Abstract
An important aspect of goal-directed action selection is differentiating between actions that are more or less likely to be reinforced. With repeated performance or psychostimulant exposure, however, actions can assume stimulus-elicited—or “habitual”—qualities that are resistant to change. We show that selective knockdown of prelimbic prefrontal cortical Brain-derived neurotrophic factor (Bdnf) increases sensitivity to response–outcome associations, blocking habit-like behavioral inflexibility. A history of adolescent cocaine exposure, however, occludes the “beneficial” effects of Bdnf knockdown. This finding highlights a challenge in treating addiction—that drugs of abuse may bias decision-making toward habit systems even in individuals with putative neurobiological resiliencies.
Both humans and rodents can learn to associate specific actions with their outcomes. Such actions are considered “goal-directed,” meaning their performance is sensitive to changes in the predictive relationship between the behavior and its outcome. Extended training, certain reinforcement schedules, and drugs of abuse can induce a shift from goal-directed to automated, or “habitual,” response strategies that are defined by insensitivity to changes in the contingency between the behavior and the outcome (Yin et al. 2008; Balleine and O'Doherty 2010). Within the prefrontal cortex, the prelimbic subregion is intimately linked with goal-directed decision-making. For example, rodents with prelimbic-selective lesions are insensitive to changes in outcome value or modifications in response–outcome associative contingencies (Balleine and Dickinson 1998; Corbit and Balleine 2003; Killcross and Coutureau 2003).
Under some conditions, overexpression of the neurotrophin BDNF within the prelimbic cortex also confers habit-like behavioral inflexibility (Graybeal et al. 2011; Gourley et al. 2012). These findings predict that site-selective Brain-derived neurotrophic factor (Bdnf) knockdown might, conversely, augment goal-directed decision-making. Moreover, site-selective knockdown may protect against habits that result from pathological stimuli such as cocaine. This is critical because stimulus–response habits are considered etiological factors in addiction (Jentsch and Taylor 1999; Everitt and Robbins 2005).
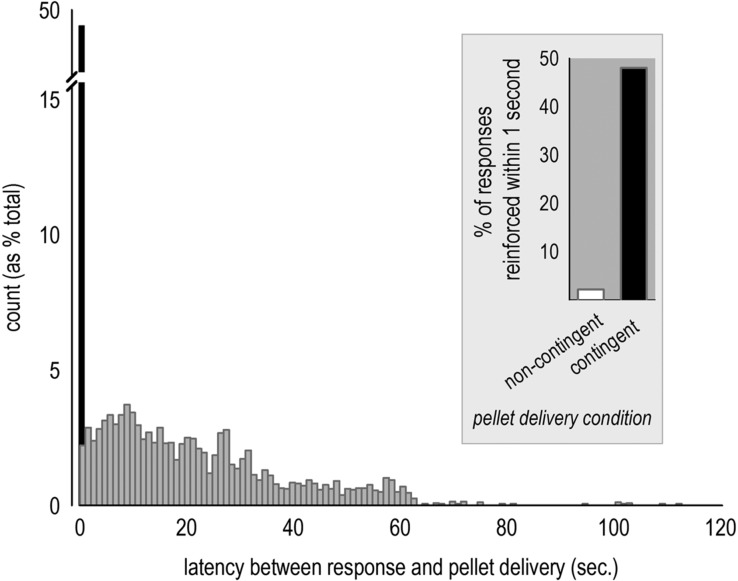
An important aspect of goal-directed decision-making is differentiating between actions that are more or less likely to be reinforced. Here we trained BALB/c mice to respond for food pellets on two distinct nose poke apertures using a continuous reinforcement schedule. We then modified the schedule such that ∼50% of the responses on one aperture were reinforced 1 sec after the response. Responding on the other aperture had no consequences; instead, food pellets were delivered noncontingently, “for free,” at a rate matched to that of the prior session (Gourley et al. 2013a,b). In this case, only ∼2.2% of pellets were delivered within 1 sec after a response (Fig. 1). We used these testing parameters (further elaborated below) to ascertain whether targeted Bdnf knockdown would enhance outcome-based decision-making in both drug-naïve and cocaine-exposed mice.
