Abstract
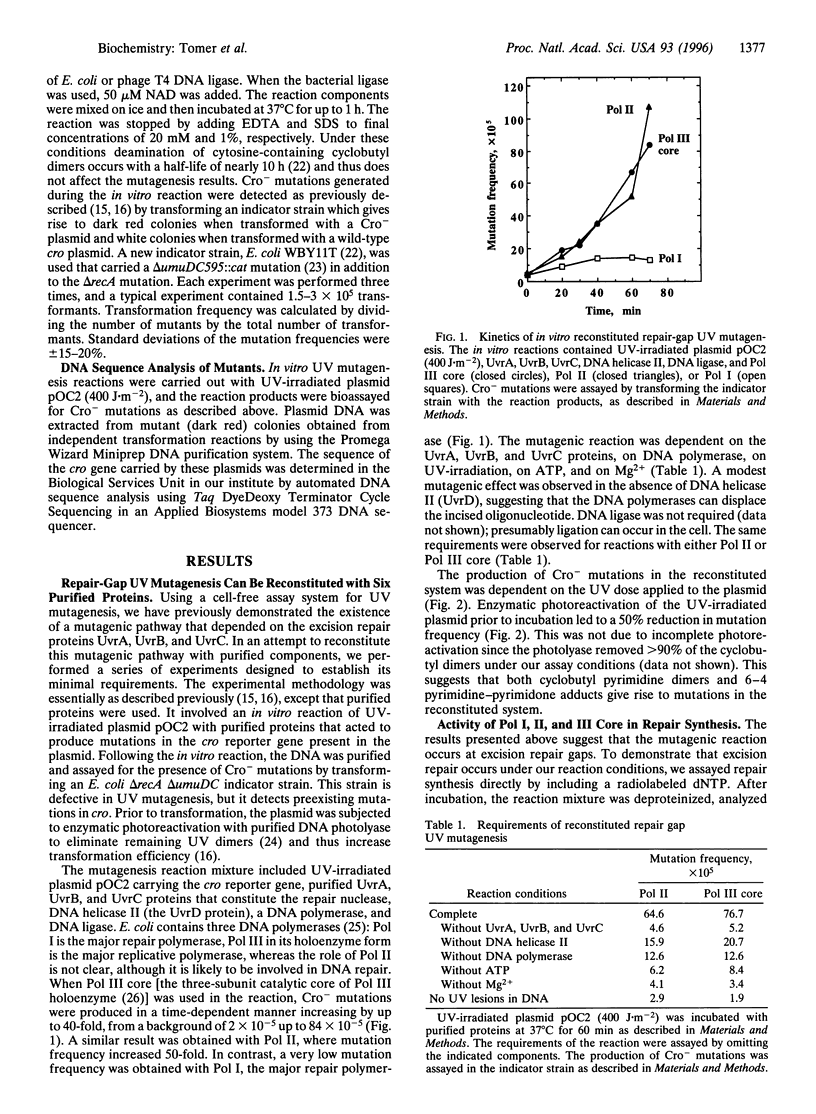
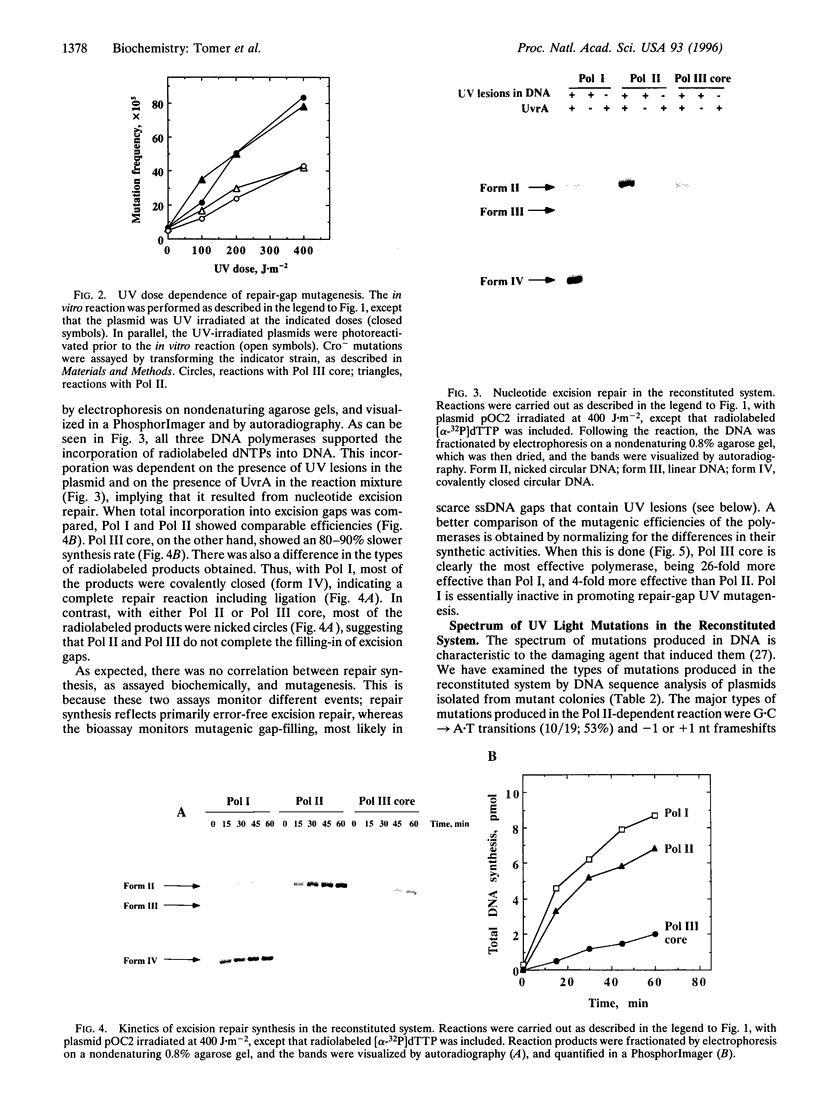
Using a cell-free system for UV mutagenesis, we have previously demonstrated the existence of a mutagenic pathway associated with nucleotide-excision repair gaps. Here, we report that this pathway can be reconstituted by using six purified proteins: UvrA, UvrB, UvrC, DNA helicase II, DNA polymerase III core, and DNA ligase. This establishes the minimal requirements for repair-gap UV mutagenesis. DNA polymerase II could replace DNA polymerase III, although less effectively, whereas DNA polymerase I, the major repair polymerase, could not. DNA sequence analysis of mutations generated in the in vitro reaction revealed a spectrum typical of mutations targeted to UV lesions. These observations suggest that repair-gap UV mutagenesis is performed by DNA polymerase III, and to a lesser extent by DNA polymerase II, by filling-in of a rare class of excision gaps that contain UV lesions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barak Y., Cohen-Fix O., Livneh Z. Deamination of cytosine-containing pyrimidine photodimers in UV-irradiated DNA. Significance for UV light mutagenesis. J Biol Chem. 1995 Oct 13;270(41):24174–24179. doi: 10.1074/jbc.270.41.24174. [DOI] [PubMed] [Google Scholar]
- Bates H., Randall S. K., Rayssiguier C., Bridges B. A., Goodman M. F., Radman M. Spontaneous and UV-induced mutations in Escherichia coli K-12 strains with altered or absent DNA polymerase I. J Bacteriol. 1989 May;171(5):2480–2484. doi: 10.1128/jb.171.5.2480-2484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner C. A., Hays S., McEntee K., Goodman M. F. DNA polymerase II is encoded by the DNA damage-inducible dinA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7663–7667. doi: 10.1073/pnas.87.19.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner C. A., Randall S. K., Rayssiguier C., Radman M., Eritja R., Kaplan B. E., McEntee K., Goodman M. F. Purification and characterization of an inducible Escherichia coli DNA polymerase capable of insertion and bypass at abasic lesions in DNA. J Biol Chem. 1988 Dec 15;263(35):18946–18952. [PubMed] [Google Scholar]
