horizontal saccade that followed the instruction cue. Saccadic reaction times (SRTs) were also recorded. At the end of the experiment, the participants were asked to rate the age of each faces with a value between 01 and 99 years, to verify that participants perceived the distracting faces as belonging to the 4 age ranges of interest (manipulation check).
Control Experiment
Participants
Twenty-four right-handed adults belonging to two different age groups (Middle-aged group: 6 females and 6 males, mean age = 40.1 ± 4.2 years; Elderly group: 6 females and 6 male, mean age = 69.7 ± 3.9 years) participated as volunteers in the study and were all unaware of the purpose of the experiment. Middle-aged adults (expect for two) were members of the Department of Psychology of the University of Milano-Bicocca; whereas elderly adults were all community dwelling and recruited through personal contacts. They did not self-report eye diseases (e.g., cataract disease, glaucoma) and had normal or corrected-to normal vision. Their good visual acuity was checked prior to the start of the experiment by means of the Italian version of the Radner's Reading Eyesight Chart. All gave their written informed consent. The study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and fulfilled the ethical standard procedure recommended by the Italian Association of Psychology (AIP). All experimental protocols were also approved by the ethical committee of the University of Milano-Bicocca.
Procedure
The middle-aged and elderly participants were tested in one experimental session, lasting about 45 minutes. The procedure was exactly the same as in the main experiment, except that middle-aged and elderly participants performed a shorter version of the experimental session that comprised 256 trials, divided into eight blocks of 32 trials each with the 16 conditions being equally probable and repeated randomly.
Author Contributions
F.C. designed, performed the experiments, prepared the stimuli, and wrote the manuscript. B.F.M.M. analyzed the data, helped running the control experiments, wrote the results section of the manuscript, and prepared the figures. R.A-G. designed the experiment and contributed to the discussion of the data. A.R. performed the experiments, prepared the stimuli, and contributed to data analysis. P.R. designed the experiments, wrote and revised the manuscript. All authors reviewed the manuscript.
Supplementary Material
Face age modulates gaze following in young adults
Acknowledgments
This work was supported by MIUR PRIN (project 2008ZN5J5S). We thank Laura Casnaghi for helping with data collection. We are grateful to Marco Perugini and Simona Sacchi for discussion of this project. The authors have no conflicts of interest.
References
- Morales M., Mundy P. & Rojas J. Following the direction of gaze and language development in 6-month-olds. Infant Behav. Dev. 21, 373–377; 10.1016/S0163-6383(98)90014-5 (1998). [Google Scholar]
- Mundy P. & Newell L. Attention, joint attention, and social cognition. Curr. Dir. Psychol. Sci. 16, 269–274; 10.1111/j.1467-8721.2007.00518.x (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischen A., Bayliss A. P. & Tipper S. P. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychol. Bull. 133, 694–724; 10.1037/0033-2909.133.4.694 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind (MIT Press, Cambridge, MA, 1997). [Google Scholar]
- Innocenti A., De Stefani E., Bernardi N. F., Campione G. C. & Gentilucci M. Gaze direction and request gesture in social interactions. PLoS One 7, e36390; 10.1371/journal.pone.0036390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierno A. C. et al. When gaze turns into grasp. J. Cognitive Neurosci. 18, 2130–2137; 10.1162/jocn.2006.18.12.2130 (2006). [DOI] [PubMed] [Google Scholar]
- Ricciardelli P., Bricolo E., Aglioti S. M. & Chelazzi L. My eyes want to look where your eyes are looking: exploring the tendency to imitate another individual's gaze. NeuroReport 13, 2259–2263 (2002). [DOI] [PubMed] [Google Scholar]
