Malignant gliomas are the most common primary intracranial tumor, with a proclivity for widespread invasion and rampant destruction of healthy parenchyma. This infiltrative process affords high-grade gliomas protection from traditional therapies and subjects the adjacent normal tissue to potential damage from nonspecific treatment modalities.1,2 Immunotherapies involving antibodies or sensitized effector cells can offer selective targeting of protein-arbohydrate complexes on tumor cell surfaces that distinguish neoplastic from noncancerous cells.1,3 Consequently, the treatment of malignant gliomas may be enhanced not only by increased specificity for tumor tissue but also from decreased toxicity to the host’s healthy cells.1 This review focuses on published findings from the use of passive immunotherapy for the treatment of high-grade gliomas, particularly glioblastoma multiforme (GBM).
PASSIVE IMMUNOTHERAPY
Passive immunotherapy can be broadly categorized into 2 treatment approaches: one that relies on the administration of antibodies that may further be coupled to a toxic counterpart molecule or one involving the adoptive transfer of an activated immune cell effector component to act against a neoplasm in the host. For cellular therapy, the most common types have included the adoptive transfer of nonspecifically activated lymphocyte-activated killer (LAK) cells or specifically sensitized cytotoxic T lymphocytes (CTLs).4,5 In adoptive immunotherapy (AIT), patients’ native immune cells are extracted and then activated ex vivo to increase antitumor activity. These cells are then reinfused back into the patients either intravenously or directly placed into the tumor resection cavity. Another technique of passive immunotherapy involves monoclonal antibodies (mAbs). Antibody-mediated immunotherapy uses mAbs to induce lymphocyte recruitment and complement system activation, thereby resulting in tumor cytotoxicity. In addition, radiolabeled antibodies may deliver localized radiation to the target-specific neoplastic tissue, with subsequent induction of cell death.
AIT: LAK CELLS
LAK cells are nonspecific effector cells that are derived from peripheral blood mononuclear cells (PBMC) and activated ex vivo with high-concentration interleukin 2 (IL-2) (T-cell growth factor) to induce antitumor properties.6–10 IL-2 is an endogenously produced cytokine that aids in the host’s natural immune system and is available in recombinant form to facilitate LAK cell generation.8–17 The LAK cell’s cytolytic properties against numerous tumor types have been demonstrated in various models, with the enhanced capability of destroying natural killer (NK) cell–resistant malignant gliomas and sparing of normal parenchyma.8,18–26 Furthermore, it has been suggested that the use of IL-2/LAK cell immunotherapy may possess preventative properties against metastasis and recurrence of disease because intraventricular administration can induce a systemic response.8 Yet, given the high toxicity of intravenous IL-2, local administration of this cytokine has been adopted for an increased therapeutic response and decreased morbidity.8,27–29 In addition, LAK cells are unable to migrate to tumor sites, necessitating local therapeutic administration at the surgical resection cavity.30 However, LAK AIT has remained limited, in part, by the need for leukapheresis to obtain significantly therapeutic numbers of LAK cells, a costly process that may inhibit its use for many patients with GBM.
Nevertheless, 12 trials8,25,26,29,31–38 including 211 patients (170 GBM) have been reported using LAK cell AIT for the treatment of recurrent high-grade gliomas. Although historically disappointing, more recent findings have demonstrated improvement in median survival for patients with GBM compared with control groups.25
In most studies, patients were included at the time of relapse and received 1 to 15 injections, containing 106 to 1010 injected LAK cells. Adverse effects included neurologic toxicity, cerebral edema, aseptic meningitis, and hypereosinophlia.7,39 However, the local presence of eosinophils has been positively correlated with long-term survival and may be an indicator of treatment response.8
Efficacy was typically reported based on radiological criteria, demonstrating 5 complete responses (CR), 13 partial responses (PR), and 6 stable diseases (SD) in a total of 118 patients.36 Of the data exclusive to 88 GBM patients, the investigators reported 3 CR (3.4%), 8 PR (11.0%), and 6 SD (6.8%). However, these figures do not include the beneficial results observed in the two most recent studies that included 73 patients with GBM.25,31 In the most promising of studies, Dillman and colleagues25 reported results of their phase II clinical trial demonstrating a 20.5-month median survival and 75% 1-year survival rate in 40 patients with GBM treated with intralesional autologous LAK cells; this has been the only report thus far investigating patients with newly diagnosed GBM treated with LAK cells. In addition, patients who received higher doses of CD3+/CD16+/CD56+ (T-NK) cells were found to have an increased survival advantage compared with those with lower T-NK cell counts that presumably resulted from steroid use during the month before leukapheresis. Given these findings, the investigators conducted a 2-arm, randomized phase II trial using either intralesional LAK cells or carmustine (Gliadel) wafers, following standard treatment with surgical resection and radio- and chemotherapy with temozolomide. Results of this study are currently pending publication.
Additionally, 3 other trials have also demonstrated improved median survival for patients with GBM compared with control groups. In a study preceding this last one, Dillman and colleagues31 reported findings of 31 patients with recurrent GBM tumors, surviving a median time of 17.5 months from the date of the original diagnosis, compared with 13.6 months for a control group. Hayes and colleagues33 reported results of 19 total patients with recurrent malignant gliomas, demonstrating a median survival for 15 cases of GBM of 53 weeks after reoperation versus 25.5 weeks for patients treated with conventional therapy alone. In a subsequent report, Hayes and colleagues8 presented results of 15 patients with recurrent GBM (28 total cases of recurrent malignant gliomas) improving median survival with similar findings as reported in their previous study.
However, findings from various other clinical trials using LAK cell immunotherapy have not indicated successful in vivo antitumor efficacy. In a study with 10 patients with recurrent malignant primary brain tumors (4 GBM), Sankhla and colleagues32 reported no improvement in overall survival compared with patients receiving standard treatments, although partial and transient clinical responses were seen in 2 patients with grade II to III astrocytomas. Similarly, Jeffes and colleagues36 failed to identify any significant relationship between clinical improvement and radiological response in 19 patients with recurrent gliomas, 14 of which had GBM. Merchant and colleagues. reported findings of 13 patients with recurrent GBM resulting in a median survival of less than 6 months and a 16% 60-day postoperative mortality.25,38 Barba and colleagues37 discussed findings for 9 patients in which 5 experienced significant toxicity and more than half were deceased within 4 months, with a 33% 60-day postoperative mortality. Similarly, Lillehei and colleagues29 evaluated 11 patients with recurrent high-grade gliomas (9 GBM) and reported a median survival of less than 5 months following LAK cell therapy. Morbidity related to vascular leak syndrome caused by high-dose IL-2 was of considerable concern.
Given the findings that responders were noted more so in patients with lower-grade glioma and that there is now a precedent for treating patients with GBM earlier, additional prospective randomized trials will be necessary to fully elucidate the therapeutic potential of nonspecifically activated LAK cells in the management of patients with GBM.
