Abstract
Introduction
1α,25-Dihydroxyvitamin D3 (1,25-D3) is antiproliferative in pre-clinical models of lung cancer, but in tumor tissues its efficacy may be limited by CYP24A1 expression. CYP24A1 is the rate limiting catabolic enzyme for 1,25-D3 and is overexpressed in human lung adenocarcinoma (AC) by unknown mechanisms.
Methods
The DNA methylation status of CYP24A1 was determined by bisulfite DNA pyrosequencing in a panel of 30 lung cell lines and 90 surgically resected lung AC. The level of CYP24A1 methylation was correlated with CYP24A1 expression in lung AC cell lines and tumors. In addition, histone modifications were assessed by quantitative chromatin immunoprecipitation-PCR (ChIP-qPCR) in A549, NCI-H460 and SK-LU-1.
Results
Bisulfite DNA pyrosequencing analysis revealed that CYP24A1 gene was heterogeneously methylated in lung AC. Expression of CYP24A1 was inversely correlated with promoter DNA methylation in lung AC cell lines and tumors. Treatment with 5-aza-2′-deoxycytidine (5-Aza) as well as trichostatin A (TSA) increased CYP24A1 expression in lung AC. We observed that CYP24A1 promoter hypermethylation decreased CYP24A1 enzyme activity in vitro, whereas treatment with 5-Aza and/or TSA increased CYP24A1 enzyme affinity for its substrate 1,25-D3. In addition, ChIP-qPCR analysis revealed specific histone modifications within the CYP24A1 promoter region. Treatment with TSA increased H3K4me2 and H3K9ac and simultaneously decreased H3K9me2 at the CYP24A1 promoter.
Conclusions
The expression of CYP24A1 gene in human lung AC is in part epigenetically regulated by promoter DNA methylation and repressive histone modifications. These findings should be taken into consideration when targeting CYP24A1 to optimize antiproliferative effects of 1,25-D3 in lung AC.
Keywords: vitamin D metabolism; CYP24A1; 1α,25-Dihydroxyvitamin-D3; DNA methylation; histone modification; lung adenocarcinoma
Introduction
1α,25-Dihydroxyvitamin D3 (1,25-D3, calcitriol) is the most active form of vitamin D and has antineoplastic activity at supraphysiologic doses in several cancers both in vitro and in vivo.1 At these doses, 1,25-D3 is associated with antiproliferative activity,2–4 induction of cell differentiation,5 cell cycle arrest,6 apoptosis,7, 8 and inhibition of angiogenesis.9 Antiproliferative activity is mediated through the vitamin D receptor (VDR) which is not only found in classic target organs (intestinal tract, kidney and bone), but also in many other epithelial and mesenchymal cells of tissues such as lung, prostate and breast. The evidence for a possible role of the vitamin D pathway in lung cancer comes largely from in vitro or in vivo studies. Higashimoto et al.,10 reported that 1,25-D3 inhibited the growth of lung cancer cell lines and affects cell cycle regulation in squamous cell carcinoma models.11, 12 1,25-D3 has been shown to inhibit lung tumor growth and lung metastases in mouse models.13, 14 The antiproliferative effect of 1,25-D3 is related to G1 cell cycle arrest and is directly and inversely proportional to the VDR15 and CYP24A1 expression respectively.16
CYP24A1 is a member of the cytochrome P450 superfamily of enzymes involved in drug metabolism and synthesis of steroids, and other lipids. CYP24A1 encodes for 24-hydroxylase, the rate limiting enzyme that catalyzes the inactivation of 1,25-D3. This gene is located on chromosome 20q13.2, and is thought to be a proto-oncogene. Albertson et al.,17 reported that gene copy number and CYP24A1 expression are increased in breast cancer. Mimori et al.,18 correlated overexpression of CYP24A1 and poor survival in esophageal cancer. We and others16, 19,20 have reported up-regulation of CYP24A1 in non-small cell lung cancer as compared to normal tissues. Furthermore, overexpression of CYP24A1 resulted in increased catabolism of 1,25-D3 in lung cancer cells compared with non-neoplastic lung.21 Our initial studies revealed that CYP24A1 mRNA was significantly overexpressed (8–50 fold) in lung adenocarcinoma (AC) as compared to non-neoplastic lung tissue and especially higher in poorly-differentiated tumors. Additionally, resected lung AC patients with CYP24A1 overexpression had significantly shorter overall survival as compared to low CYP24A1 (5 year survival rate, 42% versus 81% respectively; Log-rank p = 0.007).16 In this study, we found that one third of patients with high CYP24A1 expression showed gain in region of 20q, even though focal amplification was only noted in a small percentage of the tumors. We hypothesized that epigenetic mechanisms such as promoter methylation and histone modifications may be involved in the transcriptional regulation of CYP24A1. CYP24A1 has a promoter region that is rich in CpG islands and tissue-specific CYP24A1 promoter hypermethylation has been described in normal human tissues.22 Interestingly, transformed neoplastic cells have also demonstrated differential methylation of the CYP24A1 gene. Luo et al. showed that CYP24A1 gene expression in human prostate cancer is silenced in part by promoter DNA methylation and repressive histone modifications.23
In this study, we sought to determine whether epigenetic regulation of the CYP24A1 gene in lung AC could explain its differential expression in lung AC and whether these events result in differential affinity to the substrate 1,25-D3. Considering the importance of CYP24A1 in the regulation of 1,25-D3 levels, we elucidated the roles of DNA methylation and histone modification of CYP24A1 in human lung AC cell lines and primary lung AC..
Materials and Methods
Human Samples
Lung tumor samples were obtained from patients undergoing primary thoracic resection for lung cancer without preoperative radiation or chemotherapy, as previously described.24 Tissue specimens were banked with informed consent after approval from the University of Michigan Institutional Review Board and Ethics Committee and were frozen in liquid nitrogen and stored at −80°C. Percentage of tumor purity in sections adjacent to the regions used for RNA extraction was estimated during routine histopathologic analysis. Regions containing a minimum of 70% tumor cellularity were utilized for DNA and RNA isolation.