Figure 1.
The latency between a response and subsequent pellet delivery varies significantly when the instrumental contingency is abolished. Latencies between responses and pellet deliveries varied substantially when pellet delivery was not contingent upon an animal's response, ranging from <1 sec to nearly 2 min (gray bars). In the background, a black bar represents the percentage of responses that were reinforced within 1 sec of responding during test sessions when pellet delivery was contingent upon an animal's response. (Inset) On average, a pellet was delivered within 1 sec of a response only 2.2% of the time during noncontingent, or “degraded,” test sessions. By contrast, 48% of responses were reinforced within 1 sec during contingent test sessions. The mice represented here are control GFP-expressing mice from the experiments represented in Figures 2 and 3.
Our experiments used Emory University IACUC-approved procedures and male mice homozygous for a floxed allele (exon 5) encoding the Bdnf gene (Jackson Labs) (Rios et al. 2001). Mice were bred on a mixed BALB/c background, maintained on a 12-h light cycle (07.00 on), and provided food and water ad libitum unless otherwise noted.
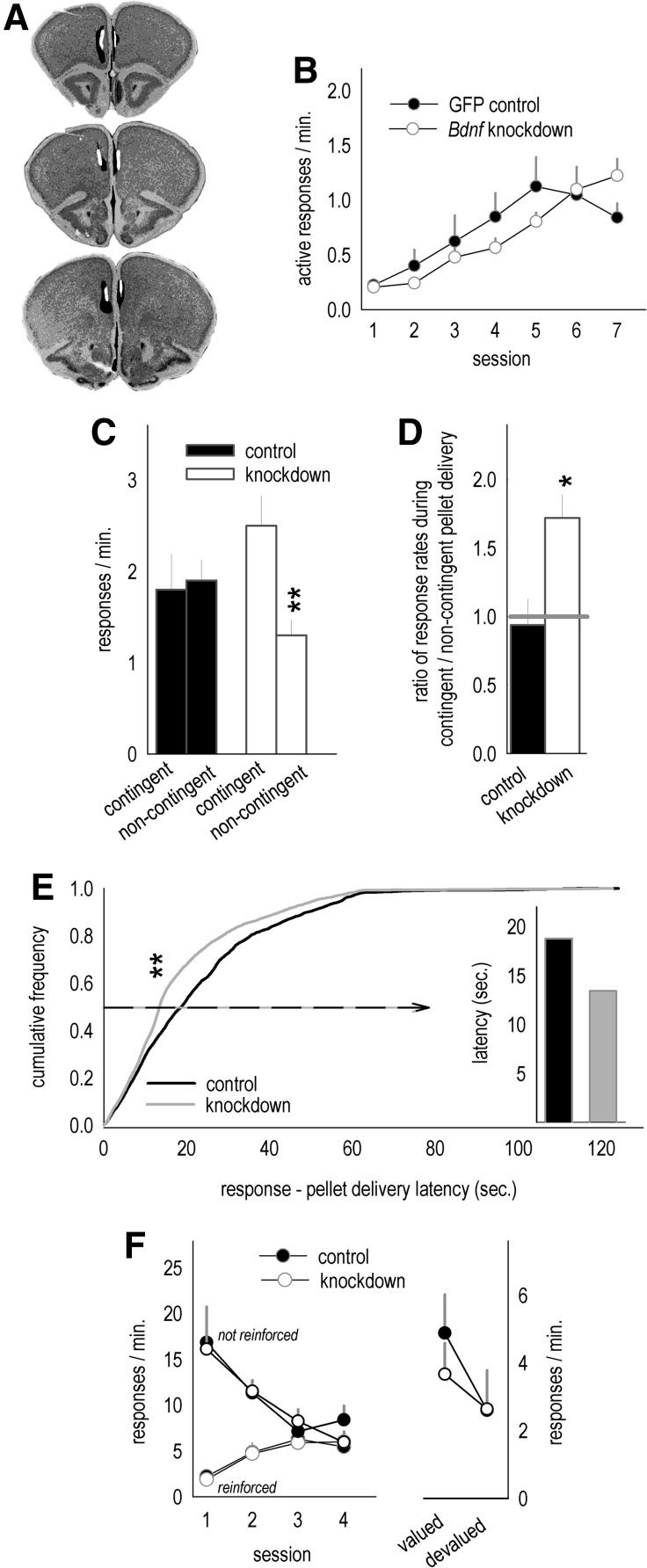
As adults aged ≥8 wk, mice were anesthetized with ketamine/xylazine, and stereotaxic coordinates corresponding to the prelimbic prefrontal cortex were located on the leveled skull. A single burr hole was drilled, and lentiviral vectors expressing either Cre Recombinase or GFP were infused (0.5 μL) at +2.0 AP, −2.8 DV, ±0.1 ML (Gourley et al. 2009) over 5 min with needles left in place for five additional minutes. Mice were sutured and provided a recovery period of ≥3 wk, allowing for Bdnf knockdown. This protocol is expected to reduce BDNF protein at the infusion site by ∼48% (Gourley et al. 2009). Infusion sites were verified by immunostaining for Cre (DePoy et al. 2013) or by imaging GFP. Mice lacking prelimbic cortex infection (n = 10) were excluded. Otherwise, viral vector infection was largely restricted to the prelimbic cortex with some spread into the medial orbitofrontal cortex in the rostral-most sections (Fig. 2A).
Figure 2.
