Abstract
The proper regulation of translation is required for the expression of long-lasting synaptic plasticity. A major site of translational control involves the phosphorylation of eukaryotic initiation factor 2 α (eIF2α) by PKR-like endoplasmic reticulum (ER) kinase (PERK). To determine the role of PERK in hippocampal synaptic plasticity, we used the Cre-lox expression system to selectively disrupt PERK expression in the adult mouse forebrain. Here, we demonstrate that in hippocampal area CA1, metabotropic glutamate receptor (mGluR)-dependent long-term depression (LTD) is associated with increased eIF2α phosphorylation, whereas stimulation of early- and late-phase long-term potentiation (E-LTP and L-LTP, respectively) is associated with decreased eIF2α phosphorylation. Interesting, although PERK-deficient mice exhibit exaggerated mGluR-LTD, both E-LTP and L-LTP remained intact. We also found that mGluR-LTD is associated with a PERK-dependent increase in eIF2α phosphorylation. Our findings are consistent with the notion that eIF2α phosphorylation is a key site for the bidirectional control of persistent forms of synaptic LTP and LTD and suggest a distinct role for PERK in mGluR-LTD.
De novo synthesis of proteins is critical for the expression of long-lasting synaptic plasticity (Kelleher et al. 2004; Costa-Mattioli et al. 2009; Richter and Klann 2009). Protein synthesis is highly regulated at the level of initiation by numerous translational control molecules, including the eukaryotic initiation factor 2 (eIF2) (Sonenberg and Dever 2003). Although phosphorylation of the α subunit of eIF2 inhibits general translation, it selectively stimulates the translation of the transcriptional modulator activating transcription factor 4 (ATF4) (Vattem and Wek 2004), which plays a role as a repressor of cAMP response element-binding protein (CREB)-mediated synaptic plasticity (Bartsch et al. 1995; Abel et al. 1998; Chen et al. 2003). Previously, it was shown that reduction of eIF2α phosphorylation in mice lacking the eIF2α kinase general control nonderepressible 2 (GCN2) and in heterozygous knockin mice with a mutation on serine 51 of eIF2α results in a lowered threshold for inducing long-lasting late-phase long-term potentiation (L-LTP) (Costa-Mattioli et al. 2005, 2007). In addition, mice harboring a deletion of the double-stranded (ds) RNA-activated protein kinase (PKR) show a similar decrease in threshold for inducing L-LTP (Zhu et al. 2011). Conversely, increased eIF2α phosphorylation in transgenic mice overexpressing PKR causes increased expression of ATF4 and impaired L-LTP (Jiang et al. 2010). Collectively, these findings suggest that the proper regulation of eIF2α phosphorylation is required for normal synaptic plasticity.
The PKR-like ER kinase (PERK) is a highly conserved protein kinase that phosphorylates eIF2α to mediate translational control in response to ER stress (Shi et al. 1998; Harding et al. 2000). Global deletion of PERK in mice impairs development of the skeletal system, postnatal growth, and pancreatic viability (Harding et al. 2001; Zhang et al. 2002; Wei et al. 2008). In humans, mutations of the PERK gene (EIF2AK3) cause Wolcott–Rallison syndrome (WRS), a rare autosomal recessive disorder characterized by early onset diabetes, liver dysfunction, and pancreas insufficiency (Delepine et al. 2000; Rubio-Cabezas et al. 2009; Julier and Nicolino 2010) and, in some cases, mental retardation (Thornton et al. 1997; Delepine et al. 2000; Senee et al. 2004; Reis et al. 2011). Together, these analyses suggest that PERK functions as a critical modulator of a number of cellular processes requiring precise translational control.
Here, we show that PERK is required for the proper expression of protein synthesis-dependent mGluR-LTD, but not L-LTP, at Schaffer–collateral CA1 synapses in hippocampal slices. Moreover, we observed that LTP-inducing stimulation decreases eIF2α phosphorylation, whereas mGluR-LTD is associated with a PERK-dependent increase in eIF2α phosphorylation, demonstrating for the first time that a translation factor is divergently regulated during different protein synthesis-dependent forms of synaptic plasticity. Taken together, our findings suggest that PERK distinctly controls the regulation of eIF2α phosphorylation during mGluR-dependent LTD in the hippocampus.