Bisulfite DNA Pyrosequencing Analysis
Genomic DNA was isolated using the genomic DNA isolation kit (Qiagen, Valencia, CA). DNA (500 ng) was converted using the EZ DNA Methylation Kit (Zymo Research Corporation, Irvine, CA), following manufacturers’ recommendations. Methylation status of the CYP24A1 promoter in human lung primary tissue samples and cell lines was assessed through pyrosequencing assay. Bisulfite pyrosequencing assays for the CYP24A1 gene were designed to assess the methylation status of 5 CpG sites corresponding to the 3′-CpG island, while spaning a putative regulatory sequence located in exon 1 within this CpG island. The sequences of the primers used for PCR amplification were +66F (5′-GGAAAAGGAAGTAAAGAGGGTTAGT-3′) and +301R (Biotin-5′-ACCAAACCCTAAAAATCAACCTTAC-3′) and the sequencing primer was 5′-TTAGTGTAAGGAGGTATTAA-3′. PCR cycling conditions were as follows: initial incubation at 95°C for 5 min, followed by 45 cycles of 95°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec. Pyrosequencing reactions were performed in a PSQ 96 Pyrosequencing System (Biotage, Charlotte, NC) using appropriate reagents and protocols. The analysis was performed in duplicate in two independent experiments and methylation value was obtained from the average of each of the 5 CpG sites included in the sequence analyzed. Controls to assess accurate bisulphite conversion of the DNA were used on each run to ensure the fidelity of the measurements.
Cell Culture
Three human lung AC cancer cell lines (A549, NCI-H460 and SK-LU-1 were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured with RPMI 1640 or DMEM-F12 medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS, Hyclon, Logan, UT) and 1% Gibco® Antibiotic-Antimycotic (Life Technologies, Carlsbad, CA) at 37°C in a humid atmosphere consisting of 5% CO2/95% air. Cell lines were authenticated and genotyped using the Identifiler Plus kit (Applied Biosystems, Carlsbad, CA).
Drug Treatments
A549, NCI-H460 and SK-LU-1 cells were seeded at 1 × 105 cells in a six-well plate overnight. For dose-response effects, cells were treated with 5-aza-2′-deoxycytidine (5-Aza, Sigma-Aldrich, St. Louis, MO) at concentrations of 0.1, 0.5, 1, 2 and 5 μM for 72 h, followed by an additional incubation for 24 h with or without 100 nM of 1,25-D3 (Sigma-Aldrich) and then were finally harvested. Cells were also treated with trichostatin A (TSA, Sigma-Aldrich) at 50, 100, 200, 300 and 400 nM for 8 h, followed by an additional incubation for 24 h with or without 1,25-D3 (100 nM), and then harvested. For 5-Aza and TSA combination treatments, cells were treated with 5 μM of 5-Aza for 72 h, followed by addition of 300 nM of TSA and 100 nM of 1,25-D3 for an additional 24 h, and then harvested.
RNA Extraction and cDNA Synthesis
Total RNA was isolated from the tissue samples and cell lines using Trizol (Invitrogen) followed by column purification using the RNeasy Mini kit (Qiagen) according to the manufacturers’ instructions. RNA was eluted from the spin column using RNase-free dH2O. cDNA was prepared from RNA samples using the High Capacity cDNA Reverse Transcription kit (Applied Biosytems, Foster City, CA) according to manufacturer’s protocols.
Quantitative real time reverse transcription polymerase chain reaction (qRT-PCR)
The qRT-PCR reaction was prepared using Power SYBR Green Master Mix (Applied Biosystems), and qRT-PCR was performed with the StepOne Real-Time PCR System (Applied Biosystems). Each sample had a final volume of 15 μl containing approximately 100 ng of cDNA. The sequences of the oligonucleotide primers for CYP24A1 (144 bp PCR product) were as follows: 5′-GCCGTATTTAAAAGCCTGTCTGAA-3′ (forward) and 5′-ACCTGGGTATTTAGCATG AGCACTG-3′ (reverse). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to standardize the CYP24A1 qRT-PCR results. Relative mRNA levels were assessed using the 2−ΔΔCt method as described previously.16
CYP24A1 Activity Assay
Cells were treated with 5 μM of 5-Aza for 72 h and/or 300 nM of TSA for 8 h and were washed with PBS twice. The cells (5 ×105) were then incubated with 1,25-D3 (100 nM) in 3 ml of medium for 48 h. The medium was collected and the cells were treated with lysis buffer and were combined with the medium. The remaining 1,25-D3 in the combined medium and cell lysate was analyzed using the 1,25-Dihydroxy Vitamin D EIA kit (Immunodiagnostic Systems Ltd, Scottsdale, AZ) according to the manufacturer’s instruction.
Quantitative chromatin immunoprecipitation PCR (ChIP-qPCR)
ChIP-qPCR was performed essentially as described by Väisänen and colleagues.25 Briefly, cells were treated with 5-Aza (5 μM) or TSA (300 nM) or both agents. One hour after adding 1,25-D3, the cells were fixed by adding formaldehyde as described previously to cross-link nuclear protein to DNA. Chromatin was sheared enzymatically (Active Motiff, Carlsbad, CA) to about 500 bp on an average length and was precipitated with the antibodies against H3K9me2, H3K4me2, H3K9ac and VDR. Antibodies against H3K9me2, H3K4me2 and H3K9ac were purchased from Active Motiff and an antibody against VDR was obtained from Santa Cruz Biotechnologies (Dallas, TX). ChIP reaction was performed two times. ChIP-qPCR primers for the CYP24A1 promoter region 1 and region 2 were −292F (5′-AGCACACCCGGTGAACTC-3′) and −152R (5′-TGGAAGGAGGATGGAGTCAG-3′); +130F (5′-TTCAAGAGGTCCCCAGACAC-3′) and +333R (5′-AGTCGGGGCTTAACGATTCT-3′). ChIP-qPCR was performed using the Power SYBR Green PCR Master Mix (Applied Biosystems) with the following reaction cycling: 95°C for 10 min, 45 cycles of 94°C for 30 sec and 57°C for 30 sec.22 ChIP-qPCR was run in triplicate and repeated twice per each ChIP reaction to confirm the reproducibility of results. Copy number was determined using a standard curve containing serial dilutions (107–100 copies) of the CYP24A1 DNA amplicon. Results were normalized to 10% input DNA amplifications after subtraction of signals obtained from the antibody isotype control.
Protein Isolation and Immunoblot Analysis
A549, NCI-H460 and SK-LU-1 cells were plated and grown until 80% confluent. The cells were harvested with lysis buffer [150 mM NaCl, 20 mM Tris (pH 7.5), 1 mM EDTA, 1 mM EGTA, 2.5 mM Na4P2O7, 1 mM β-glycerol 2-phosphate disodium salt hydrate, 1 mM Na3VO4, 1% Triton X-100] supplemented with protease inhibitor cocktail (Sigma-Aldrich). Proteins were quantified using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s protocol. Proteins (20 μg) were resolved on 10% tris-glycine gels (Invitrogen) and transferred to nitrocellulose membranes (Pall Corp., Ann Arbor, MI). The blots were probed with either anti-CYP24A1 (Santa Cruz Biotechnology), anti-DNA methyltransferase (DNMT) 1 (Cell Signaling Biotechnology, Beverly, CA) and anti-DNMT3A (Cell Signaling Biotechnology) diluted at 1:1,000, or GAPDH (Calbiochem, EMD Bioscience Inc., San Diego, CA) diluted at 1:10,000. GAPDH was used as a loading control. Each band was normalized by GAPDH. Arbitrary units in the figures represent the ratio between the normalized band density of the specific protein treated with 5-Aza and/or TSA and the corresponding untreated protein. This was repeated in three independent experiments.