- Bonner C. A., Stukenberg P. T., Rajagopalan M., Eritja R., O'Donnell M., McEntee K., Echols H., Goodman M. F. Processive DNA synthesis by DNA polymerase II mediated by DNA polymerase III accessory proteins. J Biol Chem. 1992 Jun 5;267(16):11431–11438. [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P. Mutagenic DNA repair in Escherichia coli. III. Requirement for a function of DNA polymerase III in ultraviolet-light mutagenesis. Mol Gen Genet. 1976 Feb 27;144(1):53–58. doi: 10.1007/BF00277304. [DOI] [PubMed] [Google Scholar]
- Christensen J. R., LeClerc J. E., Tata P. V., Christensen R. B., Lawrence C. W. UmuC function is not essential for the production of all targeted lacI mutations induced by ultraviolet light. J Mol Biol. 1988 Oct 5;203(3):635–641. doi: 10.1016/0022-2836(88)90198-2. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O., Livneh Z. Biochemical analysis of UV mutagenesis in Escherichia coli by using a cell-free reaction coupled to a bioassay: identification of a DNA repair-dependent, replication-independent pathway. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3300–3304. doi: 10.1073/pnas.89.8.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Escarceller M., Hicks J., Gudmundsson G., Trump G., Touati D., Lovett S., Foster P. L., McEntee K., Goodman M. F. Involvement of Escherichia coli DNA polymerase II in response to oxidative damage and adaptive mutation. J Bacteriol. 1994 Oct;176(20):6221–6228. doi: 10.1128/jb.176.20.6221-6228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L., Yeung A. T. The UvrABC endonuclease system of Escherichia coli--a view from Baltimore. Mutat Res. 1990 Sep-Nov;236(2-3):213–221. doi: 10.1016/0921-8777(90)90006-q. [DOI] [PubMed] [Google Scholar]
- Hughes A. J., Jr, Bryan S. K., Chen H., Moses R. E., McHenry C. S. Escherichia coli DNA polymerase II is stimulated by DNA polymerase III holoenzyme auxiliary subunits. J Biol Chem. 1991 Mar 5;266(7):4568–4573. [PubMed] [Google Scholar]
- Iwasaki H., Nakata A., Walker G. C., Shinagawa H. The Escherichia coli polB gene, which encodes DNA polymerase II, is regulated by the SOS system. J Bacteriol. 1990 Nov;172(11):6268–6273. doi: 10.1128/jb.172.11.6268-6273.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Kow Y. W., Faundez G., Hays S., Bonner C. A., Goodman M. F., Wallace S. S. Absence of a role for DNA polymerase II in SOS-induced translesion bypass of phi X174. J Bacteriol. 1993 Jan;175(2):561–564. doi: 10.1128/jb.175.2.561-564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Gibbs P. E., Borden A., Horsfall M. J., Kilbey B. J. Mutagenesis induced by single UV photoproducts in E. coli and yeast. Mutat Res. 1993 May;299(3-4):157–163. doi: 10.1016/0165-1218(93)90093-s. [DOI] [PubMed] [Google Scholar]