- Greene D. J., Mooshagian E., Kaplan J., Zaidel E. & Iacoboni M. The neural correlates of social attention: automatic orienting to social and non-social cues. Psychol. Res. 73, 499–511; 10.1007/s00426-009-0233-3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmaso M., Pavan G., Castelli L. & Galfano G. Social status gates social attention in humans. Biol. Lett. 8, 450–452; 10.1098/rsbl.2011.0881 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzza M. T. et al. Follow my eyes: the gaze of politicians reflexively captures the gaze of ingroup voters. PLoS One 6, e25117; 10.1371/journal.pone.0025117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardelli P., Carcagno S., Vallar G. & Bricolo E. Is gaze following purely reflexive or goal-directed instead? Revisiting the automaticity of orienting attention by gaze cues. Exp. Brain Res. 224, 93–106; 10.1007/s00221-012-3291-5 (2013). [DOI] [PubMed] [Google Scholar]
- Pavan G., Dalmaso M., Galfano G. & Castelli L. Racial group membership is associated to gaze-mediated orienting in Italy. PLoS One 6, e25608; 10.1371/journal.pone.0025608 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner R. O., Shepherd S. V. & Platt M. L. Familiarity accentuates gaze cuing in women but not men. Biol. Lett. 3, 64–67; 10.1098/rsbl.2006.0564 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungr C. J. & Hunt A. R. Physical self-similarity enhances the gaze-cueing effect. Q. J. Exp. Psychol. (Hove) 65, 1250–1259; 10.1080/17470218.2012.690769 (2012). [DOI] [PubMed] [Google Scholar]
- Jones B. C. et al. Facial cues of dominance modulate the short-term gaze cuing effect in human observers. Proc. R. Soc. B 277, 617–624; 10.1098/rspb.2009.1575 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsen G., van Zoest W. & van Vugt M. Gender and facial dominance in gaze cuing: emotional context matters in the eyes that we follow. PLoS One 8, e59471; 10.1371/journal.pone.0059471 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacci P., Ricciardelli P., Lugli L. & Pellicano A. Emotional attention: effects of emotion and gaze direction on overt orienting of visual attention. Cogn. Process. 9, 127–135 10.1007/s10339-007-0198-3 (2008). [DOI] [PubMed] [Google Scholar]
- Tipples J. Fear and fearfulness potentiate automatic orienting to eye gaze. Cogn. Emot. 20, 309–320; 10.1080/02699930500405550 (2006). [Google Scholar]
- Macrae C. N. & Bodenhausen G. V. Social cognition: thinking categorically about others. Annu. Rev. Psychol. 51, 93–120; 10.1146/annurev.psych.51.1.93 (2000). [DOI] [PubMed] [Google Scholar]
- Anastasi J. S. & Rhodes M. G. An own-age bias in face recognition for children and older adults. Psychon. Bull. Rev. 12, 1043–1047 (2005). [DOI] [PubMed] [Google Scholar]
- McGuire W. J. [The nature of attitudes and attitude change] The Handbook of Social Psychology [Lindzey, G. & Aronson, E. (Eds.)] [136–314] (Random House, New York, 1969). [Google Scholar]
- Shanteau J. & Nagy G. F. Probability of acceptance in dating choice. J. Pers. Soc. Psychol. 37, 522–533; 10.1037/0022-3514.37.4.522 (1979). [Google Scholar]
- DeBruine L. M. Trustworthy but not lust-worthy: context-specific effects of facial resemblance. Proc. R. Soc. B 272, 919–922; 10.1098/rspb.2004.3003 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp D. B., DeBruine L. M. & Barclay P. A cue of kinship promotes cooperation for the public good. Evol. Hum. Behav. 29, 49–55; 10.1016/j.evolhumbehav.2007.08.002 (2008). [Google Scholar]
- Slessor G., Phillips L. H. & Bull R. Age related declines in basic social perception: evidence from tasks assessing eye-gaze processing. Psychol. Aging 23, 812–822; 10.1037/a0014348 (2008). [DOI] [PubMed] [Google Scholar]
- Slessor G., Laird G., Phillips L. H., Bull R. & Filippou D. Age-related differences in gaze following: does the age of the face matter? J. Gerontol. B Psychol. Sci. Soc. Sci. 65, 536–541; 0.1093/geronb/gbq038 (2010). [DOI] [PubMed] [Google Scholar]