Prelimbic cortex-targeted Bdnf knockdown increases sensitivity to the relationship between a response and its outcome. (A) Viral vector infection sites were transposed onto images from the Mouse Brain Atlas (Rosen et al. 2000). GFP or Cre were detected either slightly offset from the midline (represented in the left hemisphere) or directly adjacent to the midline (represented in the right hemisphere). Black represents the largest and white the smallest infusion sites. (B) Instrumental response acquisition was unaffected by Bdnf knockdown in BALB/c mice. (C) A comparison of response rates during test sessions when pellets were delivered contingently vs. noncontingently indicated that control mice were acutely insensitive to modifications in the predictive relationship between a response and an outcome. By contrast, Bdnf knockdown mice showed precocious sensitivity, generating higher response rates when responding was more likely to be reinforced. (D) Response rates were also converted to ratios: Here, knockdown mice generated ∼1.7-fold more responses during the test session when responding was more likely to be reinforced. (E) Additionally, when pellets were delivered noncontingently at a fixed rate, responses generated by knockdown mice were more closely aligned in time with pellet delivery. (Inset) The average latencies between responses and noncontingent pellet deliveries at the 50th percentile are indicated for each group. (F) When a previously inactive aperture became active, mice acquired the new response and inhibited the previously reinforced response, but here no differences were detected between groups. Moreover, response rates dropped in both groups after prefeeding devaluation of the food outcome. Bars and symbols = means + SEMs, (*) P ≤ 0.05, (**) P ≤ 0.001.
Mice were food-restricted and trained to nose poke for food reinforcement (20-mg grain-based pellets; Bioserv) using Med-Associates conditioning chambers with three nose poke recesses and a food magazine located on the opposite wall of the chamber. Training was initiated with a continuous reinforcement schedule; 30 pellets were available for responding on the two outermost recesses, resulting in 60 pellets/session. Sessions ended when all 60 pellets were delivered, or at 70 min. Seven daily sessions were conducted, during which mice acquired the responses. Response rates did not differ between groups (P > 0.2) (Fig. 2B).