- Lawrence C. The RAD6 DNA repair pathway in Saccharomyces cerevisiae: what does it do, and how does it do it? Bioessays. 1994 Apr;16(4):253–258. doi: 10.1002/bies.950160408. [DOI] [PubMed] [Google Scholar]
- Livneh Z., Cohen-Fix O., Skaliter R., Elizur T. Replication of damaged DNA and the molecular mechanism of ultraviolet light mutagenesis. Crit Rev Biochem Mol Biol. 1993;28(6):465–513. doi: 10.3109/10409239309085136. [DOI] [PubMed] [Google Scholar]
- Maki S., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. II. A novel complex including the gamma subunit essential for processive synthesis. J Biol Chem. 1988 May 15;263(14):6555–6560. [PubMed] [Google Scholar]
- Masker W., Hanawalt P., Shizuya H. Role of DNA polymerase II in repair replication in Escherichia coli. Nat New Biol. 1973 Aug 22;244(138):242–243. doi: 10.1038/newbio244242a0. [DOI] [PubMed] [Google Scholar]
- McHenry C. S., Crow W. DNA polymerase III of Escherichia coli. Purification and identification of subunits. J Biol Chem. 1979 Mar 10;254(5):1748–1753. [PubMed] [Google Scholar]
- Miura A., Tomizawa J. I. Studies on radiation-sensitive mutants of E. coli. 3. Participation of the rec system in induction of mutation by ultraviolet irradiation. Mol Gen Genet. 1968;103(1):1–10. doi: 10.1007/BF00271151. [DOI] [PubMed] [Google Scholar]
- Modrich P. Mismatch repair, genetic stability, and cancer. Science. 1994 Dec 23;266(5193):1959–1960. doi: 10.1126/science.7801122. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. E. Accessory proteins bind a primed template and mediate rapid cycling of DNA polymerase III holoenzyme from Escherichia coli. J Biol Chem. 1987 Dec 5;262(34):16558–16565. [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Sancar A. Mechanisms of DNA excision repair. Science. 1994 Dec 23;266(5193):1954–1956. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- Sancar G. B. DNA photolyases: physical properties, action mechanism, and roles in dark repair. Mutat Res. 1990 Sep-Nov;236(2-3):147–160. doi: 10.1016/0921-8777(90)90002-m. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L., Glickman B. W. Mechanisms of ultraviolet-induced mutation. Mutational spectra in the Escherichia coli lacI gene for a wild-type and an excision-repair-deficient strain. J Mol Biol. 1987 Nov 20;198(2):187–202. doi: 10.1016/0022-2836(87)90305-6. [DOI] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Structure and function of the (A)BC excinuclease of Escherichia coli. Mutat Res. 1990 Sep-Nov;236(2-3):203–211. doi: 10.1016/0921-8777(90)90005-p. [DOI] [PubMed] [Google Scholar]