Results
PERK is expressed in the mouse hippocampus and forebrain-specific disruption of PERK preserves hippocampal morphology
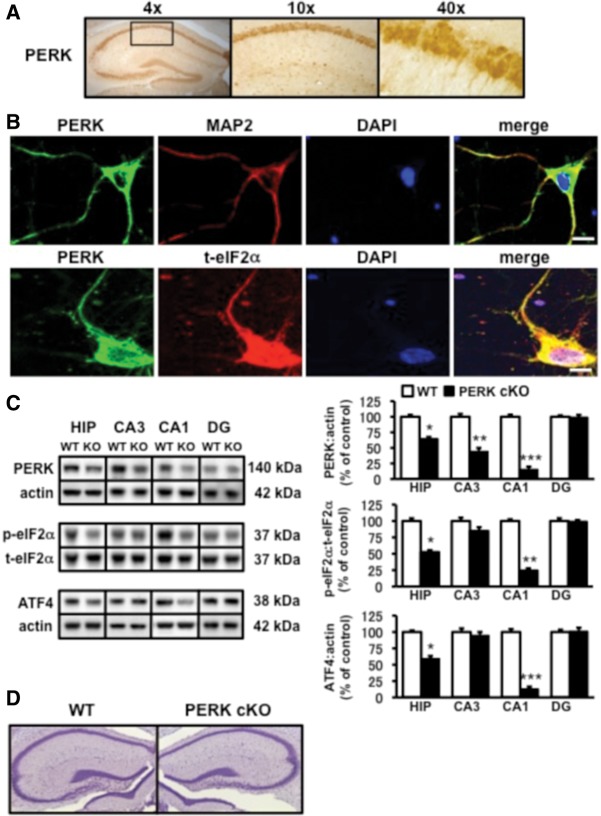
To determine whether PERK is expressed in the mouse hippocampus, we incubated coronal brain sections from 4-wk-old wild-type mice with an antibody specific to PERK and counterstained with diaminobenzidine (DAB). We observed that PERK is abundantly expressed in various regions of the brain, including the dentate gyrus, CA1, and CA3 regions of the hippocampus (Fig. 1A). To determine the subcellular localization and distribution of PERK in hippocampal neurons, we stained cultured hippocampal pyramidal neurons (DIV 9) for PERK and the endogenous dendritic marker, MAP2. We found that PERK staining was present in the cell body and colocalized with MAP2-positive dendrites (Fig. 1B). Similarly, we found that PERK also colocalized with eIF2α in these regions (Fig. 1B). To evaluate the role of PERK in translation-dependent forms of synaptic plasticity in the hippocampus, we employed Cre-lox recombination techniques to generate mice in which PERK activity is disrupted specifically in the adult forebrain (PERK cKO) (Trinh et al. 2012). Western blotting confirmed efficient Cre-mediated reduction of PERK in hippocampal area CA1, which was correlated with decreased levels of phosphorylated eIF2α and ATF4 (Fig. 1C). Despite the reduction of PERK in this region, Nissl-stained sections revealed that PERK cKO mice did not demonstrate gross alterations in hippocampal morphology compared to wild-type mice (Fig. 1D). Together, these findings indicate that PERK is expressed in the soma and dendrites of mouse hippocampal neurons and that a forebrain-specific disruption of PERK at 2–3 wk of age does not dramatically alter hippocampal morphology.
Figure 1.