The response–outcome contingencies were next modified during two 25-min test sessions. In one session, one aperture was occluded, and responding on the other aperture was reinforced using a modified variable ratio 2 schedule of reinforcement with the added requirement that mice retrieve each pellet before acquiring more (Baldwin et al. 2002). In this case, 48% (±3%) of responses in the control mice and 47% (±2%) of responses in Bdnf knockdown mice were reinforced (P > 0.1) (not shown). In the other session, the opposite aperture was occluded, and pellets were delivered at a constant rate such that the same number of pellets acquired (per minute) during the prior session were delivered noncontingently.
Noncontingent pellet delivery sessions were counter-balanced, meaning half were coupled to the final day of training and half to the contingent pellet delivery session. Pellet delivery rates did not differ between groups (control = 1/min, knockdown = 0.97/min, P > 0.1) (not shown). Nonetheless, when response rates were compared between the two reinforcement conditions, knockdown mice showed precocious sensitivity to degradation of the instrumental contingency, i.e., higher response rates when responding were more likely to be reinforced. Meanwhile, control BALB/c mice did not acutely differentiate between actions that were more vs. less predictive of reinforcement (interaction F(1,12) = 6.9, P = 0.02) (Fig. 2C). When response rates were expressed as ratios, control mice had a ratio of ∼1—equivalent responding between conditions. By contrast, knockdown mice generated ∼1.7-fold more responses when responding was reinforced vs. nonreinforced (t(12) = −3, P = 0.01) (Fig. 2D). Additionally, the latencies between responses and noncontingent pellet deliveries were shorter in knockdown mice (Kolmogorov–Smirnov P < 0.001), suggestive of heightened sensitivity to the constant rate of pellet delivery (Fig. 2E).
As a different measure of sensitivity to modifications in response–outcome associative contingencies, we stopped reinforcing responding on the outermost nose poke apertures and reinforced responding on the center, previously inactive, aperture for four 25-min sessions. Here, there were no differences between groups (F’s < 1) (Fig. 2F). We also devalued the outcome by placing mice in a clean cage with freely available pellets for 30 min prior to test. Relative to a control test session in which the cage contained no food, both groups decreased response rates, demonstrating sensitivity to the decreased value of the outcome (Fig. 2F). We again detected no differences between groups (F’s < 1).
Interactions between Bdnf knockdown and cocaine exposure
Figure 2 indicates that prelimbic-targeted Bdnf knockdown enhances animals’ ability to differentiate between actions that are more, or less, predictive of reinforcement. By contrast, prolonged cocaine exposure results in decision-making strategies biased toward stimulus-elicited habits (Schoenbaum and Setlow 2005; Zapata et al. 2010). Both chronic cocaine exposure in adulthood and a history of early-life exposure increase BDNF and Bdnf expression in the mature medial prefrontal cortex (Lu et al. 2010; McGinty et al. 2010; Sadri-Vakili et al. 2010); if this incubation confers vulnerability to cocaine-induced habits, targeted knockdown might rescue this deficiency.
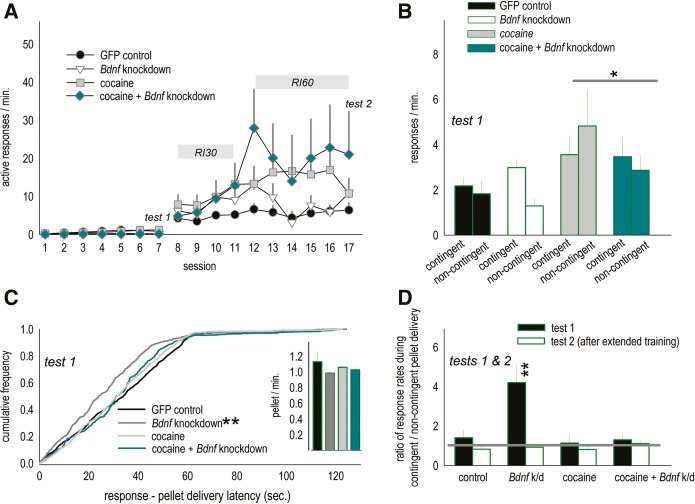
We next replicated our experiment, except here mice were exposed to either cocaine (10 mg/kg, i.p.; Sigma) or PBS from postnatal day (P) 31–35, corresponding to early adolescence in mice (Spear 2000). At P56, mice were infused with viral vectors, resulting in a 2 × 2 experimental design. Mice were trained to nose poke for food reinforcers, and then response–outcome contingencies were modified as described. Next, we further trained these mice using random interval (RI) schedules of reinforcement as indicated (Fig. 3A). This allowed us to ascertain whether mice with selective Bdnf knockdown could, with extended training, ultimately develop insensitivity to modifications in outcome-predictive contingencies (i.e., habit-like inflexibility) as expected (Dickinson et al. 1983).