- Shwartz H., Shavitt O., Livneh Z. The role of exonucleolytic processing and polymerase-DNA association in bypass of lesions during replication in vitro. Significance for SOS-targeted mutagenesis. J Biol Chem. 1988 Dec 5;263(34):18277–18285. [PubMed] [Google Scholar]
- Steinborn G. Uvm mutants of Escherichia coli K12 deficient in UV mutagenesis. I. Isolation of uvm mutants and their phenotypical characterization in DNA repair and mutagenesis. Mol Gen Genet. 1978 Sep 20;165(1):87–93. doi: 10.1007/BF00270380. [DOI] [PubMed] [Google Scholar]
- Studwell-Vaughan P. S., O'Donnell M. DNA polymerase III accessory proteins. V. Theta encoded by holE. J Biol Chem. 1993 Jun 5;268(16):11785–11791. [PubMed] [Google Scholar]
- Stukenberg P. T., Studwell-Vaughan P. S., O'Donnell M. Mechanism of the sliding beta-clamp of DNA polymerase III holoenzyme. J Biol Chem. 1991 Jun 15;266(17):11328–11334. [PubMed] [Google Scholar]
- Tait R. C., Harris A. L., Smith D. W. DNA repair in Escherichia coli mutants deficient in DNA polymerases I, II and-or 3. Proc Natl Acad Sci U S A. 1974 Mar;71(3):675–679. doi: 10.1073/pnas.71.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I. UV-induced mutagenesis of phage S13 can occur in the absence of the RecA and UmuC proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6614–6618. doi: 10.1073/pnas.82.19.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Levy M., Sancar A. Amplification and purification of UvrA, UvrB, and UvrC proteins of Escherichia coli. J Biol Chem. 1985 Aug 15;260(17):9875–9883. [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. The mutability toward ultraviolet light of recombination-deficient strains of Escherichia coli. Mutat Res. 1969 Jul-Aug;8(1):9–14. doi: 10.1016/0027-5107(69)90135-3. [DOI] [PubMed] [Google Scholar]
- Woodgate R. Construction of a umuDC operon substitution mutation in Escherichia coli. Mutat Res. 1992 Mar;281(3):221–225. doi: 10.1016/0165-7992(92)90012-7. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Yoakum G. H., Grossman L. The purification of the Escherichia coli UvrABC incision system. Nucleic Acids Res. 1986 Nov 11;14(21):8535–8556. doi: 10.1093/nar/14.21.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]