AIT: CTL
Unlike LAK cells, AIT using CTL is advantageous because of its ability to migrate to target-specific antigens following administration. Furthermore, a T-cell subset has the capability to persist as memory cells, allowing for an extended period of antitumor response.40 CTLs are most commonly generated by antigenic stimulation of PBMCs with autologous inactivated tumor cells (ATC).39,41,42 This strong ex vivo priming of T cells overcomes the weak in vivo T-cell immune response to endogenous tumor-antigen stimulation.43 Furthermore, CTLs can be expanded ex vivo to increase the numbers of effector T cells for adoptive transfer, compared with active immunotherapy relying on in situ or endogenous immune cell expansion.44
Various other methods of CTL generation have also been investigated, including the use of autologous HLA-displaying lymphocytes for allogeneic CTL stimulation.43 In addition, CTL extraction from tumor-infiltrating lymphocytes (TIL) following IL-2 amplification, as well as lymphocyte collection from lymph nodes/PBMCs following stimulation with granulocyte-macrophage colony-stimulating factor and irradiated ATCs, have all been examined as sufficient means of collecting adequate quantities of T cells.45–50 However, the lymphocytes obtained from these tumor-draining lymph nodes are pre-effector cells. As such, in vitro activation of the antitumor functions of these cells, in addition to the expansion of cells sensitized in situ, is required before their reinjection.51
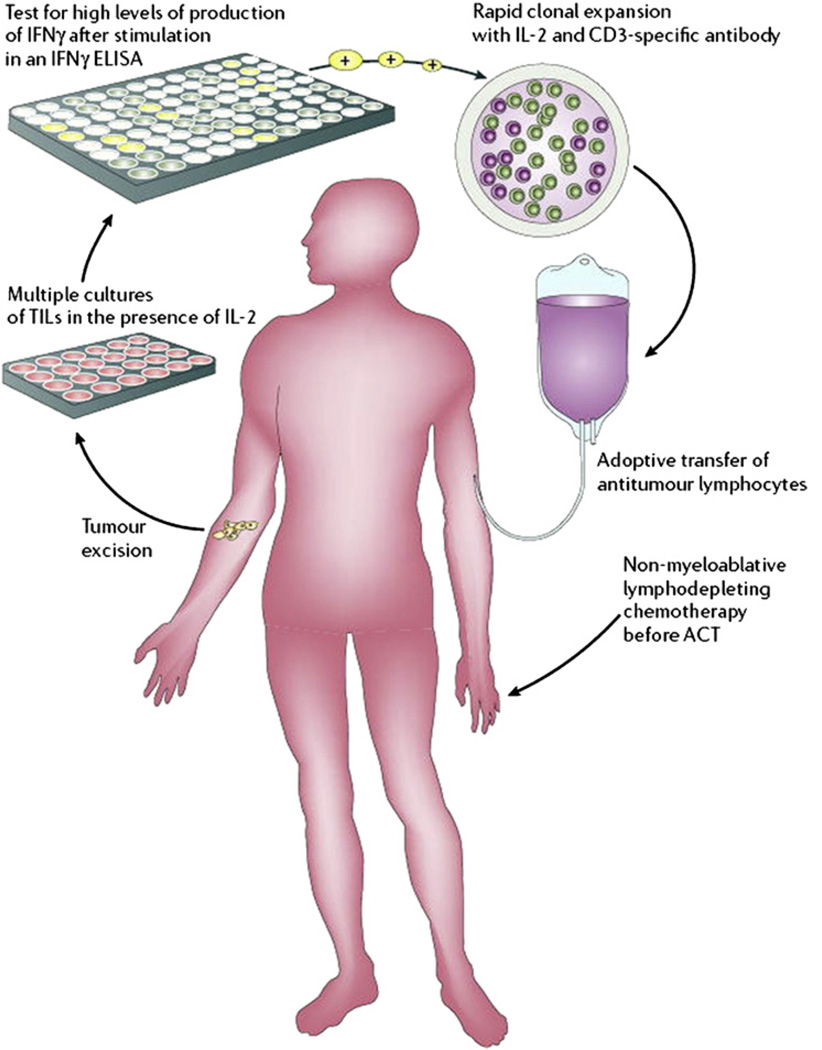
To date, 4 phase I trials examining CTLs generated from PBMCs39,41–43 and 1 pilot study using TILs45 (Fig. 1) have been reported in the literature, investigating intracranial administration in a total of 30 patients with high-grade gliomas (19 GBM). A combined approach using strategies from both active and passive immunotherapy was examined in 3 phase I46–48 and 2 pilot studies49,50 in which CTLs of 62 patients (49 GBM) were extracted from lymph nodes or PBMCs after intradermal vaccination and reinjected either intravenously or by intracarotid infusion. In contrast to passive immunotherapy, active immunotherapeutic strategies attempt to sensitize the immune system using tumor-associated antigen vaccinations to activate endogenous tumor-specific T cells. Of these 10 total CTL immunotherapy clinical trials, patients received between 1 and 13 injections of CTL cells, ranging from 3 × 107 to 10 × 1010 cells,7 and there were no grade III/IV adverse events.
Fig. 1.
A protocol for AIT using tumor-infiltrating lymphocytes in a patient with melanoma. A similar protocol may be used for patients with GBM, with adoptive transfer of CTLs directly into the tumor resection cavity. ACT, adoptive cell transfer; ELISA, enzyme-linked immunosorbent assay; IFN, interferon. (From Gattinoni L, Powell DJ Jr, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 2006;6(5):383–93; This figure was reproduced with the kind permission of the Nature Publishing Group.)
Clinical trials using CTL AIT for the treatment of high-grade gliomas in a total of 92 patients (68 GBM) have resulted in 3 CR, 27 PR, and 16 SD.49,52 However, of the data exclusively with 52 patients with GBM, the investigators reported 11 PR (21.2%) and 4 SD (7.7%). Although Sloan and colleagues50 did not distinguish between tumor grades when reporting immunotherapeutic responses for their 19 patients (16 GBM), a favorable total of 1 CR (5.3%), 7 PR (36.8%), and 9 SD (47.4%) were documented for all of their patients.
Although a few studies demonstrated a survival benefit, many of these small phase I or I/II trials were not clinically designed or supported to effectively analyze survival outcomes against a control group.7 Sloan and colleagues50 reported an improved median survival of 12 months after tumor recurrence compared with 6 months for their historical controls. In addition, they demonstrated a correlation between increased survival with radiological response and a positive delayed-type hypersensitivity reaction. Likewise, Wood and colleagues49 described a positive association between the concentration of CD8+ T cells in vaccine injections and clinical response. In their study using autologous TIL, Quattrocchi and colleagues45 suggested that the immunotherapeutic benefits of AIT may be patient-dependent because their case of complete response revealed a unique population of CD8+CD56+ cells. Kitahara and colleagues41 found 1 of 4 patients with GBM to display a PR. Kruse and colleagues43 found that 3 of 3 patients with World Health Organization grade III recurrent glioma demonstrated a long-term response, but no response was displayed by the 3 patients with GBM treated with intratumoral alloreactive CTL. The 12 patients with GBM treated by Holladay and colleagues46 showed no responders; however, there was a significant relationship between adoptive T-cell immunotherapy and delayed recurrence of gliomas. Plautz and colleagues47 reported on the limited efficacy of CTL immunotherapy; only 2 of 9 patients with GBM demonstrated partial tumor regression. In a subsequent study, they identified only 1 of 6 patients with GBM to display a PR.40 Similarly, Wood and colleagues49 reported findings in 6 patients with recurrent GBM in which only 1 displayed partial transient decreased tumor growth.
Genetic Modulation of Adoptive T Cells
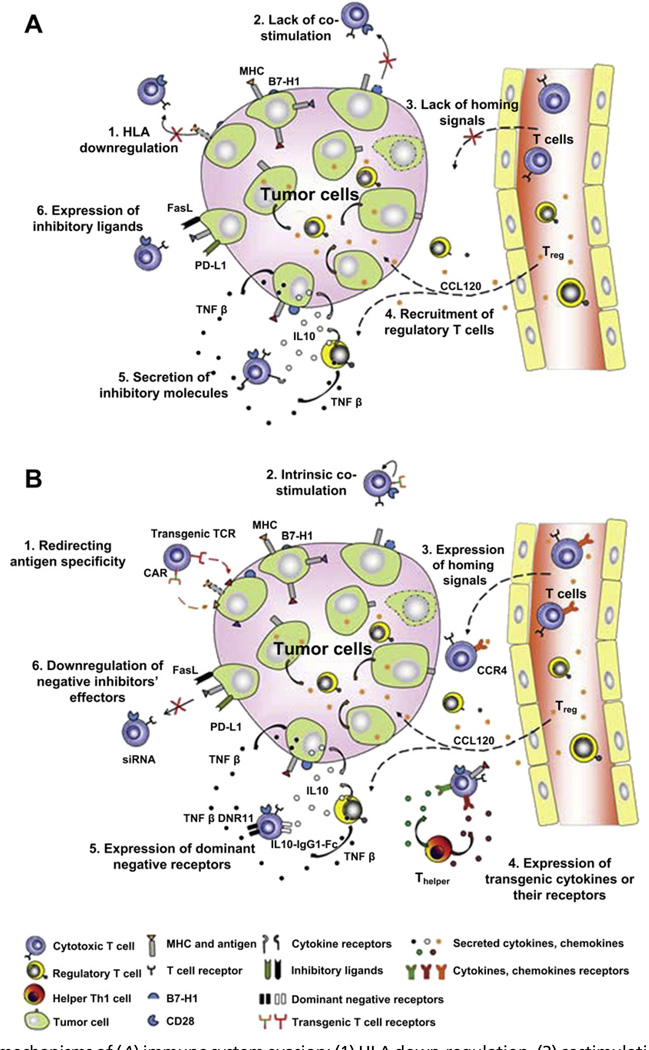
Ngo and colleagues suggested that the inability of AIT to produce more consistently promising results may be caused by functionally variable strengths of transferred cells and the proclivity of solid tumors to evade the human immune system by various techniques (Fig. 2).53 Passive down-regulation of major histocompatibility complex (MHC) or costimulatory molecules conceal tumors from T-cell targeting, whereas active expression of inhibitory ligands and secretions allow for tumor escape from immune surveillance.54 Furthermore, it may also be necessary to target brain tumor stem cells that display unique antigens.
Fig. 2.