Localization of PERK in hippocampal neurons and characterization of PERK cKO mice. (A) PERK is highly expressed in the cell body layer of hippocampal area CA1. Representative light micrograph (4× magnification) is shown to the left and the boxed-in area is shown at higher powers (10× and 40× magnification) to the right. (B) PERK is also present in dendrites of hippocampal pyramidal neurons. (Top panel) Representative confocal images show dual staining for PERK (green) and the dendritic marker MAP2 (red). (Lower panel) Hippocampal pyramidal neurons costained with PERK (green) and total eIF2α (t-eIF2α, red). DAPI nuclear stain is shown in blue. Merge of immunofluorescence staining is shown in yellow. Scale bar, 25 μm. (C) Reduction of PERK expression in hippocampal area CA1 of PERK cKO mice is correlated with reduced levels of phosphorylated eIF2α and ATF4. (Left panel) Representative Western blots showing PERK, phospho-eIF2α (p-eIF2α), and ATF4 in the hippocampus and hippocampal subregions from wild-type (WT) and PERK cKO (KO) mice. (Right panel) Quantification of PERK, eIF2α phosphorylation, and ATF4 immunoreactivity. WT, n = 5; cKO, n = 5 ([*] P < 0.05, [**] P < 0.01, [***] P < 0.005, one-way ANOVA). All data represent mean values ± SEM (for all figures). (D) Representative Nissl-staining of hippocampal cell bodies in slices obtained from wild-type (left) and PERK cKO (right) mice show no difference in gross morphology.
PERK cKO mice exhibit normal basal synaptic transmission and LTP
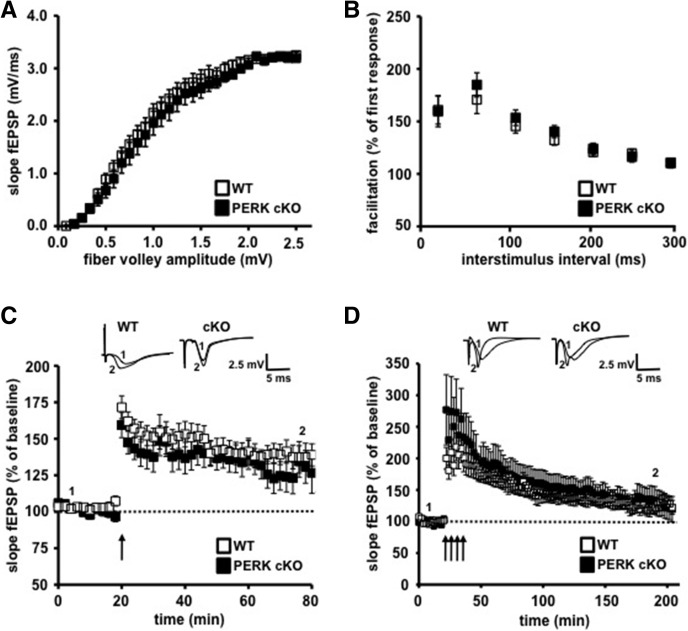
We next investigated whether the disruption of PERK altered hippocampal plasticity by recording fEPSPs in area CA1 of hippocampal slices from PERK mutant and their wild-type littermates. We first examined basal synaptic function in 4- to 5 wk-old mice by eliciting synaptic responses with a range of stimulus intensities and observed similar synaptic input–output relationships in slices from wild-type and PERK cKO mice (Fig. 2A). We next examined paired-pulse facilitation (PPF), a calcium-dependent form of presynaptic plasticity. Similar to the input–output functions, we observed no significant difference in PPF between the two genotypes (Fig. 2B). Together, these findings demonstrate that genetic disruption of the eIF2α kinase PERK does not exert an adverse effect on either basal synaptic transmission or short-lasting presynaptic plasticity. We proceeded to examine LTP in area CA1 of hippocampal slices from PERK mutant and wild-type mice. To our surprise, we found no significant differences in either early phase LTP (E-LTP) or L-LTP between PERK cKO mice and their wild-type littermates (Fig. 2C,D). These findings indicate that genetic disruption of the eIF2α kinase PERK in hippocampal neurons has no effect on the expression of either E-LTP or L-LTP.
Figure 2.