Statistical Analysis
Chi-square and analysis of variance (ANOVA) tests were used to identify statistically significant differences between different clinical variables and the level of CYP24A1 methylation. The significance of differences between in vitro experimental conditions was determined using the ANOVA tests and p value < 0.05 was considered significant. The outcome variables for survival analysis were disease-free survival (DFS) and overall survival (OS). DFS was measured from the date of surgery to the time of recurrence, death or censoring at 5 years. OS was measured from date of surgery to the time of death or censoring at 5 year. Survival curves were constructed using the method of Kaplan–Meier and survival differences were assessed using the Log-rank test. The univariate and multivariate Cox proportional hazards model including the covariates of age, gender, smoking history, disease stage and adjuvant treatment were used for assessing the prognostic value of CYP24A1 methylation on DFS and OS. The Pearson’s method was used to test the correlation between CYP24A1 expression and CYP24A1 promoter DNA methylation.
Results
CYP24A1 Promoter Methylation in Lung Cell Lines and Lung Adenocarcinoma tumors
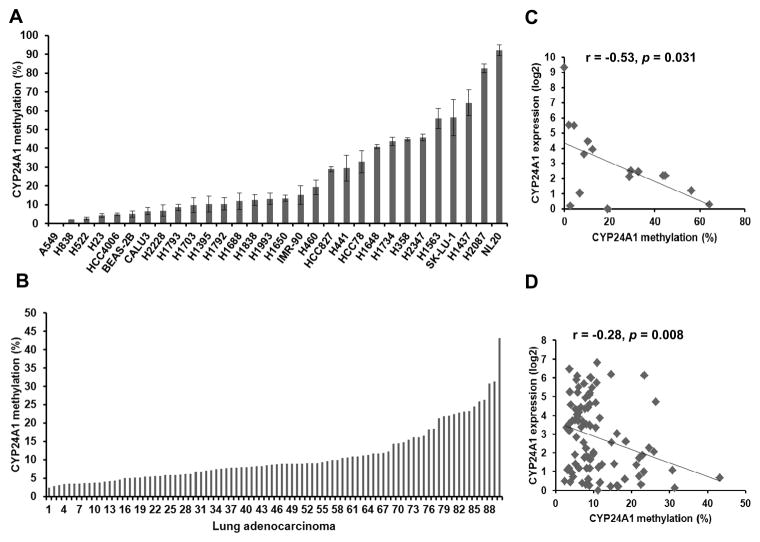
We used bisulfite DNA pyrosequencing assays to determine the methylation status of CYP24A1 in a panel of 27 lung cancer cell lines, 3 immortalized lung cell lines and 90 lung AC tumors. Based on the NCBI Epigenomics database26 the human CYP24A1 gene contains two CpG islands (580 and 1,231 bp, respectively) that span its transcription start site.27 Interestingly, the 5′-CpG island of the CYP24A1 gene encompasses the promoter region and contains two known vitamin D response elements (VDREs): the proximal element VDRE (VDREp; −172/−143) and the distal element VDRE (VDREd; −293/−273).28 We assessed the methylation status of the 3′-CpG island, since it was previously reported that this region undergoes higher levels of DNA methylation.23 The 3′-CpG island of CYP24A1 was heterogeneously methylated among the 30 lung cancer cell lines and lung AC tumors (Fig. 1A and 1B). Using a 10% cutoff, 20 cell lines (66.7%) and 32 primary tumors (35.5%) harbored CYP24A1 hypermethylation. Using DNA methylation array data from a previous study,29 we observed that non-malignant lung samples had significantly higher β-value at multiple CpG sites located at CYP24A1 promoter (Supplementary Fig. 1).
Figure 1.
CYP24A1 promoter DNA methylation in lung cancer cell lines and primary lung AC. Bisulfite pyrosequencing analysis covering 5 CpG sites of the CYP24A1 promoter in 27 lung cancer cell lines and 3 lung immortalized lines (A) and 90 lung AC tumors (B). The average methylation percentage from 5 CpGs was used for calculating the total proportion of methylated CpGs in the CYP24A1 promoter region. Scatterplot of CYP24A1 mRNA expression and CYP24A1 promoter methylation in a subset of 16 lung cancer cell lines (C) and 90 lung AC (D). An inverse correlation among CYP24A1 expression and methylation was found in lung cancer cell lines and lung AC tumors.
Next, we assessed the correlation between CYP24A1 mRNA expression and CYP24A1 promoter DNA methylation in a subset of 16 lung cancer cell lines expressing variable levels of CYP24A1 and the same cohort of 90 lung AC tumors. We found a statistically significant negative correlation between CYP24A1 expression and methylation in the lung cancer cell lines (r = −0.53, p = 0.031, Fig. 1C) and in the lung AC tumors (r = −0.28, p = 0.008, Fig. 1D), suggesting that DNA methylation plays an important role in the regulation of CYP24A1 expression. However we observed some tumors and cell lines with low CYP24A1 methylation expressed low levels of CYP24A1, suggesting that other mechanisms such as copy number or post-translational modifications might also be involved in CYP24A1 regulation.