Various mechanisms of (A) immune system evasion: (1) HLA down-regulation, (2) costimulation suppression, (3) homing signal suppression, (4) activation of Treg and Th2 subsets, (5) production of immunosuppressive cytokines, and (6) upregulation of inhibitory ligands; and (B) genetic modulations to counter the glioma microenvironment: (1) transgenic TCRs or CARs, (2) intrinsic costimulatory signals, (3) upregulation of homing signals, (4) production of transgenic cytokines, (5) dominant-negative receptors, and (6) depression of negative inhibition. CARs, chimeric antigen receptors; TCR, T-cell receptors; Th2, T helper type 2; Treg, T regulatory. (From Ngo MC, Rooney CM, Howard JM, et al. Ex vivo gene transfer for improved adoptive immunotherapy of cancer. Hum Mol Genet 2011;20(R1):R93–9. This figure was reproduced with the kind permission of Oxford University Press.)
Genetically modified CTLs may possess improved antitumor efficacy by their ability to counter the glioma’s immunosuppressive microenvironment.53 Specifically, augmentation with transgenic T-cell receptors (TCRs) or chimeric antigen receptors (CARs) may facilitate increased quantities of tumor-specific T cells with a decreased reliance on tumor cell MHC expression. Morgan and colleagues55 genetically engineered autologous T cells with a retroviral vector to display TCRs targeting the melanoma antigen recognized by T-cells (MART-1) melanoma antigen. Lymphocyte presence was detected up to a year following infusion, and tumor regression was documented in 4 of the 31 patients treated. However, because TCRs are limited in their function to MHC-matched tumors that have not yet evolved downregulation of their human leukocyte antigens (HLA), CARs may offer an alternative solution. These non–HLA-restricted synthetic receptors allow for targeted specificity without the disadvantage of dimerization with endogenous TCRs that may lead to loss of function in transgenic TCRs. CARs confer the added benefits of antigen recognition within a spectrum of posttranslational modifications,56 with an increased binding affinity and a more stable immunologic synapse than those created by TCRs.57 A phase I/II clinical trial investigating the effects of cytomegalovirus-specific CTLs expressing CARs targeting human epidermal growth factor receptor 2 (HER-2) in patients with GBM is currently underway, which may have the potential of destroying HER-2–positive CD133+ glioma cells.58 However, concerns may arise from the potential binding to low-avidity off-target antigens59 and adverse effects from supraphysiologic signaling activation induced by on-target cytokine expression.53 Consequently, various safety mechanisms have been considered. For instance, suicide genes, such as the herpes simplex viral thymidine kinase gene or the inducible caspase 9 transgene (iCaspase9), are being incorporated to provide for their elimination should serious adverse reactions occur.60,61
Other genetic modifications to TCRs have also been postulated. Receptors specific for tumor-secreted chemokines may enhance T-cell homing to optimal tumor-specific sites.53 Furthermore, T cells may be modified for transgenic expression of activating cytokines, such as IL-2 and IL-15. This action frees lymphocyte reliance on endogenous costimulatory factors for the activation and maintenance of functionality. In preclinical models, this technique applied in vivo has demonstrated increased antigen-specific T-cell expansion and enhanced antitumor activity.62
Dominant-negative receptors63,64 and other genetic modifications53 may also be used to enable T cells to overcome immunosuppressive factors present in the tumor microenvironment or immunosuppressive drug therapies. Transforming growth factor β (TGFβ) is one of the most potent inhibitory cytokines and TGFβ2 is notably upregulated in patients with GBM.65,66 In vitro studies and murine models of TGFβ-secreting Epstein-Barr virus–positive lymphoma have demonstrated T-cell resilience following the transgenic expression of dominant-negative TGFβ type II receptors.67 A similar approach to circumvent the immunosuppressive effects of IL-10 is also being investigated.68
Despite the inconclusive results reported in the literature, adoptive transfer of CTL immunotherapy may be a promising treatment of GBM, necessitating further prospective trials to elucidate its potential effects. Studies that combine active immunotherapy with passive immunotherapy are also showing promise.69 Furthermore, genetic modifications of CTLs may be a worthwhile approach to optimize the benefits of this technique for enhanced patient outcomes.
ANTIBODY-MEDIATED IMMUNOTHERAPY
Another passive immunotherapy strategy involves the use of monoclonal antibodies, which possess the capability of targeted tumor-antigen specificity with high binding affinity.70 mAbs may be used either alone or coupled to radiation-emitting particles or toxins.1 Yet, in order for mAbs immunotherapy to be effective, it has been suggested that GBM cells should display an epitope with a minimum of 105 surface markers per cell and maintain a low turnover time. Furthermore, the antigen should be glioma-associated to prevent damage to healthy brain parenchyma.71
Unlabeled Monoclonal Antibodies
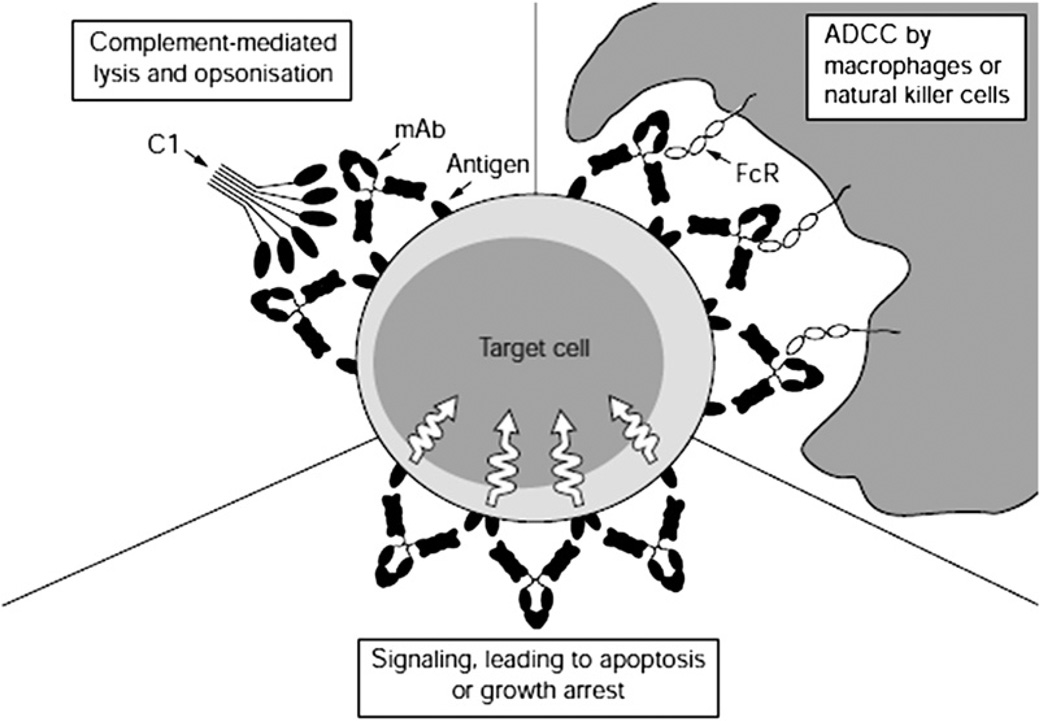
The proposed mechanism of action for unlabeled mAbs involves the combination of several processes (Fig. 3). Although one of the major functions of this immunotherapeutic technique allows for the opsonization of glioma cells and induction of antibody-dependent cellular cytotoxicity (ADCC), mAb binding may also result in cross-linking or blocking of membrane receptors, with subsequent modulation of transmembrane molecular pathway signaling. Such activity may promote further cascades leading to decreased tumor growth and cellular apoptosis.72–74 These concepts were supported by Bleeker and colleagues; they demonstrated anti–epidermal growth factor receptor (EGFR) mAb-induced cell death resulting from a combination of ADCC and a disruption of EGF signaling.75
Fig. 3.
Mechanisms of action for unlabeled monoclonal antibodies used in passive immunotherapy for the treatment of GBM. Antigen binding can induce subsequent C1 complement binding, activate antibody-dependent cellular cytotoxicity, or alter signaling pathways leading to reduced tumor growth or apoptosis. ADCC, antibody-dependent cellular cytotoxicity. (From Cragg MS, French RR, Glennie MJ. Signaling antibodies in cancer therapy. Cur Opin Immunol 1999;11(5):541–7; This figure was reproduced with the kind permission of Elsevier.)