Basal synaptic transmission and early- and late-phase long-term potentiation (LTP) are normal in PERK cKO mice. (A) Input–output relationships plotted as fEPSP slope vs. fiber volley amplitude show no difference in basal synaptic transmission between genotypes. WT, n = 11; cKO, n = 9 (P > 0.05, two-way ANOVA). (B) Paired-pulse facilitation (PPF) is unaltered in PERK cKO mice compared to their wild-type (WT) littermates. WT, n = 11; cKO, n = 9 (P > 0.05, two-way ANOVA). The percentage of facilitation is shown at interpulse intervals ranging from 10 to 300 msec. (C) Early-phase LTP evoked by a single 100-Hz train (1 sec) was unaltered in PERK cKO slices compared to WT slices. WT, n = 7; cKO, n = 7 (P > 0.05, measured 5 min before and 30 min after HFS for both genotypes, two-way ANOVA). (D) Late-phase LTP evoked by four trains of HFS was normal in both genotypes. WT, n = 10; cKO, n = 11 (P > 0.05, measured 5 min before and 1.5 h after HFS, two-way ANOVA). Representative traces are shown in each panel.
PERK cKO mice display enhanced mGluR-LTD that is protein synthesis-dependent
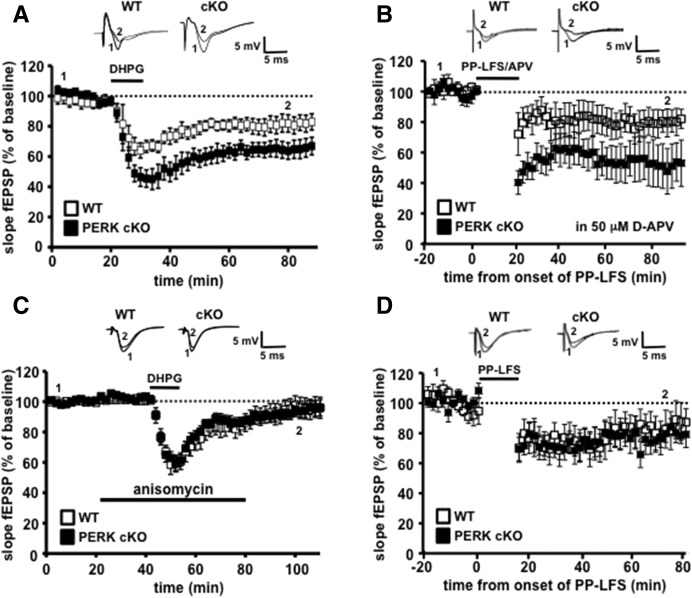
Activation of metabotropic glutamate receptor (mGluR)-dependent signaling is tightly coupled to the regulation of translation in hippocampal neurons (Huber et al. 2000; Aschrafi et al. 2005; Banko et al. 2006; Ronesi and Huber 2008). Because the regulation of translation initiation involves eIF2α phosphorylation by PERK, we determined whether genetic disruption of PERK in the hippocampus impacted mGluR-LTD. Hippocampal slices from PERK cKO and wild-type mice were incubated with DHPG (50 μM, 10 min) to induce protein synthesis-dependent mGluR-LTD. In contrast to our LTP studies, PERK cKO slices exhibited enhanced mGluR-LTD compared to their wild-type littermates (Fig. 3A). Similarly, another paradigm of mGluR-LTD induced by paired-pulse low-frequency stimulation (PP-LFS) in the presence of D-(–)-2-amino-5-phosphonopentanoic acid (D-APV) (Huber et al. 2000), was enhanced in slices from PERK cKO mice when compared to wild-type mice (Fig. 3B). Together, these findings suggest that PERK-directed eIF2α phosphorylation is required for the normal expression of chemically and electrically induced mGluR-LTD.
Figure 3.
PERK cKO mice express enhanced mGluR-LTD that is protein synthesis-dependent. (A) DHPG application (50 µM, 10 min) induced mGluR-LTD in wild-type (WT) slices that was enhanced in PERK cKO slices. WT, n = 8 slices; cKO, n = 8 slices; seven mice per genotype (P < 0.01, two-way ANOVA followed by Bonferroni's post hoc test). (B) mGluR-LTD induced by paired-pulse low-frequency stimulation (PP-LFS) (1-Hz, 50-msec interstimulus interval, 20 min) in the presence of D-APV (50 µM) was enhanced in PERK cKO slices. WT, n = 9 slices; cKO, n = 7 slices (P < 0.05, two-way ANOVA followed by Bonferroni's post hoc test). (C) Measurement of mGluR-LTD in slices from PERK cKO and WT littermates in the presence of anisomycin (40 µM) 20 min prior to, during, and 30 min after DHPG (50 µM, 10 min) treatment indicates that enhanced mGluR-LTD in PERK cKO slices is sensitive to anisomycin. WT, n = 8 slices; cKO, n = 8 slices; seven mice per genotype (P > 0.05, two-way ANOVA). (D) NMDA receptor-dependent LTD induced by paired-pulse low-frequency stimulation (PP-LFS) (1-Hz, 50-msec interstimulus interval, 15 min) was not altered in PERK cKO slices compared with WT slices. WT, n = 9 slices; cKO, n = 12 slices (P > 0.05, two-way ANOVA).