- Wright D. B. & Stroud J. S. Age differences in line up identification accuracy: people are better with their own age. Evol. Hum. Behav. 26, 641–654; 10.1023/A,1020981501383 (2002). [DOI] [PubMed] [Google Scholar]
- Wolff N., Wiese H. & Schweinberger S. R. Face recognition memory across the adult life span: event-related potential evidence from the own-age bias. Psychol. Aging 27, 1066–1081; 10.1037/a0029112 (2012). [DOI] [PubMed] [Google Scholar]
- Cuddy A. J. & Fiske S. T. [Doddering, but dear: process, content, and function in stereotyping of older persons]. Ageism: Stereotyping and Prejudice Against Older Persons [Nelson, T. D. (Ed.)] [3–26] (MIT Press, Cambridge, MA, 2002). [Google Scholar]
- Rhodes M. G. Age estimation of faces: A review. Appl. Cogn. Psychol. 23, 1–12; 10.1002/acp.1442 (2009). [Google Scholar]
- Kuhn G. & Kingstone A. Look away! Eyes and arrows engage oculomotor responses automatically. Atten. Percept. Psychophys. 71, 314–327; 10.3758/APP.71.2.314 (2009). [DOI] [PubMed] [Google Scholar]
- Ricciardelli P., Betta E., Pruner S. & Turatto M. Is there a direct link between gaze perception and joint attention behaviours? Effects of gaze contrast polarity on oculomotor behaviour. Exp. Brain. Res. 194, 347–357; 10.1007/s00221-009-1706-8 (2009). [DOI] [PubMed] [Google Scholar]
- Kuefner D., Macchi Cassia V., Vescovo E. & Picozzi M. Experience acquired in adulthood enhances holistic face processing: evidence from the other-age effect. Vis. Cogn. 18, 11–25; 10.1080/13506280802396507 (2010). [Google Scholar]
- Arnett J. J. Emerging adulthood: A theory of development from the late teens through the twenties. Am. Psychol. 55, 469–480; 10.1037/0003-066X.55.5.469 (2000). [PubMed] [Google Scholar]
- Stone A. A., Schwartz J. E., Broderick J. E. & Deaton A. A snapshot of the age distribution of psychological well-being in the United States. Proc. Natl. Acad. Sci. USA 107, 9985–9990; 10.1073/pnas.1003744107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardo F., Marino B. F. M., Rossetti A., Actis-Grosso R. & Ricciardelli P. [Face age and social status exert different modulatory effects on gaze following behaviour]. Proceedings of the 35th Annual Conference of the Cognitive Science Society [Knauff, M., Pauen,M., Sebanz, N., & Wachsmuth, I. (Eds.)] [2058–2062] (Cognitive Science Society, Austin, TX, 2013). [Google Scholar]
- Wiese H., Schweinberger S. R. & Hansen K. The age of the beholder: ERP evidence of an own-age bias in face memory. Neuropsychologia 46, 2973–2985; 10.1016/j.neuropsychologia.2008.06.007 (2008). [DOI] [PubMed] [Google Scholar]
- Macchi Cassia V. Age biases in face processing: The effects of experience across development. Br. J. Psychol. 102, 816–829; 10.1111/j.2044-8295.2011.02046.x (2011). [DOI] [PubMed] [Google Scholar]
- Ebner N. C. & Johnson M. K. Young and older emotional faces: are there age group differences in expression identification and memory? Emot. 9, 329–339; 10.1037/a0015179 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti V., Pisacane A. & Macchi Cassia V. Natural experience modulates the processing of older adult faces in young adults and 3-year-old children. PLoS One 8, e57499; 10.1371/journal.pone.0057499 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischen A. & Tipper S. P. Orienting attention via observed gaze shift evokes longer term inhibitory effects: implications for social interactions, attention, and memory. J. Exp. Psychol. 133, 516–533; 10.1037/0096-3445.133.4.516 (2004). [DOI] [PubMed] [Google Scholar]
- Tipples J. Orienting to eye gaze and face processing. J. Exp. Psychol. Hum. Percept. Perform 3, 843–856; 10.1037/0096-1523.31.5.843 (2005). [DOI] [PubMed] [Google Scholar]
- Minear M. & Park D. C. A lifespan database of adult facial stimuli. Behav. Res. Methods Instrum. Comput. 36, 630–633 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Face age modulates gaze following in young adults