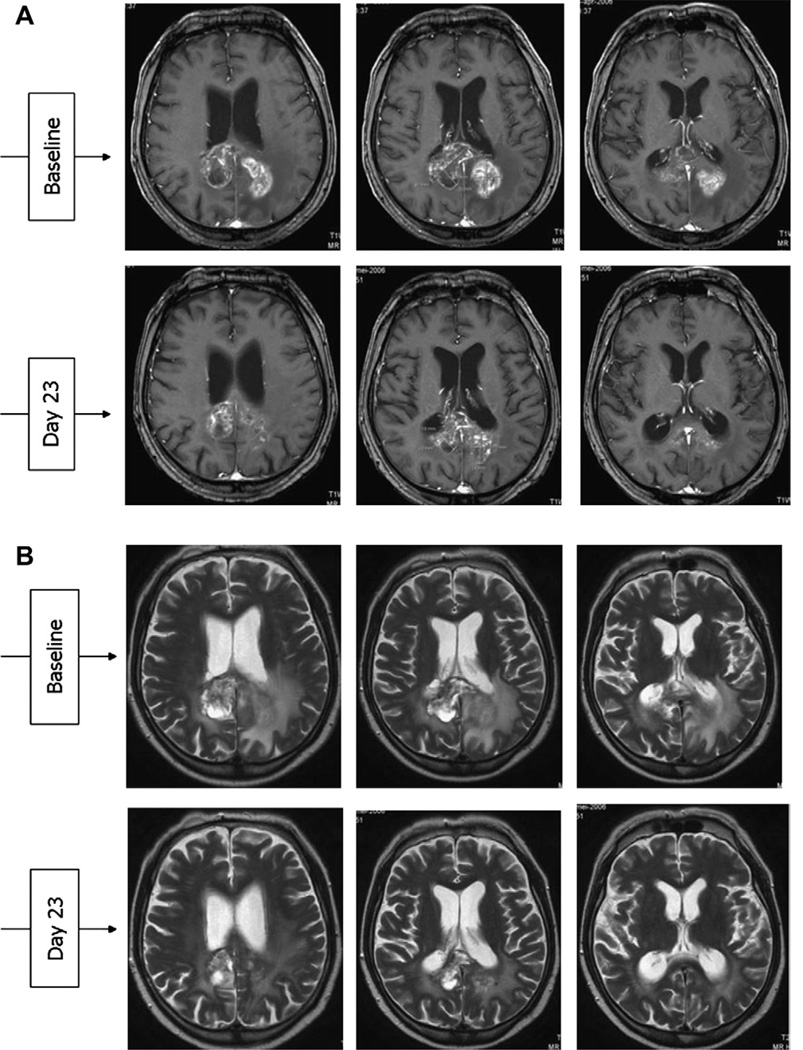
In high-grade gliomas, EGFR is estimated to be overexpressed or mutated in 40% to 50% of all tumors.1 EGFR activation is thought to induce cellular proliferation, motility, and increased tumor cell survival via downstream signaling related to the PI3K/Akt, Ras/Raf/Mek/ERK, and PLC-gamma/ PKC pathways.76 Cetuximab is a mAb that has been demonstrated to inhibit the conformational changes necessary for EGFR to dimerize, thus preventing aberrant ligand-independent activation and signaling.77 Confirmation of cetuximab’s effects have been suggested by preclinical studies reporting GBM growth inhibition and increased apoptosis.78,79 In a phase I/II trial with 17 patients with GBM, anti-EGFR mAb therapy demonstrated a median follow-up of 13 months. The investigators reported a 6-month progression-free survival (PFS) of 81%, whereas 87%of the patients were still alive at 1 year.1 Neyns and colleagues80 investigated the effects of cetuximab in 55 patients (Fig.4)with recurrent GBM (28 with EGFR amplification and 27 without), noting evidence of some radiographic response (3 PR, 16 SD) but no overall improved outcome in survival. In a phase II trial combining cetuximab, bevacizumab (vascular endothelial growth factor [VEGF] inhibitor), and irinotecan (topoisomerase-1 inhibitor) for the treatment of 32 patients with recurrent GBM, available data for 27 patients demonstrated 1 CR (3.7%), 8 PR (30.0%), and 5 minor responses (18.5%, defined as 25%–50% regression and clinical improvement).1
Fig. 4.
(A) T1-weighted and (B) T2-weighted axial postcontrast magnetic resonance imaging of a patient with GBM at baseline and day 23 posttreatment with cetuximab. (From Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol 2009;20(9):1596–603. This figure was reproduced with the kind permission of Oxford University Press.)
Another promising antigen includes the EGFR variant III (EGFRvIII) protein.81–84 EGFRvIII is restricted to only cancer cells and has been expressed in approximately 40% of all GBM cases. Furthermore, this EGFR mutation confers constitutively active signaling, resulting in increased tumor proliferation, invasion, and apoptotic resistance.85,86 In animal models of brain tumors, the administration of anti-EGFRvIII mAbs have resulted in decreased tumor volume and increased survival.85,87–90 Similarly, intratumoral administration of Y10 (mAb to an EGFRvIII murine homolog) for the treatment of EGFRvIII-expressing B16 melanoma increased the median survival by 286%.91 Despite these promising preclinical results, there have not yet been any reports from clinical trials evaluating mAb for EGFRvIII targeting of GBM.1 However, one phase I trial of 7 patients with various tumor types, including one anaplastic astrocytoma, showed the effects of a chimeric form of mAb 806 (ch806, one of the most tumor-specific EGFRvIII mAb), to demonstrate excellent target specificity, no evidence of normal tissue uptake, no significant toxicity, and stabilization of the patient’s glioma.92 Furthermore, an EGFRvIII-targeted dsFv-PE38K-DEL single fragment chain Pseudomonas exotoxin construct (MR1-1) is being used in a clinical trial for the treatment of patients with GBM.85
Other immunotherapeutic targets include the VEGF receptor. Literature suggests that glioma angiogenesis is the manifestation of definitive genetic mutations resulting in characteristic microvascular proliferation seen in GBM histopathology.93,94 VEGF plays a role in endothelial cell survival, proliferation, invasion, and migration, which all participate in angiogenesis and tumor progression.95 GBM have high levels of VEGF compared with other malignancies; high expression correlates with poor prognosis.96,97 Accordingly, several studies have examined the therapeutic value of antiangiogenic mAbs, particularly bevacizumab, for GBM treatment.
In 2009, the Food and Drug Administration (FDA) approved bevacizumab for recurrent GBM based on its phase II demonstration of high treatment response rates and promising clinical improvements.98 The first use of this therapy was by Dr Stark-Vance who treated 21 patients with recurrent GBM; this group documented a 43% response rate (1 CR, 8 PR).99 Vredenburgh and colleagues100 reported the administration of bevacizumab and irinotecan in 32 recurrent malignant gliomas (23 GBM) resulting in radiographic responses in 14 patients with GBM (61% of 23), with a 20-week median PFS and nearly doubled PFS at 6 months compared with control groups. However, overall survival was not significantly improved. These findings were reaffirmed in a retrospective review of 55 patients (33 GBM) that also included irinotecan.101 However, Fine and colleagues102 reported results from a phase II study of 79 patients with recurrent GBM treated with bevacizumab alone, establishing a 60% response rate, 30% PFS at 6 months, and a reduction in toxicity. Given the decreased adverse events and similar therapeutic effects of bevacizumab alone compared with other studies that have included the concurrent administration of irinotecan, the added benefits of this topoisomerase-1 inhibitor have been a point of investigation.