As mentioned above, group I mGluR activation elicits a persistent form of LTD in wild-type mice that requires de novo protein synthesis (Huber et al. 2000; Hou and Klann 2004; Ronesi and Huber 2008). In contrast, in Fmr1 KO mice that lack the translational repressor fragile X mental retardation protein (FMRP), this mGluR-induced LTD is enhanced and independent of new protein synthesis (Hou et al. 2006; Nosyreva and Huber 2006; Sharma et al. 2010). Therefore, we investigated whether the enhanced mGluR-LTD in the PERK cKO mice required de novo protein synthesis. Unlike mGluR-LTD in Fmr1 knockout mice, we found that the LTD in the PERK cKO mice was blocked with application of the general protein synthesis inhibitor anisomycin (20 µM) (Fig. 3C). These results demonstrate that de novo protein synthesis is required for the enhanced mGluR-LTD in PERK cKO mice. Furthermore, we found that NMDA receptor-dependent LTD (NMDAR-LTD) was similar between wild-type and PERK cKO slices (Fig. 3D). These results suggest a specific role for PERK-dependent translational control mechanisms in mGluR-dependent LTD vs. NMDAR-LTD.
Differential regulation of eIF2α phosphorylation following stimulation that induces LTP and mGluR-LTD
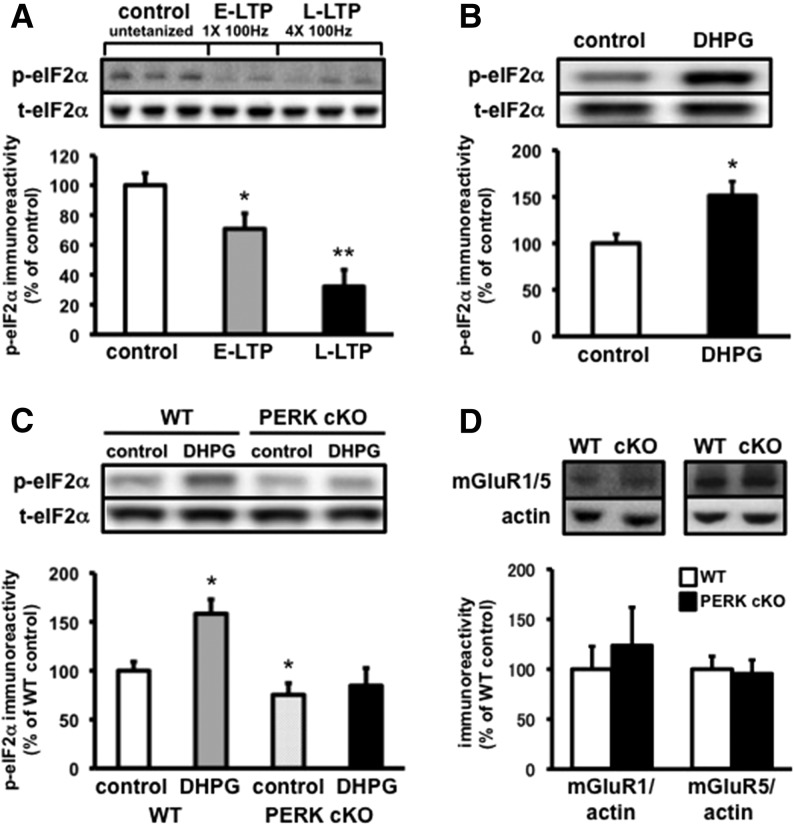
To better understand the regulation of eIF2α phosphorylation during persistent changes in synaptic strength, we first investigated the effects of LTP-inducing stimulation on eIF2α phosphorylation in hippocampal slices. Protein synthesis-dependent increases in synaptic strength, induced by tetanic stimulation and with application of either brain-derived neurotrophic factor (BDNF) or the cyclic AMP (cAMP) activator forskolin, have been shown to decrease the phosphorylation of eIF2α (Takei et al. 2001; Costa-Mattioli et al. 2005). Consistent with these findings, we found that phosphorylation of eIF2α was reduced following both one and four trains of high-frequency stimulation (HFS) that induces E-LTP and L-LTP, respectively (Fig. 4A). These results indicate that increases in synaptic strength are associated with a reduction of eIF2α phosphorylation in hippocampal area CA1.