CYP24A1 Promoter Methylation Is Associated with Clinical Outcome in Lung Adenocarcinoma
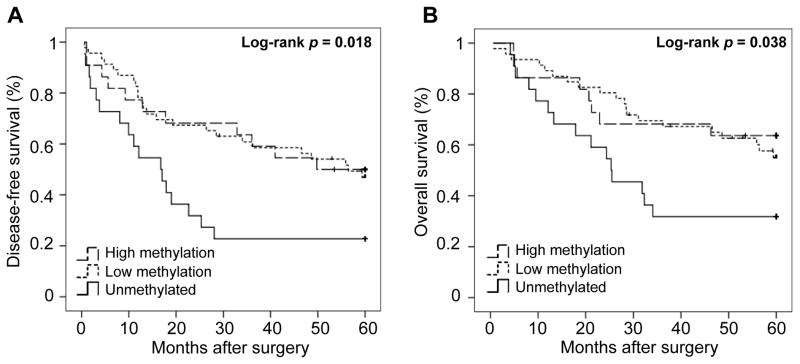
The correlation between the clinicopathological variables and the level of CYP24A1 methylation was assessed and is shown in Table 1. No statistically significant differences were observed according to age, gender, smoking history, tumor differentiation, disease stage and adjuvant treatment. Although smokers had a significantly higher CYP24A1 expression (n = 80; mean, 16.91; standard error (S.E.), 2.61) as compared with non-smokers (n = 10; mean, 5.87; S.E., 3.43; t-test p = 0.018), no significant differences were observed in the CYP24A1 methylation status according to the tobacco history. Interestingly, unmethylated CYP24A1 was associated with higher proportion of death (p = 0.076) and recurrence (p = 0.039). Survival analysis according to the level of CYP24A1 methylation revealed that patients with unmethylated CYP24A1 had significantly worse disease-free survival (DFS, Log-rank p = 0.018, Fig. 2A) and overall survival (OS, Log-rank p = 0.038, Fig. 2B).
Table 1.
Correlation between clinicopathological characteristics and CYP24A1 DNA methylation levels categorized as unmethylated (<25 percentile, <5.7%), low methylation (25–75 percentile, 5.7% – 12.9%) and high methylation (>75 percentile, >12.9%).
| Characteristics | Non methylated (n=22) | Low methylation (n=46) | High methylation (n=22) | All patients (n=90) | P-value |
|---|---|---|---|---|---|
| Age- years | |||||
| Mean (SD) | 66.2 (± 9.9) | 67.4 (± 9.9) | 67.8 (± 9.7) | 67.2 (± 9.7) | 0.851 |
| Gender –n (%) | |||||
| Male | 15 (68%) | 22 (48%) | 8 (36%) | 45 (50%) | |
| Female | 7 (32%) | 24 (52%) | 14 (64%) | 45 (50%) | 0.099 |
| Smoking –n (%) | |||||
| Never smokers | 1 (4.5%) | 8 (17%) | 1 (4.5%) | 10 (11%) | |
| Smokers (<35 PY) | 8 (36.5%) | 18 (39%) | 8 (36.5%) | 34 (38%) | |
| Smokers (≥35 PY) | 13 (59%) | 20 (43%) | 13 (59%) | 46 (51%) | 0.348 |
| Stage 7th –n (%) | |||||
| I | 10 (45%) | 25 (54%) | 10 (46%) | 45 (50%) | |
| II | 6 (27%) | 10 (22%) | 8 (36%) | 24 (27%) | |
| III | 3 (14%) | 8 (17%) | 4 (18%) | 15 (17%) | |
| IV | 3 (14%) | 3 (7%) | 0 (0%) | 6 (7%) | 0.571 |
| Differentiation –n (%) | |||||
| Well | 6 (27%) | 12 (26%) | 5 (23%) | 23 (26%) | |
| Moderate | 6 (27%) | 15 (33%) | 12 (54%) | 33 (37%) | |
| Poor | 10 (46%) | 19 (41%) | 5 (23%) | 34 (38%) | 0.335 |
| Adjuvant treatment –n (%) | |||||
| Yes | 9 (41%) | 14 (31%) | 8 (36%) | 31 (35%) | |
| No | 13 (59%) | 31 (69%) | 14 (64%)) | 58 (65%) | 0.721 |
| Life status –n (%) | |||||
| Dead | 15 (68%) | 20 (43.5%) | 8 (36%) | 43 (48%) | |
| Alive | 7 (32%) | 26 (56.5%) | 14 (64%) | 47 (52%) | 0.076 |
| Recurrence –n (%) | |||||
| Yes | 13 (62%) | 14 (30%) | 11 (50%) | 38 (43%) | |
| No | 8 (38%) | 32 (70%) | 11 (50%) | 51 (57%) | 0.039 |
Figure 2.
Kaplan-Meier plot of disease-free survival (DFS) overall survival (OS) according to level of CYP24A1 methylation. (A) Patients with unmethylated CYP24A1 (n=22) had significantly median shorter DFS (16.8, CI 95% 8.9 – 24.6) as compared to low and high CYP24A1 methylation (n=46 and 22, 56.5 and median not reached respectively, Log-rank P = 0.018). (B) Patients with unmethylated CYP24A1 (n=22) had significantly median shorter OS (25.3, CI 95% 12.9 – 37.7) as compared to low and high CYP24A1 methylation (n=46 and 22 respectively, median not reached, Log-rank P = 0.038).
The statistically significant clinical variables in the univariate Cox regression for DFS and OS were included in the multivariate Cox analysis (disease stage, adjuvant treatment and smoking history). Remarkably, unmethylated CYP24A1 was associated with worse DFS (HR = 2.55, 95% CI 1.17 – 5.58, p = 0.019) and OS (HR = 2.65, 95% CI 1.11 – 6.37, p = 0.029) in the multivariate analysis. These results imply that CYP24A1 unmethylation might be an independent prognostic marker in lung AC and are consistent with our previous findings where CYP24A1 overexpression was associated with poor outcome in lung AC patients.
Effects of Epigenetic Regulation of CYP24A1 Gene Expression in Lung AC Cells
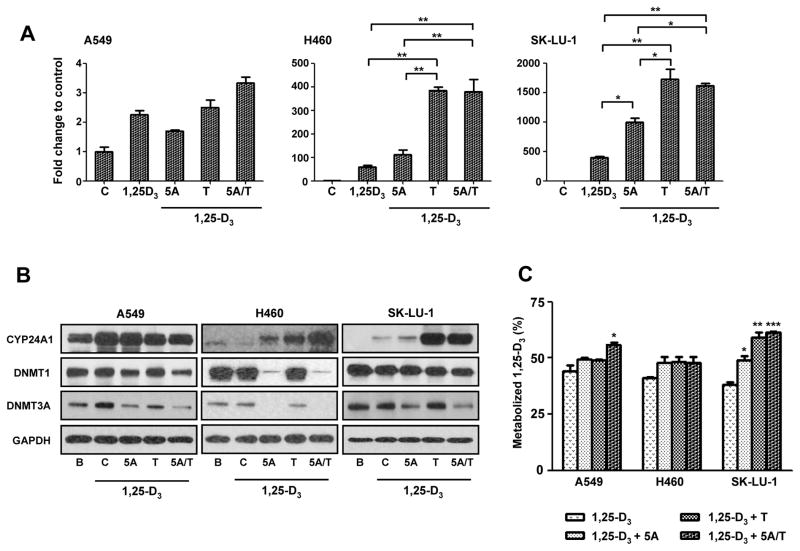
To determine the effect of CYP24A1 promoter methylation in CYP24A1 gene expression, three lung AC cells (A549, NCI-H460 and SK-LU-1) with various levels of CYP24A1 methylation and expression were treated with 5-Aza, a DNA methyltransferase (DNMT) inhibitor or TSA, a histone deacetylease inhibitor. Quantitative RT-PCR revealed an up-regulation of CYP24A1 gene expression in NCI-H460 and SK-LU-1 cells when treated with 5-Aza or TSA in combination with 1,25-D3 (Fig. 3A). In contrast, A549 which has an unmethylated CYP24A1 CpG promoter showed no increase in CYP24A1 expression with 5-Aza and/or TSA treatment (Fig. 3A). Restoration of CYP24A1 mRNA expression by 5-Aza and TSA was dose dependent in NCI-H460 and SK-LU-1 (Supplementary Fig. 2A). Consistent with the activation of CYP24A1 mRNA expression by 5-Aza or TSA, both drugs induced CYP24A1 protein expression in NCI-H460 and SK-LU-1 cells as well (Fig. 3B). However, combination treatment with 5-Aza and TSA did not induce a significant increase in CYP24A1 mRNA (Fig. 3A) or CYP24A1 protein (Fig. 3B) expression in these cells.