In a study of 167 patients with recurrent GBM assigned to either bevacizumab alone or in combination with irinotecan, response rates, PFS at 6 months, and median overall survival were 28% and 38%, 43% and 50%, and 9.2 months and 8.7 months, respectively.103 However, despite these statistics, the median overall survival did not demonstrate clinically substantial improvements.104–106 In a randomized trial by de Groot and colleagues,99 they demonstrated an improvement in both the response rate and PFS with the addition of irinotecan, yet the median overall survival for both groups did not differ from historical controls. In a study evaluating bevacizumab alone, then in combination with irinotecan, Kreisl and colleagues107 reported findings for 48 patients with recurrent GBM. Median PFS was 16 weeks, PFS at 6 months was 29%, and median overall survival was 31 weeks. Similar findings were reported in a recent study regarding outcomes in 225 patients with recurrent high-grade glioma (176 GBM) treated with bevacizumab alone or in combination with chemotherapy.108 In summary, many investigations have evaluated the potential of chemotherapeutic agents in conjunction with bevacizumab, with most demonstrating clinical outcomes equivalent to those produced by anti-VEGF monotherapy.100,103,109–112
Although many previous studies have failed to identify an improvement in overall survival with the incorporation of bevacizumab to their treatment regimen, other trials have demonstrated more success.95,100,103,107,110 Vredenburg and colleagues96 later evaluated 75 patients with newly diagnosed GBM in a phase II trial for treatment with bevacizumab and irinotecan. Despite moderate toxicity in which 19 patients (25%) had to withdraw from the study early, results were promising. Compared with historical controls, this investigation demonstrated an improvement in median overall survival (21.2 months) and median PFS (14.2 months). These findings were similar to those of Lai and colleagues in which their phase II study of bevacizumab in patients with newly diagnosed GBM demonstrated an improved median overall survival of 19.6 months.96,113 The experience of the University of California, Los Angeles group with bevacizumab has largely shown benefit radiographically, thus indicating that it may replace the need for high-dose steroids. In addition, a recent study of 14 recurrent high-grade gliomas (11 GBM) treated with bevacizumab and irinotecan within the Chinese population demonstrated an overall response detected within 9 patients with GBM (3 CR, 6PR), a median PFS of 6 months, a PFS at 6 months of 64%, and a median overall survival of 17 months.114
Since its inception, overall radiographic response and PFS following bevacizumab administration have achieved improvements up to fourfold greater than historical controls.103,107,115–117 However, given the general lack of improvement to median overall survival, the true benefits of this antiangiogenic mAb remain controversial. Wong and colleagues98 conducted a 15-study meta-analysis of 548 patients with recurrent GBM treated with bevacizumab. Their efforts demonstrated a 45% PFS at 6 months, 76% 6-month survival rate, and a 9.3-month overall survival, with no clear evidence of a dose-response benefit. Their 84% response rate included 6% CR, 49% PR, and 29% with SD. However, it has been suggested that radiological responses, on which demonstration of antitumor efficacy has been traditionally based, may represent the normalization of blood-brain-barrier function and resultant decreased contrast enhancement rather than valid glioma stability or regression. This concept has been supported by the findings of Norden and colleagues in which patients treated with bevacizumab demonstrated a lack of contrast enhancement, yet still displayed significant tumor dissemination.101,104 This notion was the basis for which the European Medicines Agency denied approval for bevacizumab for recurrent GBM, stating that radiological response rates may not be the most appropriate measure of drug efficacy.118 Furthermore, it has been postulated that the use of anti-VEGF treatment may induce a more invasive lesion with normal vessel cooption.96,99 Given the continued controversy regarding the use of bevacizumab and its therapeutic benefits, further investigations will be necessary to establish the true value of this approach, with several phase III trials currently underway.119 With the recent withdrawal of bevacizumab for breast cancer and the fast-tracked FDA approval of it for GBM, we may see withdrawal of this approval soon until better data become available to demonstrate its effects.
Radiolabeled Monoclonal Antibodies
Radiolabeled monoclonal antibodies confer added advantage over their unlabeled counterparts by providing delivered therapeutics. Similar to radiotherapy, treatment with radiolabeled mAbs uses radiation to induce cell death, with the enhanced benefit of an increased target specificity.
Emrich and colleagues120 evaluated the use of 125I-coupled mAbs against human A431 carcinoma cells, which have been demonstrated to display high concentrations of EGFR. In their phase II study of 180 patients of which 118 had a diagnosis of GBM, the overall median survival was 13.4 months for the patients with GBM, demonstrating a significant improvement in outcome. Furthermore, GBM patients less than 40 years old with a Karnofsky Performance Score greater than 70 had a median survival of 25.4 months. Casaco and colleagues121 investigated the role of 188Re (beta and gamma radionuclide) paired with nimotuzumab (anti-EGFR mAb) in 11 patients with recurrent malignant gliomas, 8 of which were GBM; there were 2 patients with CR (1 GBM), 1 with PR (1 GBM), and 2 with SD (1 GBM). However, no improvement in overall survival was reported, and 2 of 4 patients experienced severe adverse events, including hemorrhagic brain necrosis. Yet, in a recent study representing the largest series evaluating the use of radioimmunotherapy, Li and colleagues122 reported findings of their phase II trial investigating 125I-mAb 425 (anti-EGFR) for the treatment of newly diagnosed GBM in 192 patients. They demonstrated no National Cancer Institute common toxicities at grades 3/4 and an overall median survival of 15.7 months. Subgroup analysis determined that although those treated with 125I-mAb 425 alone had an overall median survival of 14.5 months, those treated concurrently with temozolomide survived 20.2 months, indicating there may be no interference in the therapeutic effects of both agents when given simultaneously.
Another target of interest involves the extracellular matrix protein, tenascin-C, expressed in more than 90% of all GBM cases and implicated in glioma-associated angiogenesis.71,123 Its function has been implicated in adhesion, migration, and proliferation, with increased expression being correlated with higher grades of tumor malignancy.124–127 Riva and colleagues128 reported findings for the treatment of 105 patients (58 GBM) with 131I-labeled antitenascin mAbs (81C6). Their study identified a statistically significant improvement in survival (23 months) compared with controls, whereas others have demonstrated similar responses with increased stabilization of disease.128–130 In another investigation of 21 patients with newly diagnosed malignant glioma (16 GBM) treated with 81C6, median overall survival was 91 weeks, with 87% of GBM patients alive at 1 year.131,132 On later follow-up, the investigators found an average time to progression of 18 months and median overall survival of nearly 2 years.1
In a study by Zalutsky and colleagues,133 18 patients with recurrent high-grade gliomas (14 GBM) were treated with maximal surgical resection followed by 211At (alpha-particle emitter) coupled with chimeric antitenascin mAbs. Alpha particles enable high-intensity radiation over short distances of 1 to 2 mm, thus targeting tumor cells at the resected margin. The investigators reported no grade 3/4 adverse reactions and a favorable 52-week median overall survival compared with 23 to 31 weeks observed for patients receiving conventional therapies.
Despite these promising results, there are still many obstacles that must be overcome before the treatment of GBM using monoclonal antibodies is optimized. One problem involves the host’s immune system forming endogenous antibodies against the transferred mAbs. Furthermore, mAbs from passive immunotherapy may react with antigen-positive normal tissue causing collateral damage to healthy brain parenchyma. However, approaches that use intratumoral infusion of mAb may be capable of minimizing these potential complications.71
SUMMARY
The use of passive immunotherapeutic approaches for the treatment of GBM represents a promising adjuvant to current management strategies. However, given inconsistent findings between various studies, future prospective randomized trials will be necessary to validate the added benefits that the administration of LAK cells, CTLs, and mAbs may confer to this patient population.
KEY POINTS.
Glioblastoma multiforme has a proclivity for widespread invasion and destruction of healthy parenchyma, displaying a poor outcome despite aggressive conventional treatment.
Immunotherapy offers the potential to selectively target tumor cells, thereby decreasing collateral damage to normal brain.
Passive immunotherapy includes administration of monoclonal antibodies and the adoptive transfer of lymphocyte-activated killer cells or cytotoxic T lymphocytes.
Although many clinical trials have demonstrated promising results, further prospective randomized studies will be necessary to validate the effects of various passive immunotherapeutic approaches.
Acknowledgments
Daniel Nagasawa (first author) was partially supported by an American Brain Tumor Association Medical Student Summer Fellowship in Honor of Connie Finc. Carol Kruse (sixth author) was supported in part by NIH R01CA121258, R01CA125244, and R01CA154256. Isaac Yang (senior author) was partially supported by an Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research UCLA Scholars in Translational Medicine Program Award, Visionary Fund Grant, and the Stein Oppenheimer Endowment Award.