Figure 3.
Adolescent cocaine exposure occludes Bdnf-mediated behavioral plasticity. (A) BALB/c mice were exposed to cocaine or saline prior to Bdnf knockdown. Mice were later trained to respond for food reinforcers, then the response–outcome contingencies were modified as above. After an initial test, mice were shifted from a continuous reinforcement schedule to random interval (RI) schedules of reinforcement as indicated. A second test is annotated as “test 2.” (B) Response rates during different pellet delivery conditions during the initial test are shown: A history of cocaine exposure nonspecifically increased response rates, regardless of whether pellet delivery was contingent or noncontingent upon responding. (C) When pellets were delivered noncontingently at a fixed rate, responses generated by knockdown mice were temporally more closely aligned with pellet delivery (as in Fig. 2E). Prior cocaine exposure blocked this effect. This was despite pellet delivery rates that did not differ between groups (inset). (D) Response ratios indicated that drug-naïve Bdnf knockdown mice were highly sensitive to response–outcome contingencies (black bars, as in Fig. 2D); however, a history of cocaine exposure entirely occluded the effect of targeted Bdnf knockdown, resulting in insensitivity to degradation of the instrumental contingency. With extended training, all mice ultimately developed habitual response strategies, as annotated by white bars and the gray line at 1. Thus, Bdnf knockdown in drug-naïve mice delays, but does not fully block, habit formation. Bars and symbols = means + SEMs, (*) P ≤ 0.05, (**) P ≤ 0.001 vs. all other groups. (k/d) Knockdown, (RI30) random interval 30 sec, (RI60) random interval 60 sec.
Instrumental response training is shown; here, a trend for an effect of cocaine was detected (P = 0.07) (Fig. 3A), and a history of adolescent cocaine exposure had several significant consequences as well: It nonspecifically increased response rates at test (F(1,26) = 4.2, P ≤ 0.05) (Fig. 3B), and it also blocked the attenuating effect of Bdnf knockdown on the latencies between responding and noncontingent pellet delivery (Kolmogorov–Smirnov P < 0.001) (Fig. 3C).
When we generated response ratios as above and compared response patterns with test session, cocaine history, and Bdnf status as factors, cocaine history and Bdnf status interacted (F(1,26) = 5.3, P < 0.05) (Fig. 3D). Bdnf knockdown in drug-naïve mice increased sensitivity to response–outcome contingencies, as in Figure 2C, D, but a history of cocaine exposure entirely occluded the “beneficial” effects of Bdnf silencing—instead, cocaine-exposed Bdnf-deficient mice were insensitive to modifications in the response–outcome associative contingencies, indicated by a response ratio of ∼1 even during the initial test (Fig. 3D). Notably, because the contingent and the noncontingent test sessions were counter-balanced, half of the noncontingent pellet delivery rates were matched to the final day of continuous reinforcement training, and half to the contingent test session. Our findings are thus unlikely to be biased by differences in noncontingent food delivery rates, since counter-balancing eliminated differences in noncontingent pellet delivery rates across groups (F’s < 1) (Fig. 3C, inset).
With extended training, all mice ultimately developed habit-like response strategies, indicated by response ratios of ∼1 during a “late test” (Fig. 3D). This pattern indicates that, although Bdnf knockdown increases sensitivity to response–outcome contingencies, Bdnf knockdown mice are nonetheless capable of developing habitual response strategies with extensive training.