Figure 3.
Restoration of CYP24A1 gene expression and protein expression by 5 μM of 5-aza-2′-deoxycytidine (5-Aza) or 300 nM of trichostatin A (TSA) treatment followed by 100 nM of 1α,25-dihydroxyvitamin D3 (1,25-D3) in lung AC cells. (A) Quantitative RT-PCR of CYP24A1 expression in A549, NCI-H460 and SK-LU-1 lung cancer cells treated with 5-Aza (5A), TSA (T) and 5-Aza plus TSA (5A/T) followed by 1,25-D3. (B) Western blot analysis of CYP24A1, DNA methyltransferase 1 (DNMT1) and 3A (DNMT3A) protein expression in A549, NCI-H460 and SK-LU-1 cells treated with 5-Aza (5A), TSA (T) and 5-Aza plus TSA (5A/T) followed by 1,25-D3. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. (C) CYP24A1 enzymatic activity in A549, NCI-H460 and SK-LU-1 lung cancer cells treated with 5-Aza (5A), TSA (T) and 5-Aza plus TSA (5A/T) followed by 1,25-D3. Barplots and error bars represent mean and standard deviation of triplicate data points. P-values: < 0.05, *; < 0.01, **; < 0.001, ***.
To determine whether DNMTs plays a key role in the regulation of CYP24A1 DNA promoter methylation, we analyzed DNMT1 and DNMT3A protein expression in A549, NCI-H460 and SK-LU-1 cells with 5-Aza and/or TSA treatment (Fig. 3B). Western blot analysis revealed that the basal expression levels of DNMT1 and DNMT3A were higher in NCI-H460 and SK-LU-1 cells compared to A549 cells (Supplementary Fig. 2B). DNMT1 was inhibited by 5-Aza in A549 and NCI-H460 cells but not in SK-LU-1 cells, whereas DNMT3A was inhibited by 5-Aza in all three cells (Fig. 3B).
To confirm whether increased CYP24A1 expression by 5-Aza or TSA led to increased CYP24A1 activity, CYP24A1 enzyme activity assay was performed. CYP24A1 enzyme activity was significantly increased in SK-LU-1 cells treated with 5-Aza, TSA or both (Fig. 3C). In A549 cells, no significant increase in enzyme activity was observed when treated with 5-aza alone, but the combination of 5-Aza with TSA led to a significant increase of CYP24A1 enzyme activity (Fig. 3C).
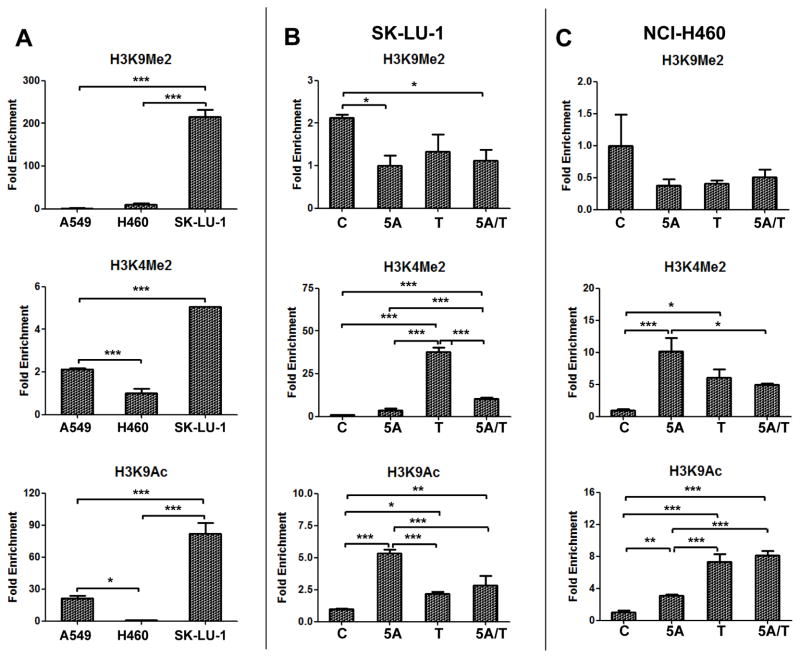
Histone Modifications at the CYP24A1 Promoter
To investigate the role of histone modifications in the regulation of CYP24A1 gene, the active histone marks (H3K4me2 and H3K9ac) and the repressive histone mark (H3K9me2) associated with the CYP24A1 promoter region were assessed using ChIP-qPCR. CYP24A1 mRNA and protein is not expressed in SK-LU-1 cells which have high level of CYP24A1 promoter hypermethylation (56.3%) and showed relatively high level of the repressive histone mark (H3K9me2) as compared to A549 and NCI-460 (Fig. 4A). Surprisingly, the active histone markers (H3K4me2 and H3K9ac) were also relatively high in SK-LU-1 as compared to the other cells. NCI-H460 cells that expresses low CYP24A1 which is hypermethylated (19.3%) showed the lowest levels of active histone marks (H3K4me2 and H3K9ac) relative to the other cells (Fig. 4A). Additionally, we observed that the profile of histone marks at the CYP24A1 promoter was modified by treating SK-LU-1 and NCI-H460 cells with 5-Aza and/or TSA (Fig. 4B and 4C). In SK-LU-1 cells, the ChIP-qPCR assay showed that 5-Aza, TSA or both significantly increased H3K9ac and H3K4me2 levels and decreased H3K9me2 level (Fig. 4B). Similar histone changes were observed in NCI-H460 cells after treatment with 5-Aza and/or TSA as well (Fig. 4C).