REFERENCES
- 1.Mitra S, Li G, Harsh GR. Passive antibody-mediated immunotherapy for the treatment of malignant gliomas. Neurosurg Clin North Am. 2010;21(1):67–76. doi: 10.1016/j.nec.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 3.Bolesta E, Kowalczyk A, Wierzbicki A, et al. DNA vaccine expressing the mimotope of GD2 ganglio-side induces protective GD2 cross-reactive antibody responses. Cancer Res. 2005;65(8):3410–3418. doi: 10.1158/0008-5472.CAN-04-2164. [DOI] [PubMed] [Google Scholar]
- 4.Herrlinger U, Weller M, Schabet M. New aspects of immunotherapy of leptomeningeal metastasis. J Neurooncol. 1998;38(2–3):233–239. doi: 10.1023/a:1005948722912. [DOI] [PubMed] [Google Scholar]
- 5.Tjoa BA, Murphy GP. Progress in active specific immunotherapy of prostate cancer. Semin Surg Oncol. 2000;18(1):80–87. doi: 10.1002/(sici)1098-2388(200001/02)18:1<80::aid-ssu10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Quan WD, Jr, Palackdharry CS. Common cancers–immunotherapy and multidisciplinary therapy: parts III and IV. Dis Mon. 1997;43(11):745–808. doi: 10.1016/s0011-5029(97)90035-3. [DOI] [PubMed] [Google Scholar]
- 7.Vauleon E, Avril T, Collet B, et al. Overview of cellular immunotherapy for patients with glioblastoma. Clin Dev Immunol. 2010;2010:689171. doi: 10.1155/2010/689171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes RL, Arbit E, Odaimi M, et al. Adoptive cellular immunotherapy for the treatment of malignant gliomas. Crit Rev Oncol Hematol. 2001;39(1–2):31–42. doi: 10.1016/s1040-8428(01)00122-6. [DOI] [PubMed] [Google Scholar]
- 9.Lotze MT, Grimm EA, Mazumder A, et al. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- 10.Grimm EA, Mazumder A, Zhang HZ, et al. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang A, Lu SD, Mark DF. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 14.West WH, Tauer KW, Yannelli JR, et al. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- 15.Oldham RK. Cancer biotherapy: the first year. Cancer Biol Ther. 1994;9(3):179–181. doi: 10.1089/cbr.1994.9.179. [DOI] [PubMed] [Google Scholar]
- 16.Oldham RK, Blumenschein G, Schwartzberg L, et al. Combination biotherapy utilizing interleukin-2 and alpha interferon in patients with advanced cancer: a National Biotherapy Study Group trial. Mol Biother. 1992;4(1):4–9. [PubMed] [Google Scholar]
- 17.Dillman RO, Church C, Oldham RK, et al. Inpatient continuous-infusion interleukin-2 in 788 patients with cancer. The National Biotherapy Study Group experience. Cancer. 1993;71(7):2358–2370. doi: 10.1002/1097-0142(19930401)71:7<2358::aid-cncr2820710730>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Rosenstein M, Yron I, Kaufmann Y, et al. Lymphokine-activated killer cells: lysis of fresh syngeneic natural killer-resistant murine tumor cells by lymphocytes cultured in interleukin 2. Cancer Res. 1984;44(5):1946–1953. [PubMed] [Google Scholar]
- 19.Mule JJ, Shu S, Schwarz SL, et al. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984;225(4669):1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- 20.Mazumder A, Rosenberg SA. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J Exp Med. 1984;159(2):495–507. doi: 10.1084/jem.159.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ettinghausen SE, Lipford EH, 3rd, Mule JJ, et al. Recombinant interleukin 2 stimulates in vivo proliferation of adoptively transferred lymphokine-activated killer (LAK) cells. J Immunol. 1985;135(5):3623–3635. [PubMed] [Google Scholar]
- 22.Lafreniere R, Rosenberg SA. Successful immunotherapy of murine experimental hepatic metastases with lymphokine-activated killer cells and recombinant interleukin 2. Cancer Res. 1985;45(8):3735–3741. [PubMed] [Google Scholar]
- 23.Kaaijk P, Troost D, Dast PK, et al. Cytolytic effects of autologous lymphokine-activated killer cells on organotypic multicellular spheroids of gliomas in vitro. Neuropathol Appl Neurobiol. 1995;21(5):392–398. doi: 10.1111/j.1365-2990.1995.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 24.George RE, Loudon WG, Moser RP, et al. In vitro cytolysis of primitive neuroectodermal tumors of the posterior fossa (medulloblastoma) by lymphokine-activated killer cells. J Neurosurg. 1988;69(3):403–409. doi: 10.3171/jns.1988.69.3.0403. [DOI] [PubMed] [Google Scholar]
- 25.Dillman RO, Duma CM, Ellis RA, et al. Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J Immunother. 2009;32(9):914–919. doi: 10.1097/CJI.0b013e3181b2910f. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs SK, Wilson DJ, Kornblith PL, et al. Interleukin-2 or autologous lymphokine-activated killer cell treatment of malignant glioma: phase I trial. Cancer Res. 1986;46(4 Pt 2):2101–2104. [PubMed] [Google Scholar]
- 27.Papa MZ, Vetto JT, Ettinghausen SE, et al. Effect of corticosteroid on the antitumor activity of lymphokine-activated killer cells and interleukin 2 in mice. Cancer Res. 1986;46(11):5618–5623. [PubMed] [Google Scholar]
- 28.Mulvin DW, Kruse CA, Mitchell DH, et al. Lymphokine-activated killer cells with interleukin-2: dose toxicity and localization in isolated perfused rat lungs. Mol Biother. 1990;2(1):38–43. [PubMed] [Google Scholar]
- 29.Lillehei KO, Mitchell DH, Johnson SD, et al. Long-term follow-up of patients with recurrent malignant gliomas treated with adjuvant adoptive immunotherapy. Neurosurgery. 1991;28(1):16–23. doi: 10.1097/00006123-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Hook GR, Greenwood MA, Barba D, et al. Morphology of interleukin-2-stimulated human peripheral blood mononuclear effector cells killing glioma-derived tumor cells in vitro. J Natl Cancer Inst. 1988;80(3):171–177. doi: 10.1093/jnci/80.3.171. [DOI] [PubMed] [Google Scholar]
- 31.Dillman RO, Duma CM, Schiltz PM, et al. Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother. 2004;27(5):398–404. doi: 10.1097/00002371-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Sankhla SK, Nadkarni JS, Bhagwati SN. Adoptive immunotherapy using lymphokine-activated killer (LAK) cells and interleukin-2 for recurrent malignant primary brain tumors. J Neurooncol. 1996;27(2):133–140. doi: 10.1007/BF00177476. [DOI] [PubMed] [Google Scholar]
- 33.Hayes RL, Koslow M, Hiesiger EM, et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995;76(5):840–852. doi: 10.1002/1097-0142(19950901)76:5<840::aid-cncr2820760519>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 34.Boiardi A, Silvani A, Ruffini PA, et al. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol Immunother. 1994;39(3):193–197. doi: 10.1007/BF01533386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blancher A, Roubinet F, Grancher AS, et al. Local immunotherapy of recurrent glioblastoma multiforme by intracerebral perfusion of interleukin-2 and LAK cells. Eur Cytokine Netw. 1993;4(5):331–341. [PubMed] [Google Scholar]
- 36.Jeffes EW, 3rd, Beamer YB, Jacques S, et al. Therapy of recurrent high grade gliomas with surgery, and autologous mitogen activated IL-2 stimulated killer (MAK) lymphocytes: I. Enhancement of MAK lytic activity and cytokine production by PHA and clinical use of PHA. J Neurooncol. 1993;15(2):141–155. doi: 10.1007/BF01053935. [DOI] [PubMed] [Google Scholar]
- 37.Barba D, Saris SC, Holder C, et al. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg. 1989;70(2):175–182. doi: 10.3171/jns.1989.70.2.0175. [DOI] [PubMed] [Google Scholar]
- 38.Merchant RE, Grant AJ, Merchant LH, et al. Adoptive immunotherapy for recurrent glioblastoma multiforme using lymphokine activated killer cells and recombinant interleukin-2. Cancer. 1988;62(4):665–671. doi: 10.1002/1097-0142(19880815)62:4<665::aid-cncr2820620403>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Tsuboi K, Saijo K, Ishikawa E, et al. Effects of local injection of ex vivo expanded autologous tumor-specific T lymphocytes in cases with recurrent malignant gliomas. Clin Cancer Res. 2003;9(9):3294–3302. [PubMed] [Google Scholar]
- 40.Plautz GE, Shu S. Adoptive immunotherapy of CNS malignancies. Cancer Chemother Biol Response Modif. 2001;19:327–338. [PubMed] [Google Scholar]
- 41.Kitahara T, Watanabe O, Yamaura A, et al. Establishment of interleukin 2 dependent cytotoxic T lymphocyte cell line specific for autologous brain tumor and its intracranial administration for therapy of the tumor. J Neurooncol. 1987;4(4):329–336. doi: 10.1007/BF00195603. [DOI] [PubMed] [Google Scholar]
- 42.Tsurushima H, Liu SQ, Tuboi K, et al. Reduction of end-stage malignant glioma by injection with autologous cytotoxic T lymphocytes. Jpn J Cancer Res. 1999;90(5):536–545. doi: 10.1111/j.1349-7006.1999.tb00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruse CA, Cepeda L, Owens B, et al. Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin-2. Cancer Immunol Immunother. 1997;45(2):77–87. doi: 10.1007/s002620050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheever MA, Chen W. Therapy with cultured T cells: principles revisited. Immunol Rev. 1997;157:177–194. doi: 10.1111/j.1600-065x.1997.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 45.Quattrocchi KB, Miller CH, Cush S, et al. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol. 1999;45(2):141–157. doi: 10.1023/a:1006293606710. [DOI] [PubMed] [Google Scholar]