Coordination of action selection by medial prefrontal cortical BDNF
Our findings show that Bdnf knockdown within the prelimbic prefrontal cortex enhances sensitivity to modifications in the predictive relationship between an action and an outcome. Infection sites here were generally more restricted to the prelimbic compartment of the medial prefrontal cortex than in our previous reports regarding the effects of prelimbic cortical Bdnf knockdown on stimulus–outcome and response–outcome conditioning (Gourley et al. 2009, 2012; Choi et al. 2012). These reports together suggest that BDNF expression within the prelimbic cortex, selectively, plays a critical role in appetitive decision-making. And notably, while Bdnf knockdown inhibited nonreinforced responses here, the nonreinforced responses that were nonetheless generated were temporally closely aligned with noncontingent pellet delivery. This observation suggests that Bdnf knockdown increased sensitivity to the regular intervals of noncontingent pellet delivery.
The rodent medial prefrontal cortex contains cytoarchitectonically distinct subregions that can be differentiated based on efferent and afferent projection patterns, with dorsal regions—including the prelimbic cortex—sharing similar functions that differ from those of the ventromedial prefrontal cortex, which includes, for example, the infralimbic cortex. Behaviorally, dorsal/ventral medial prefrontal cortical networks have been termed “go” and “stop” systems, respectively, that coincidentally guide behavior (Heidbreder and Groenewegen 2003). For example, the prelimbic cortex is essential for maintaining instrumental responding for food when reinforcement availability is uncertain (Corbit and Balleine 2003; Gourley et al. 2010), and for outcome-based decision-making, as discussed at the beginning of this report. By contrast, recent models argue that the adjacent infralimbic cortex promotes stimulus–response habits by mitigating the influence of contextual cues that are incongruent with the habit, promoting, for example, the performance of a familiar stimulus-elicited behavior even in an unfamiliar environment (Haddon and Killcross 2011).
Unlike the infralimbic cortex, the prelimbic cortex sends substantial projections to the dorsomedial striatum (Heidbreder and Groenewegen 2003), a structure essential for outcome-based decision-making (Yin et al. 2005). Through these interactions, the prelimbic cortex is thought to organize goal-directed decision-making in both rodents and humans (Balleine and O'Doherty 2010). Nonetheless, prelimbic-selective Bdnf knockdown facilitated goal-directed decision-making by increasing sensitivity to modifications in the predictive relationship between a response and an outcome. These findings extend our prior work indicating that mixed C57BL/6 mice with Bdnf knockdown have intact consolidation of response–outcome conditioning (Gourley et al. 2012); because of strain differences, the use of BALB/c mice here allowed us the resolution to detect evidence that the acquisition of novel response–outcome contingencies is enhanced.
Our findings are also in line with evidence that in healthy mice, BDNF overexpression blocks sensitivity to changes in response–outcome associations (Gourley et al. 2012). Why might supraphysiological BDNF occlude outcome-based decision-making? Supraphysiological levels of BDNF induce structural destabilization and neuronal remodeling (Horch et al. 1999; Horch and Katz 2002), as well as activation of cortical interneurons (Rutherford et al. 1998), potentially negating the native function of excitatory prelimbic cortical neurons. According to this model, physiological BDNF levels would support goal-directed decision-making more effectively than supraphysiological levels.