Figure 4.
CYP24A1 promoter-associated histone modifications. (A) Quantitative chromatin immunoprecipitation PCR (ChIP-qPCR) of H3K9me2, H3K4me2 and H3K9ac at the CYP24A1 promoter in A549, NCI-H460 and SK-LU-1 lung cancer cells. ChIP-qPCR of H3K9me2, H3K4me2 and H3K9ac at the CYP24A1 promoter in SK-LU-1 (B) and NCI-H460 (C) lung cancer cells treated with 5 μM of 5-aza-2′-deoxycytidine (5A), 300 nM of trichostatin A (T) or combination of both (5A/T) followed by 100 nM of 1α,25-dihydroxyvitamin D3 (1,25-D3). The data from immunoprecipitated DNA was normalized to 10% DNA input. Barplots and error bars represent mean and standard deviation of triplicate data points. P-values: < 0.05, *; < 0.01, **; < 0.001, ***.
Binding of VDR to the CYP24A1 Promoter by Epigenetic Modulatory Drugs
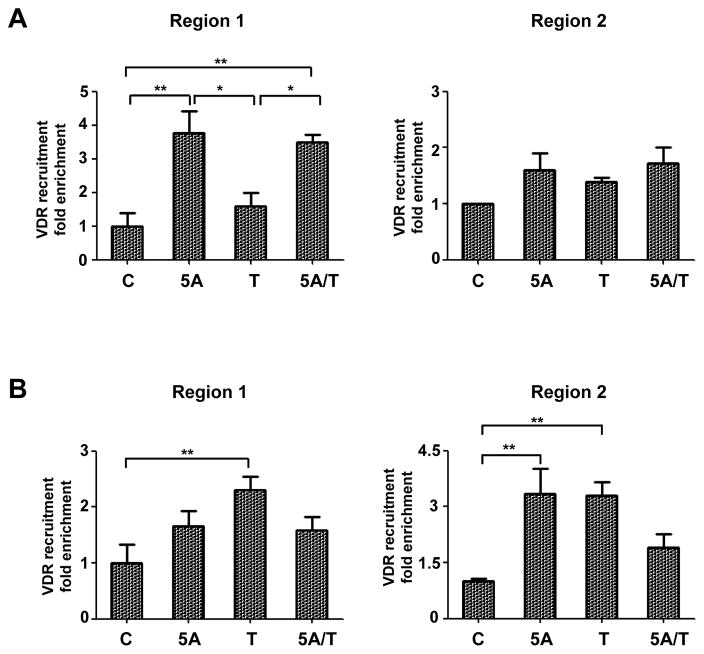
To confirm whether these epigenetic changes induced by 5-Aza and/or TSA may affect the binding of VDR to the CYP24A1 promoter region, ChIP-qPCR analysis was performed using a VDR-specific antibody. A significant increase in VDR binding to the CYP24A1 promoter was detected in NCI-H460 (Fig. 5A) and SK-LU-1 cells (Fig. 5B) upon treatment with 5-Aza and TSA, in addition to 1,25-D3. These data support that decreased recruitment of VDR to the CYP24A1 promoter in NCI-H460 and SK-LU-1 cells, due to hypermethylation and repressive histone modifications, result in the suppression of CYP24A1 expression in lung cancer cells.
Figure 5.
Quantitative chromatin immunoprecipitation PCR (ChIP-qPCR) analysis of vitamin D receptor (VDR) recruitment to the CYP24A1 promoter in NCI-H460 (A) and SK-LU-1 (B) cells treated with 5 μM of 5-aza-2′-deoxycytidine (5A), 300 nM of trichostatin A (T) or combination of both (5A/T) followed by 100 nM of 1α,25-dihydroxyvitamin D3 (1,25-D3). The data from immunoprecipitated DNA was normalized to 10% DNA input. Barplots and error bars represent mean and standard deviation of triplicate data points. P-values: < 0.05, *; < 0.01, **; < 0.001, ***.
Discussion
There has been increasing interest in incorporating 1,25-D3 in cancer therapy and for prevention. However before designing large clinical trials, it is imperative to understand the pharmacokinetics as well as pharmacodynamic properties of 1,25-D3. It appears that metabolism of 1,25-D3 might be strongly influenced by tissue context. In particular, both CYP27B1 (enzyme that converts 25(OH)-D3 to 1,25-D3) as well as CYP24A1 (rate limiting enzyme that converts 1,25-D3 to inactive metabolite) lend themselves to epigenetic regulation. Several studies have suggested a differential epigenetic regulation in normal versus neoplastic cells.30–32 Our study focuses on the epigenetic regulation of CYP24A1 in lung AC, where there is a differential expression of CYP24A1 mRNA.16 In the present study, we demonstrate that DNA methylation of CYP24A1 gene is a plausible molecular mechanism for down-regulation of CYP24A1 gene expression in human lung cancer based on the following observations from our study results. An inverse correlation was found between CYP24A1 expression and promoter hypermethylation in lung AC cell lines and primary tumors. In addition, CYP24A1 expression was restored when lung cell lines endogenously expressing low CYP24A1 were treated with the demethylating agent (5-Aza). Accordingly, when the unmethylated and high CYP24A1 expressing cells (A549) were treated with 5-Aza, CYP24A1 expression remained unchanged. Evidence in the current study and previous reports22, 31–33 supports the role of promoter DNA hypermethylation in inducing CYP24A1 silencing.
We found that lung AC patients with unmethylated CYP24A1 promoter had significantly worse DFS and OS, independently of other clinical covariates such as disease stage, smoking status and adjuvant therapy. These results are consistent with our previous study that reported that CYP24A1 overexpression was associated with poor outcome for patients with resected lung AC and further validation using an independent cohort is warranted.