- 46.Holladay FP, Heitz-Turner T, Bayer WL, et al. Autologous tumor cell vaccination combined with adoptive cellular immunotherapy in patients with grade III/IV astrocytoma. J Neurooncol. 1996;27(2):179–189. doi: 10.1007/BF00177482. [DOI] [PubMed] [Google Scholar]
- 47.Plautz GE, Barnett GH, Miller DW, et al. Systemic T cell adoptive immunotherapy of malignant gliomas. J Neurosurg. 1998;89(1):42–51. doi: 10.3171/jns.1998.89.1.0042. [DOI] [PubMed] [Google Scholar]
- 48.Plautz GE, Miller DW, Barnett GH, et al. T cell adoptive immunotherapy of newly diagnosed gliomas. Clin Cancer Res. 2000;6(6):2209–2218. [PubMed] [Google Scholar]
- 49.Wood GW, Holladay FP, Turner T, et al. A pilot study of autologous cancer cell vaccination and cellular immunotherapy using anti-CD3 stimulated lymphocytes in patients with recurrent grade III/IV astrocytoma. J Neurooncol. 2000;48(2):113–120. doi: 10.1023/a:1006456421177. [DOI] [PubMed] [Google Scholar]
- 50.Sloan AE, Dansey R, Zamorano L, et al. Adoptive immunotherapy in patients with recurrent malignant glioma: preliminary results of using autologous whole-tumor vaccine plus granulocyte-macrophage colony-stimulating factor and adoptive transfer of anti-CD3-activated lymphocytes. Neurosurg Focus. 2000;9(6):e9. doi: 10.3171/foc.2000.9.6.10. [DOI] [PubMed] [Google Scholar]
- 51.Arca MJ, Mule JJ, Chang AE. Genetic approaches to adoptive cellular therapy of malignancy. Semin Oncol. 1996;23(1):108–117. [PubMed] [Google Scholar]
- 52.Lokhorst HM, Liebowitz D. Adoptive T-cell therapy. Semin Hematol. 1999;36(1) Suppl 3:26–29. [PubMed] [Google Scholar]
- 53.Ngo MC, Rooney CM, Howard JM, et al. Ex vivo gene transfer for improved adoptive immunotherapy of cancer. Hum Mol Genet. 2011;20(R1):R93–R99. doi: 10.1093/hmg/ddr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez GG, Kruse CA. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther Mol Biol. 2006;10(A):133–146. [PMC free article] [PubMed] [Google Scholar]
- 55.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beckman RA, Weiner LM, Davis HM. Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumors. Cancer. 2007;109(2):170–179. doi: 10.1002/cncr.22402. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed N, Salsman VS, Kew Y, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16(2):474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heslop HE. Safer CARS. Mol Ther. 2010;18(4):661–662. doi: 10.1038/mt.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonini C, Brenner MK, Heslop HE, et al. Genetic modification of T cells. Biol Blood Marrow Transplant. 2011;17(Suppl 1):S15–S20. doi: 10.1016/j.bbmt.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tey SK, Dotti G, Rooney CM, et al. Inducible caspase 9 suicide gene to improve the safety of allo-depleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(8):913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quintarelli C, Vera JF, Savoldo B, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110(8):2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bollard CM, Rossig C, Calonge MJ, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99(9):3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 64.Westwood JA, Kershaw MH. Genetic redirection of T cells for cancer therapy. J Leukoc Biol. 2010;87(5):791–803. doi: 10.1189/jlb.1209824. [DOI] [PubMed] [Google Scholar]
- 65.Olofsson A, Miyazono K, Kanzaki T, et al. Transforming growth factor-beta 1, -beta 2, and -beta 3 secreted by a human glioblastoma cell line. Identification of small and different forms of large latent complexes. J Biol Chem. 1992;267(27):19482–19488. [PubMed] [Google Scholar]
- 66.Kuppner MC, Hamou MF, Sawamura Y, et al. Inhibition of lymphocyte function by glioblastoma-derived transforming growth factor beta 2. J Neurosurg. 1989;71(2):211–217. doi: 10.3171/jns.1989.71.2.0211. [DOI] [PubMed] [Google Scholar]
- 67.Foster AE, Dotti G, Lu A, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother. 2008;31(5):500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weijtens ME, Willemsen RA, Valerio D, et al. Single chain Ig/gamma gene-redirected human T lymphocytes produce cytokines, specifically lyse tumor cells, and recycle lytic capacity. J Immunol. 1996;157(2):836–843. [PubMed] [Google Scholar]
- 69.Hickey MJ, Malone CC, Erickson KL, et al. Cellular and vaccine therapeutic approaches for gliomas. J Transl Med. 2010;8:100. doi: 10.1186/1479-5876-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson LA, Sampson JH. Immunotherapy approaches for malignant glioma from 2007 to 2009. Curr Neurol Neurosci Rep. 2010;10(4):259–266. doi: 10.1007/s11910-010-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wikstrand CJ, Cokgor I, Sampson JH, et al. Monoclonal antibody therapy of human gliomas: current status and future approaches. Cancer Metastasis Rev. 1999;18(4):451–464. doi: 10.1023/a:1006354102377. [DOI] [PubMed] [Google Scholar]
- 72.Vitetta ES, Uhr JW. Monoclonal antibodies as agonists: an expanded role for their use in cancer therapy. Cancer Res. 1994;54(20):5301–5309. [PubMed] [Google Scholar]
- 73.Nadler LM, Stashenko P, Hardy R, et al. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res. 1980;40(9):3147–3154. [PubMed] [Google Scholar]
- 74.Cragg MS, French RR, Glennie MJ. Signaling antibodies in cancer therapy. Curr Opin Immunol. 1999;11(5):541–547. doi: 10.1016/s0952-7915(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 75.Bleeker WK, Lammerts van Bueren JJ, van Ojik HH, et al. Dual mode of action of a human anti-epidermal growth factor receptor monoclonal antibody for cancer therapy. J Immunol. 2004;173(7):4699–4707. doi: 10.4049/jimmunol.173.7.4699. [DOI] [PubMed] [Google Scholar]
- 76.Ohno M, Natsume A, Ichiro Iwami K, et al. Retrovirally engineered T-cell-based immunotherapy targeting type III variant epidermal growth factor receptor, a glioma-associated antigen. Cancer Sci. 2010;101(12):2518–2524. doi: 10.1111/j.1349-7006.2010.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferguson KM. Active and inactive conformations of the epidermal growth factor receptor. Biochem Soc Trans. 2004;32(Pt 5):742–745. doi: 10.1042/BST0320742. [DOI] [PubMed] [Google Scholar]
- 78.Eller JL, Longo SL, Hicklin DJ, et al. Activity of anti-epidermal growth factor receptor monoclonal antibody C225 against glioblastoma multiforme. Neurosurgery. 2002;51(4):1005–1013. doi: 10.1097/00006123-200210000-00028. [discussion: 1013–4]. [DOI] [PubMed] [Google Scholar]
- 79.Eller JL, Longo SL, Kyle MM, et al. Anti-epidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo. Neurosurgery. 2005;56(1):155–162. doi: 10.1227/01.neu.0000145865.25689.55. [discussion: 162]. [DOI] [PubMed] [Google Scholar]
- 80.Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-gradeglioma. Ann Oncol. 2009;20(9):1596–1603. doi: 10.1093/annonc/mdp032. [DOI] [PubMed] [Google Scholar]
- 81.Bigner SH, Burger PC, Wong AJ, et al. Gene amplification in malignant human gliomas: clinical and histopathologic aspects. J Neuropathol Exp Neurol. 1988;47(3):191–205. doi: 10.1097/00005072-198805000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Bigner SH, Humphrey PA, Wong AJ, et al. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res. 1990;50(24):8017–8022. [PubMed] [Google Scholar]
- 83.Humphrey PA, Gangarosa LM, Wong AJ, et al. Deletion-mutant epidermal growth factor receptor in human gliomas: effects of type II mutation on receptor function. Biochem Biophys Res Commun. 1991;178(3):1413–1420. doi: 10.1016/0006-291x(91)91051-d. [DOI] [PubMed] [Google Scholar]
- 84.Humphrey PA, Wong AJ, Vogelstein B, et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenografts. Cancer Res. 1988;48(8):2231–2238. [PubMed] [Google Scholar]
- 85.Zalutsky MR, Boskovitz A, Kuan CT, et al. Radioimmunotargeting of malignant glioma by monoclonal antibody D2C7 reactive against both wild-type and variant III mutant epidermal growth factor receptors. Nucl Med Biol. 2012;39(1):23–34. doi: 10.1016/j.nucmedbio.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lund-Johansen M, Bjerkvig R, Humphrey PA, et al. Effect of epidermal growth factor on glioma cell growth, migration, and invasion in vitro. Cancer Res. 1990;50(18):6039–6044. [PubMed] [Google Scholar]