Figure 4.
PERK is required for the distinct regulation of eIF2α phosphorylation following mGluR-LTD induction. (A) E-LTP and L-LTP stimulation decreases eIF2α phosphorylation in hippocampal area CA1. Untetanized (control) slices vs. 1 × 100 Hz (E-LTP) and 4 × 100 Hz (L-LTP) slices are compared (n = 5 slices per condition, [*] P < 0.05, [**] P < 0.01, one-way ANOVA). (B) DHPG application (50 µM, 10 min) increases eIF2α phosphorylation in hippocampal area CA1 (n = 6 slices per condition, [*] P < 0.05, Student's t-test). (C) The DHPG-induced increase in eIF2α phosphorylation in WT slices is blocked in PERK cKO slices. Hippocampal slices from PERK cKO and WT mice were treated with or without DHPG (50 µM, 10 min, n = 6 slices per condition, [*] P < 0.05, one-way ANOVA). (D) mGluR1 and mGluR5 expression were unaltered in hippocampal lysates from PERK cKO mice compared to wild-type littermates (mGluR1, n = 4, P = 0.6; mGluR5, n = 6, P = 0.8, Student's t-test). Representative Western blots are shown in each panel.
To determine whether a protein synthesis-dependent decrease in synaptic strength also altered eIF2α phosphorylation, we next examined whether activation of group I mGluRs, which induces LTD in the hippocampus, modulated eIF2α phosphorylation. We treated hippocampal slices from wild-type mice with DHPG (50 µM, 10 min) or vehicle (control), and then measured the levels of phosphorylated eIF2α in homogenates from area CA1. Interestingly, we found that treatment of hippocampal slices with DHPG significantly increased eIF2α phosphorylation (Fig. 4B). Thus, our findings indicate that an mGluR- and protein synthesis-dependent decrease in synaptic strength promotes eIF2α phosphorylation in hippocampal area CA1. Collectively, these findings provide evidence for the differential regulation of eIF2α phosphorylation during long-lasting LTD and LTP.
mGluR-induced increase in eIF2α phosphorylation is dependent on PERK
As mentioned earlier, mGluR-LTD requires local protein synthesis (Huber et al. 2000). Because the expression of mGluR-LTD was enhanced in PERK cKO mice, we examined whether the LTD-associated increase in eIF2α phosphorylation was still present in slices from PERK cKO mice. In hippocampal slices from wild-type mice, DHPG-induced LTD was associated with an increase in eIF2α phosphorylation 10 min after DHPG application (Fig. 4C). Notably, the enhanced level of phosphorylated eIF2α was blocked following LTD induction in the PERK cKO mice (Fig. 4C). Neither mGluR1 nor mGluR5 expression were altered in hippocampal lysates from PERK cKO mice compared to wild-type littermates, suggesting that the enhanced mGluR-LTD in the PERK cKO mice is not due to increased mGluR-dependent signaling (Fig. 4D). Taken together, these findings support the idea that DHPG induces PERK-dependent phosphorylation of eIF2α, which normally limits mGluR-LTD.