Histone modifications could provide an alternative regulatory mechanism that affects CYP24A1 gene expression in vitro and in vivo.34–36 Previous studies have shown that hypermethylated promoters often involve recruitment of methyl-binding domain proteins and histone-modifying enzymes such as histone deacetylases.37–40 Indeed, lower expression of CYP24A1 observed in non-smokers is not fully explained by DNA methylation, since the differences in the CYP24A1 methylation level were not statistically significant between smokers and non-smokers. ChIP-qPCR showed that H3K9me2, a repressive histone modification, was significantly higher in SK-LU-1 cells. When SK-LU-1 cells were treated with 5-Aza and/or TSA, the recruitment of VDR on the CYP24A1 promoter was increased, resulting in a higher CYP24A1 mRNA expression as well. Previous studies support our findings that hypermethylation of these regions could lead to a chromatin state in which the VDR is prevented from binding to the VDREs in the CYP24A1 promoter, resulting in transcriptional silencing of CYP24A1.23 In contrast, low H3K9me2 (repressive histone modification) in A549 cells, which have the highest CYP24A1 expression, suggests an association of an open chromatin structure with active gene expression.41, 42
These data are consistent with the hypothesis that aberrant DNA methylation and repressive histone modifications function in combination to silence important genes in human cancers.23, 43–45 In colon cancer, however, the CYP24A1 gene is also reactivated by methyltransferase inhibitor in a cell line-dependent manner, and this effect does not correlate with the methylation state of the promoter, suggesting that other genes located upstream of CYP24A1 may affect the methylation status of CYP24A1.46
Collectively, our findings support that CYP24A1 promoter DNA hypermethylation and histone modifications are key mechanisms regulating CYP24A1 expression in human lung AC. Transcriptional silencing of the CYP24A1 gene, resulting from hypermethylation and or repressive histones, would be advantageous to 1,25-D3 antiproliferative effects in lung cancer; whereas CYP24A1 promoter hypomethylation associated with higher mRNA expression would lead to increased metabolism of 1,25-D3. Patients with CYP24A1 promoter hypomethylation may potentially be benefitted by a micronutrient diet rich in methyl donors, earlier in the pathogenesis of lung cancer.47 Indeed, genome-wide DNA hypomethylation coupled with genome instability are the earliest events to occur in the genesis of cancer.48, 49 Taken together, the observation from this study gives us an insight regarding the mechanisms regulating CYP24A1 gene expression in lung AC tissues and this could facilitate the development of strategies to optimize vitamin D based therapies for lung cancer.
Supplementary Material
Acknowledgments
This work was supported by NIH R21CA128193-01-A1 and VA Merit I01CX000333-02 (N Ramnath), PR185/13 Mutua Madrileña grant, HEALTH-F2-2010-258677-CURELUNG Project from the European Community’s 7th Framework Programme (FP7/2007-2013) and 2012R1A1A3010123 [Basic Science Research Program of National Research Foundation of Korea (NRF) funded by Ministry of Education, Science and Technology, Korea, SH Kim]. E.N. was supported by a Spanish Society of Medical Oncology Fellowship and J.S. by an ISCIII Miguel Servet contract. We thank Dr. Guoan Chen for helpful discussion and expert technical assistance for DNA methylation and confirmation of cell line genotype. We also thank Diana Garcia and Carles Arribas for their technical support.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest.
References
- 1.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nature reviews Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 2.Hershberger PA, Modzelewski RA, Shurin ZR, et al. 1,25-Dihydroxycholecalciferol (1,25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21(Waf1/Cip1) in vitro and in vivo. Cancer research. 1999;59:2644–2649. [PubMed] [Google Scholar]
- 3.Koshizuka K, Koike M, Asou H, et al. Combined effect of vitamin D3 analogs and paclitaxel on the growth of MCF-7 breast cancer cells in vivo. Breast cancer research and treatment. 1999;53:113–120. doi: 10.1023/a:1006123819675. [DOI] [PubMed] [Google Scholar]
- 4.McElwain MC, Modzelewski RA, Yu WD, et al. Vitamin D: an antiproliferative agent with potential for therapy of squamous cell carcinoma. American journal of otolaryngology. 1997;18:293–298. doi: 10.1016/s0196-0709(97)90022-3. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, Lee MH, Cohen M, et al. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes & development. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 6.Yee SW, Campbell MJ, Simons C. Inhibition of Vitamin D3 metabolism enhances VDR signalling in androgen-independent prostate cancer cells. The Journal of steroid biochemistry and molecular biology. 2006;98:228–235. doi: 10.1016/j.jsbmb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Bernardi RJ, Trump DL, Yu WD, et al. Combination of 1alpha,25-dihydroxyvitamin D(3) with dexamethasone enhances cell cycle arrest and apoptosis: role of nuclear receptor cross-talk and Erk/Akt signaling. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:4164–4173. [PubMed] [Google Scholar]
- 8.McGuire TF, Trump DL, Johnson CS. Vitamin D(3)-induced apoptosis of murine squamous cell carcinoma cells. Selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. The Journal of biological chemistry. 2001;276:26365–26373. doi: 10.1074/jbc.M010101200. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa K, Sasaki Y, Kato S, et al. 22-Oxa-1alpha,25-dihydroxyvitamin D3 inhibits metastasis and angiogenesis in lung cancer. Carcinogenesis. 2005;26:1044–1054. doi: 10.1093/carcin/bgi049. [DOI] [PubMed] [Google Scholar]
- 10.Higashimoto Y, Ohata M, Nishio K, et al. 1 alpha, 25-dihydroxyvitamin D3 and all-trans-retinoic acid inhibit the growth of a lung cancer cell line. Anticancer research. 1996;16:2653–2659. [PubMed] [Google Scholar]
- 11.Light BW, Yu WD, McElwain MC, et al. Potentiation of cisplatin antitumor activity using a vitamin D analogue in a murine squamous cell carcinoma model system. Cancer research. 1997;57:3759–3764. [PubMed] [Google Scholar]
- 12.Hershberger PA, McGuire TF, Yu WD, et al. Cisplatin potentiates 1,25-dihydroxyvitamin D3-induced apoptosis in association with increased mitogen-activated protein kinase kinase kinase 1 (MEKK-1) expression. Molecular cancer therapeutics. 2002;1:821–829. [PubMed] [Google Scholar]
- 13.Young MR, Ihm J, Lozano Y, et al. Treating tumor-bearing mice with vitamin D3 diminishes tumor-induced myelopoiesis and associated immunosuppression, and reduces tumor metastasis and recurrence. Cancer immunology, immunotherapy : CII. 1995;41:37–45. doi: 10.1007/BF01788958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa K, Kawaura A, Kato S, et al. 1 alpha,25-Dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis. 2005;26:429–440. doi: 10.1093/carcin/bgh332. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Chen G, King AN, et al. Characterization of vitamin D receptor (VDR) in lung adenocarcinoma. Lung cancer. 2012;77:265–271. doi: 10.1016/j.lungcan.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Kim SH, King AN, et al. CYP24A1 is an independent prognostic marker of survival in patients with lung adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:817–826. doi: 10.1158/1078-0432.CCR-10-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albertson DG, Ylstra B, Segraves R, et al. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nature genetics. 2000;25:144–146. doi: 10.1038/75985. [DOI] [PubMed] [Google Scholar]