Why might Bdnf knockdown enhance sensitivity to response–outcome contingencies? Gourley et al. (2009) previously reported that prelimbic cortical Bdnf knockdown facilitates appetitive extinction conditioning. We argued then that prelimbic Bdnf knockdown allows for the dominance of competing infralimbic cortex-based behavioral response strategies since the infralimbic cortex is strongly associated with response extinction. However, the infralimbic cortex is also associated with stimulus–response habits (Coutureau and Killcross 2003; Haddon and Killcross 2007), and mice here appeared resilient to developing habits. How might we reconcile these findings? It may be that the unique infralimbic cortex contributions to extinction learning or sensitivity to reinforcer delivery (see Burgos-Robles et al. 2013) supersede its role in promoting stimulus–response habits, particularly when animals have not been so extensively trained as to develop habits. This interpretation is consistent with our findings here, potentially including heightened sensitivity to the timing of noncontingent pellet delivery in Bdnf knockdown mice. Also, prelimbic cortical Bdnf-deficient mice generate lower break points in a progressive ratio task (Gourley et al. 2012), which by nature results in diminishing reinforcer availability.
One caveat is the failure of Bdnf knockdown to impact response extinction in an instrumental redirection—or “reversal”—task. Here and previously (Gourley et al. 2009), both response acquisition and extinction were unaffected. Both cortical and hippocampal regions coordinate response selection in this task (Gourley et al. 2010), so it is possible that recruitment of the hippocampus ensures optimal response flexibility.
Conclusions: medial prefrontal cortical BDNF—a marker of resilience or vulnerability?
As in prior studies focused on cocaine-induced incubation of medial prefrontal cortical BDNF (Lu et al. 2010), we exposed mice to cocaine early in life, roughly corresponding to early adolescence in humans (Spear 2000), then tested them in adulthood. Although it is difficult to directly compare our findings with experiments reported by other groups, previous work anticipates that medial prefrontal cortical BDNF expression may be up to ∼140% of control levels in our cocaine-exposed mice at the time of knockdown in adulthood (Lu et al. 2010). We hypothesized that mitigating this incubation response via targeted Bdnf knockdown in adulthood would protect cocaine-exposed mice against developing habits. However, this was not the case: We report instead that a history of cocaine exposure occluded the behavioral benefits of Bdnf knockdown. Thus, cocaine may increase vulnerability to habit formation via BDNF incubation in even small populations of neurons, in this case, prelimbic neurons that were spared viral vector infection. Alternatively, BDNF incubation may irreversibly remodel adolescent cortical development (Vigers et al. 2012).
Additionally, our findings do not eliminate the possibility that drug-induced incubation of prefrontal cortical BDNF has both adverse and neuroprotective consequences (Corominas et al. 2007). Consistent with the possibility that incubation of medial prefrontal cortical BDNF has certain protective effects, prelimbic cortical Bdnf knockdown increases progressive ratio responding for cocaine in cocaine-experienced rats (Sadri-Vakili et al. 2010; see also Gourley et al. 2012). Further, BDNF overexpression in the dorsomedial prefrontal cortex (enhancing BDNF incubation) suppresses the reinstatement of cocaine seeking (Berglind et al. 2007; McGinty et al. 2010). These and the present findings highlight a major challenge in treating addiction—that a clearer delineation of cocaine-induced neuromodulations associated with vulnerability vs. resilience is needed, and that these dissociations may result in improved treatment strategies.
Acknowledgments
We thank Amanda Allen for her assistance and members of the Gourley lab for their feedback. This work was supported by Children's Healthcare of Atlanta, the Emory University Research Council, DA015040 (Kuhar), and DA034808 (Gourley). The Yerkes National Primate Research Center is supported by the Office of Research Infrastructure Programs/OD P51OD11132-53. The Emory Viral Vector Core is supported by an NINDS Core Facilities grant, P30NS055077.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.033290.113.