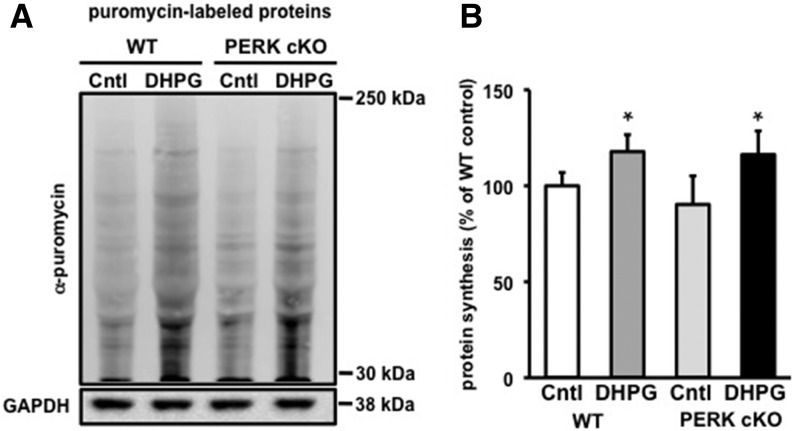
To determine whether the enhanced mGluR-LTD in the PERK cKO slices is due to changes in de novo protein synthesis, we next examined whether activation of group I mGluRs by DHPG induced de novo protein synthesis. To measure protein synthesis, we used SUnSET (Schmidt et al. 2009), a nonradioactive puromycin end-labeling assay. Following puromycin treatment, we incubated hippocampal slices from 3-mo-old wild-type and PERK cKO mice with DHPG (100 μM, 10 min) or vehicle (control), and measured the levels of protein synthesis in lysates from area CA1. We found that DHPG caused an increase in de novo protein synthesis in the PERK cKO slices that appeared to be the same magnitude as was observed in wild-type slices (Fig. 5A,B). These findings suggest that the enhanced mGluR-LTD in PERK cKO slices is due to changes in transcript-specific translation rather than changes in general protein synthesis.
Figure 5.
DHPG-induced mGluR-LTD in slices from PERK cKO mice is associated with an increase of de novo protein synthesis similar to that in wild-type mice. (A) Representative Western blot of lysates from wild-type (WT) and PERK cKO hippocampal slices treated with puromycin (5 μg/mL, 35 min) and incubated with either ACSF (Cntl) or DHPG (100 μM) for 10 min to measure protein synthesis at baseline levels and in response to DHPG treatment. (B) Cumulative graph with percentages expressed relative to WT control (Cntl), n = 3 mice per genotype, 2–3 slices per condition ([*] P < 0.05, Student's t-test), All data represent mean values ± SEM.
Discussion
Multiple lines of evidence strongly support the notion that tight regulation of translational control is critical for the expression of hippocampal mGluR-LTD (Costa-Mattioli et al. 2009; Richter and Klann 2009; Waung and Huber 2009). First, translation is required as several general protein synthesis inhibitors block mGluR-LTD (Huber et al. 2000). Second, pharmacological and genetic disruption of PI3K, mTORC1, and ERK signaling pathways alter the phosphorylation of multiple initiation factors and prevent the expression of normal mGluR-LTD (Gallagher et al. 2004; Hou and Klann 2004; Banko et al. 2006; Ronesi and Huber 2008). For example, mice lacking 4E-BP2, a translational repressor of eIF4F complex assembly, display enhanced mGluR-LTD (Banko et al. 2006), suggesting that repression of translation initiation normally limits the magnitude of mGluR-LTD. Consistent with these findings, we found that a reduction of eIF2α phosphorylation by genetic inactivation of PERK resulted in an enhancement of mGluR-dependent LTD (Fig. 3A). Thus, these findings suggest that, similar to 4E-BP2, PERK normally modulates the level of long-lasting depression following the activation of group I mGluRs. It should be noted that despite the lack of an increase in eIF2α phosphorylation in the PERK cKO mice following induction of mGluR-LTD, it is possible that the effects of PERK deletion on LTD could be independent of eIF2α and translation. However, the most parsimonious explanation for the present findings is that PERK-dependent phosphorylation of eIF2α is required for translational control that limits mGluR-LTD in the hippocampus.