- 18.Mimori K, Tanaka Y, Yoshinaga K, et al. Clinical significance of the overexpression of the candidate oncogene CYP24 in esophageal cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2004;15:236–241. doi: 10.1093/annonc/mdh056. [DOI] [PubMed] [Google Scholar]
- 19.Parise RA, Egorin MJ, Kanterewicz B, et al. CYP24, the enzyme that catabolizes the antiproliferative agent vitamin D, is increased in lung cancer. International journal of cancer Journal international du cancer. 2006;119:1819–1828. doi: 10.1002/ijc.22058. [DOI] [PubMed] [Google Scholar]
- 20.Kim B, Lee HJ, Choi HY, et al. Clinical validity of the lung cancer biomarkers identified by bioinformatics analysis of public expression data. Cancer Res. 2007;67:7431–7438. doi: 10.1158/0008-5472.CAN-07-0003. [DOI] [PubMed] [Google Scholar]
- 21.Hansdottir S, Monick MM, Hinde SL, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. Journal of immunology. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novakovic B, Sibson M, Ng HK, et al. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. The Journal of biological chemistry. 2009;284:14838–14848. doi: 10.1074/jbc.M809542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo W, Karpf AR, Deeb KK, et al. Epigenetic regulation of vitamin D 24-hydroxylase/CYP24A1 in human prostate cancer. Cancer research. 2010;70:5953–5962. doi: 10.1158/0008-5472.CAN-10-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nature medicine. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 25.Vaisanen S, Dunlop TW, Sinkkonen L, et al. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-Dihydroxyvitamin D3. Journal of molecular biology. 2005;350:65–77. doi: 10.1016/j.jmb.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 26.Fingerman IM, McDaniel L, Zhang X, et al. NCBI Epigenomics: a new public resource for exploring epigenomic data sets. Nucleic acids research. 2011;39:D908–912. doi: 10.1093/nar/gkq1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milanesi L, D’Angelo D, Rogozin IB. GeneBuilder: interactive in silico prediction of gene structure. Bioinformatics. 1999;15:612–621. doi: 10.1093/bioinformatics/15.7.612. [DOI] [PubMed] [Google Scholar]
- 28.Chen KS, DeLuca HF. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochimica et biophysica acta. 1995;1263:1–9. doi: 10.1016/0167-4781(95)00060-t. [DOI] [PubMed] [Google Scholar]
- 29.Sandoval J, Mendez-Gonzalez J, Nadal E, et al. A prognostic DNA methylation signature for stage I non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4140–4147. doi: 10.1200/JCO.2012.48.5516. [DOI] [PubMed] [Google Scholar]
- 30.Wjst M, Heimbeck I, Kutschke D, et al. Epigenetic regulation of vitamin D converting enzymes. The Journal of steroid biochemistry and molecular biology. 2010;121:80–83. doi: 10.1016/j.jsbmb.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 31.Chung I, Karpf AR, Muindi JR, et al. Epigenetic silencing of CYP24 in tumor-derived endothelial cells contributes to selective growth inhibition by calcitriol. The Journal of biological chemistry. 2007;282:8704–8714. doi: 10.1074/jbc.M608894200. [DOI] [PubMed] [Google Scholar]
- 32.Khorchide M, Lechner D, Cross HS. Epigenetic regulation of vitamin D hydroxylase expression and activity in normal and malignant human prostate cells. The Journal of steroid biochemistry and molecular biology. 2005;93:167–172. doi: 10.1016/j.jsbmb.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Ohyama Y, Kusada T, Yamasaki T, et al. Extensive methylation of CpG island of CYP24 gene in osteoblastic ROS17/2.8 cells. Nucleic acids research Supplement. 2002:249–250. doi: 10.1093/nass/2.1.249. [DOI] [PubMed] [Google Scholar]
- 34.Bachman KE, Park BH, Rhee I, et al. Histone modifications and silencing prior to DNA methylation of a tumor suppressor gene. Cancer cell. 2003;3:89–95. doi: 10.1016/s1535-6108(02)00234-9. [DOI] [PubMed] [Google Scholar]
- 35.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nature reviews Genetics. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 36.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 37.Bakker J, Lin X, Nelson WG. Methyl-CpG binding domain protein 2 represses transcription from hypermethylated pi-class glutathione S-transferase gene promoters in hepatocellular carcinoma cells. The Journal of biological chemistry. 2002;277:22573–22580. doi: 10.1074/jbc.M203009200. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Serra L, Ballestar E, Fraga MF, et al. A profile of methyl-CpG binding domain protein occupancy of hypermethylated promoter CpG islands of tumor suppressor genes in human cancer. Cancer research. 2006;66:8342–8346. doi: 10.1158/0008-5472.CAN-06-1932. [DOI] [PubMed] [Google Scholar]
- 39.Nan X, Ng HH, Johnson CA, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 40.Jones PL, Veenstra GJ, Wade PA, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature genetics. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes & development. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 42.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nature reviews Molecular cell biology. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 43.Kondo Y, Shen L, Issa JP. Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer. Molecular and cellular biology. 2003;23:206–215. doi: 10.1128/MCB.23.1.206-215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawamoto K, Hirata H, Kikuno N, et al. DNA methylation and histone modifications cause silencing of Wnt antagonist gene in human renal cell carcinoma cell lines. International journal of cancer Journal international du cancer. 2008;123:535–542. doi: 10.1002/ijc.23514. [DOI] [PubMed] [Google Scholar]
- 45.Podvinec M, Kaufmann MR, Handschin C, et al. NUBIScan, an in silico approach for prediction of nuclear receptor response elements. Mol Endocrinol. 2002;16:1269–1279. doi: 10.1210/mend.16.6.0851. [DOI] [PubMed] [Google Scholar]
- 46.Hobaus J, Fetahu I, Khorchide M, et al. Epigenetic regulation of the 1,25-dihydroxyvitamin D3 24-hydroxylase (CYP24A1) in colon cancer cells. The Journal of steroid biochemistry and molecular biology. 2013;136:296–299. doi: 10.1016/j.jsbmb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips JM, Goodman JI. Inhalation of cigarette smoke induces regions of altered DNA methylation (RAMs) in SENCAR mouse lung. Toxicology. 2009;260:7–15. doi: 10.1016/j.tox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Esteller M, Herman JG. Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. The Journal of pathology. 2002;196:1–7. doi: 10.1002/path.1024. [DOI] [PubMed] [Google Scholar]
- 49.Feinberg AP, Tycko B. The history of cancer epigenetics. Nature reviews Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.