- 87.Sampson JH, Crotty LE, Lee S, et al. Unarmed, tumor-specific monoclonal antibody effectively treats brain tumors. Proc Natl Acad Sci U S A. 2000;97(13):7503–7508. doi: 10.1073/pnas.130166597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang W, Barth RF, Wu G, et al. Development of a syngeneic rat brain tumor model expressing EGFRvIII and its use for molecular targeting studies with monoclonal antibody L8A4. Clin Cancer Res. 2005;11(1):341–350. [PubMed] [Google Scholar]
- 89.Yang W, Barth RF, Wu G, et al. Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin Cancer Res. 2006;12(12):3792–3802. doi: 10.1158/1078-0432.CCR-06-0141. [DOI] [PubMed] [Google Scholar]
- 90.Perera RM, Narita Y, Furnari FB, et al. Treatment of human tumor xenografts with monoclonal antibody 806 in combination with a prototypical epidermal growth factor receptor-specific antibody generates enhanced antitumor activity. Clin Cancer Res. 2005;11(17):6390–6399. doi: 10.1158/1078-0432.CCR-04-2653. [DOI] [PubMed] [Google Scholar]
- 91.Mitchell DA, Fecci PE, Sampson JH. Immunotherapy of malignant brain tumors. Immunol Rev. 2008;222:70–100. doi: 10.1111/j.1600-065X.2008.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scott IU, Edwards AR, Beck RW, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Birlik B, Canda S, Ozer E. Tumour vascularity is of prognostic significance in adult, but not paediatric astrocytomas. Neuropathol Appl Neurobiol. 2006;32(5):532–538. doi: 10.1111/j.1365-2990.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 94.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer. 1996;77(2):362–372. doi: 10.1002/(SICI)1097-0142(19960115)77:2<362::AID-CNCR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 95.Pope WB, Lai A, Nghiemphu P, et al. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66(8):1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 96.Vredenburgh JJ, Desjardins A, Reardon DA, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res. 2011;17(12):4119–4124. doi: 10.1158/1078-0432.CCR-11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19(56):6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 98.Wong ET, Gautam S, Malchow C, et al. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw. 2011;9(4):403–407. doi: 10.6004/jnccn.2011.0037. [DOI] [PubMed] [Google Scholar]
- 99.de Groot JF, Yung WK. Bevacizumab and irinotecan in the treatment of recurrent malignant gliomas. Cancer J. 2008;14(5):279–285. doi: 10.1097/PPO.0b013e3181867bd6. [DOI] [PubMed] [Google Scholar]
- 100.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 101.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 102.Fine HA. Promising new therapies for malignant gliomas. Cancer J. 2007;13(56):349–354. doi: 10.1097/PPO.0b013e31815b18db. [DOI] [PubMed] [Google Scholar]
- 103.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 104.Salmaggi A, Gaviani P, Botturi A, et al. Bevacizumab at recurrence in high-grade glioma. Neurol Sci. 2011;32(Suppl 2):S251–S253. doi: 10.1007/s10072-011-0799-6. [DOI] [PubMed] [Google Scholar]
- 105.Addeo R, Caraglia M, De Santi MS, et al. A new schedule of fotemustine in temozolomide-pretreated patients with relapsing glioblastoma. J Neurooncol. 2011;102(3):417–424. doi: 10.1007/s11060-010-0329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scoccianti S, Detti B, Sardaro A, et al. Second-line chemotherapy with fotemustine in temozolomide-pretreated patients with relapsing glioblastoma: a single institution experience. Anticancer Drugs. 2008;19(6):613–620. doi: 10.1097/CAD.0b013e3283005075. [DOI] [PubMed] [Google Scholar]
- 107.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hofer S, Elandt K, Greil R, et al. Clinical outcome with bevacizumab in patients with recurrent high-grade glioma treated outside clinical trials. Acta Oncol. 2011;50(5):630–635. doi: 10.3109/0284186X.2011.572913. [DOI] [PubMed] [Google Scholar]
- 109.Reardon DA, Desjardins A, Peters KB, et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol. 2012;107(1):155–164. doi: 10.1007/s11060-011-0722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 111.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer. 2009;101(12):1986–1994. doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hasselbalch B, Lassen U, Hansen S, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol. 2010;12(5):508–516. doi: 10.1093/neuonc/nop063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29(2):142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pu JK, Chan RT, Ng GK, et al. Using bevacizumab in the fight against malignant glioma: first results in Asian patients. Hong Kong Med J. 2011;17(4):274–279. [PubMed] [Google Scholar]
- 115.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6(3):227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu W, Lamborn KR, Buckner JC, et al. Joint NCCTG and NABTC prognostic factors analysis for high-grade recurrent glioma. Neuro Oncol. 2010;12(2):164–172. doi: 10.1093/neuonc/nop019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9(1):29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Franceschi E, Brandes AA. Clinical end points in recurrent glioblastoma: are antiangiogenic agents friend or foe? Expert Rev Anticancer Ther. 2011;11(5):657–660. doi: 10.1586/era.11.44. [DOI] [PubMed] [Google Scholar]
- 119.Thompson EM, Frenkel EP, Neuwelt EA. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology. 2011;76(1):87–93. doi: 10.1212/WNL.0b013e318204a3af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Emrich JG, Brady LW, Quang TS, et al. Radioiodinated (I-125) monoclonal antibody 425 in the treatment of high grade glioma patients: ten-year synopsis of a novel treatment. Am J Clin Oncol. 2002;25(6):541–546. doi: 10.1097/00000421-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 121.Casaco A, Lopez G, Garcia I, et al. Phase I single-dose study of intracavitary-administered nimotuzumab labeled with 188 Re in adult recurrent high-grade glioma. Cancer Biol Ther. 2008;7(3):333–339. doi: 10.4161/cbt.7.3.5414. [DOI] [PubMed] [Google Scholar]
- 122.Li L, Quang TS, Gracely EJ, et al. A phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J Neurosurg. 2010;113(2):192–198. doi: 10.3171/2010.2.JNS091211. [DOI] [PubMed] [Google Scholar]
- 123.Zagzag D, Friedlander DR, Dosik J, et al. Tenascin-C expression by angiogenic vessels in human astrocytomas and by human brain endothelial cells in vitro. Cancer Res. 1996;56(1):182–189. [PubMed] [Google Scholar]
- 124.Behrem S, Zarkovic K, Eskinja N, et al. Distribution pattern of tenascin-C in glioblastoma: correlation with angiogenesis and tumor cell proliferation. Pathol Oncol Res. 2005;11(4):229–235. doi: 10.1007/BF02893856. [DOI] [PubMed] [Google Scholar]
- 125.Jallo GI, Friedlander DR, Kelly PJ, et al. Tenascin-C expression in the cyst wall and fluid of human brain tumors correlates with angiogenesis. Neurosurgery. 1997;41(5):1052–1059. doi: 10.1097/00006123-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 126.Leins A, Riva P, Lindstedt R, et al. Expression of tenascin-C in various human brain tumors and its relevance for survival in patients with astrocytoma. Cancer. 2003;98(11):2430–2439. doi: 10.1002/cncr.11796. [DOI] [PubMed] [Google Scholar]
- 127.Sarkar S, Nuttall RK, Liu S, et al. Tenascin-C stimulates glioma cell invasion through matrix metalloproteinase-12. Cancer Res. 2006;66(24):11771–11780. doi: 10.1158/0008-5472.CAN-05-0470. [DOI] [PubMed] [Google Scholar]
- 128.Riva P, Franceschi G, Arista A, et al. Local application of radiolabeled monoclonal antibodies in the treatment of high grade malignant gliomas: a six-year clinical experience. Cancer. 1997;80(Suppl 12):2733–2742. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2733::aid-cncr53>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 129.Brown JM, Coates DM, Phillpotts RJ. Evaluation of monoclonal antibodies for generic detection of flaviviruses by ELISA. J Virol Methods. 1996;62(2):143–151. doi: 10.1016/s0166-0934(96)02095-2. [DOI] [PubMed] [Google Scholar]
- 130.Bigner DD, Brown MT, Friedman AH, et al. Iodine-131-labeled antitenascin monoclonal antibody 81C6 treatment of patients with recurrent malignant gliomas: phase I trial results. J Clin Oncol. 1998;16(6):2202–2212. doi: 10.1200/JCO.1998.16.6.2202. [DOI] [PubMed] [Google Scholar]
- 131.Reardon DA, Akabani G, Coleman RE, et al. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: phase II study results. J Clin Oncol. 2006;24(1):115–122. doi: 10.1200/JCO.2005.03.4082. [DOI] [PubMed] [Google Scholar]
- 132.Reardon DA, Nabors LB, Stupp R, et al. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17(8):1225–1235. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zalutsky MR, Reardon DA, Akabani G, et al. Clinical experience with alpha-particle emitting 211At: treatment of recurrent brain tumor patients with 211 At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med. 2008;49(1):30–38. doi: 10.2967/jnumed.107.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]