References
- Baldwin AE, Sadeghian K, Kelley AE 2002. Appetitive instrumental learning requires activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J Neurosci 22: 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A 1998. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37: 407–419 [DOI] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP 2010. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35: 48–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Miller SW, McGinty JF 2007. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci 26: 757–766 [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Bravo-Rivera H, Quirk GJ 2013. Prelimbic and infralimbic neurons signal distinct aspects of appetitive instrumental behavior. PLoS One 8: e57575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Gourley SL, Ressler KJ 2012. Prelimbic BDNF and trkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transl Psychiatry 2: e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW 2003. The role of the prelimbic cortex in instrumental conditioning. Behav Brain Res 146: 145–157 [DOI] [PubMed] [Google Scholar]
- Corominas M, Roncero C, Ribases M, Castells X, Casas M 2007. Brain-derived neurotrophic factor and its intracellular signaling pathways in cocaine addiction. Neuropsychobiology 55: 2–13 [DOI] [PubMed] [Google Scholar]
- Coutureau E, Killcross S 2003. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res 146: 167–174 [DOI] [PubMed] [Google Scholar]
- DePoy LM, Noble B, Allen AG, Gourley SL 2013. Developmentally divergent effects of ρ-kinase inhibition on cocaine- and BDNF-induced behavioral plasticity. Behav Brain Res 243: 171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Nicholas DJ, Adams CD 1983. The effect of the instrumental training contingency on susceptibility to reinforce devaluation. Q J Exp Psychol 35B: 35–51 [Google Scholar]
- Everitt BJ, Robbins TW 2005. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489 [DOI] [PubMed] [Google Scholar]
- Gourley SL, Howell JL, Rios M, DiLeone RJ, Taylor JR 2009. Prelimbic cortex bdnf knock-down reduces instrumental responding in extinction. Learn Mem 16: 756–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR 2010. Dissociable regulation of goal-directed action within mouse prefrontal cortex. Eur J Neurosci 32: 1726–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, DiLeone RJ, Koleske AJ, Taylor JR 2012. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci 109: 20714–20719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Zimmermann KS, Ressler KJ, DiLeone RJ, Taylor JR 2013a. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur J Neurosci 38: 2382–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Gordon J, Taylor JR 2013b. Cytoskeletal determinants of stimulus–response habits. J Neurosci 33: 11811–11816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A 2011. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci 14: 1507–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon JE, Killcross S 2007. Contextual control of choice performance: behavioral neurobiological and neurochemical influences. Ann N Y Acad Sci 1104: 250–269 [DOI] [PubMed] [Google Scholar]
- Haddon JE, Killcross S 2011. Inactivation of the infralimbic prefrontal cortex in rats reduces the influence of inappropriate habitual responding in a response-conflict task. Neuroscience 199: 205–212 [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ 2003. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27: 555–579 [DOI] [PubMed] [Google Scholar]
- Horch HW, Katz LC 2002. BDNF released from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci 5: 1177–1184 [DOI] [PubMed] [Google Scholar]
- Horch HW, Krüttgen A, Portbury SD, Katz LC 1999. Destabilization of cortical dendrites and spines by BDNF. Neuron 23: 353–364 [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR 1999. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146: 373–390 [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E 2003. Coordination of actions and habits in the medial prefrontal cortex in rats. Cereb Cortex 13: 400–408 [DOI] [PubMed] [Google Scholar]
- Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM 2010. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron 67: 821–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Berglind WJ 2010. Brain-derived neurotrophic factor and cocaine addiction. Brain Res 1314: 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R 2001. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol 15: 1748–1757 [DOI] [PubMed] [Google Scholar]
- Rosen G, Williams AG, Capra JA, Connolly MT, Cruz B, Lu L, Airey DC, Kulkarni K, Williams RW 2000. The mouse brain library @ www.Mbl.Org. International Mouse Genome Conference 14: 166 [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG 1998. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron 21: 521–530 [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, et al. 2010. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci 30: 11735–11744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B 2005. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal–amygdalar function. Cereb Cortex 15: 1162–1169 [DOI] [PubMed] [Google Scholar]
- Spear LP 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24: 417–463 [DOI] [PubMed] [Google Scholar]
- Vigers AJ, Amin DS, Talley-Farnham T, Gorski JA, Xu B, Jones KR 2012. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience 212: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW 2005. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 22: 513–523 [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW 2008. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of the cortico-basal ganglia networks. Eur J Neurosci 28: 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS 2010. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci 30: 15457–15463 [DOI] [PMC free article] [PubMed] [Google Scholar]