Previous studies demonstrated that mice lacking the translational regulator FMRP exhibit enhanced hippocampal mGluR-LTD and are insensitive to protein synthesis inhibitors (Huber et al. 2002; Hou et al. 2006; Nosyreva and Huber 2006). In contrast, in our LTD studies we found that the enhanced mGluR-LTD in PERK cKO mice were sensitive to protein synthesis inhibition (Fig. 3C), more in keeping with mGluR-LTD in wild-type mice (Huber et al. 2000). One possible explanation for these differences is that FMRP and PERK are acting to trigger distinct proteomic changes during mGluR-dependent LTD.
In this study, we found that DHPG-induced mGluR-LTD triggered an increase in phosphorylated eIF2α, which was prevented in the absence of PERK (Fig. 4C). As noted above, phosphorylation of eIF2α inhibits general translation while preferentially stimulating the gene-specific translation of mRNAs with upstream open reading frame (uORF) sequences such as ATF4. Therefore, we hypothesize that PERK normally either decreases general protein synthesis or increases the translation of specific synaptic mRNAs to limit mGluR-LTD. Future studies investigating the identities of the newly synthesized LTD proteins following synaptic activation of mGluRs in these mice should provide insight into the different regulatory mechanisms that control mGluR-LTD.
We observed that eIF2α phosphorylation was differentially regulated upon activation of LTP and LTD (Fig. 4A,B). These findings suggest that distinct pools of eIF2α are phosphorylated and mRNAs are translated during NMDA-receptor dependent LTP and mGluR-LTD. In support of this notion, a recent study showed that DHPG stimulation causes preferential recruitment of certain locally translated mRNAs, without alterations to the size or number of translational hotspots (Kim et al. 2013). Moreover, mGluR-LTD, which is enhanced in PERK cKO mice (Fig. 3A), was unaffected in mice lacking the eIF2α kinase GCN2 (Costa-Mattioli et al. 2005). In contrast, L-LTP, which had a lowered threshold for induction in GCN2-deficient mice (Costa-Mattioli et al. 2005), was unaffected in PERK cKO mice (Fig. 2C,D). Thus, it appears that GCN2-dependent translational control is specific to NMDA receptor-dependent LTP, whereas PERK-dependent translational control is specific to mGluR-LTD. Moreover, our findings provide the first direct evidence of a translation factor that is differentially regulated by protein synthesis-dependent LTP and LTD and indicate a distinct role for the proper regulation of eIF2α by PERK during mGluR-dependent LTD.
Precise translational control mechanisms in dendrites are considered critical for long-lasting plasticity of synaptic connections and for the expression of cognitive functions that rely on such plasticity (Kelleher et al. 2004; Sutton and Schuman 2006; Costa-Mattioli et al. 2009; Richter and Klann 2009). Consistent with this, we recently reported that disruption of PERK-dependent translational control mechanisms in the prefrontal cortex of PERK cKO mice results in multiple cognitive impairments, including severe behavioral inflexibility (Trinh et al. 2012). In the present study, our findings suggest that PERK-directed translational control of eIF2α phosphorylation distinctly limits the expression of mGluR-dependent LTD in the hippocampus. Based on these results, we speculate that disruption of PERK-dependent phosphorylation of eIF2α following activation of group I mGluRs may serve as a mechanism for the behavioral manifestations observed in PERK-deficient mice. Future studies investigating the specific role of PERK-regulated translation in the brain will prove useful to elucidate the molecular underpinnings of normal cognitive processes and how they are altered in neurological disorders and neurodegenerative diseases.
Materials and Methods
Materials
Alexa Fluor 488-coupled goat anti-rabbit, Alexa Fluor 568-coupled goat anti-mouse, and anti-rabbit phospho-eIF2αS52 antibodies were from Invitrogen. Rabbit anti-PKR-like endoplasmic reticulum kinase (PERK) antibody was from Rockland. Mouse anti-eIF2α (L57A5), and rabbit anti-PERK (C33E10) and rabbit anti-mGluR1 antibodies were from Cell Signaling Technology. Anti-puromycin (12D10) antibody was kindly provided by Dr. P. Pierre. Anti-mGluR5 antibody was from R&D Systems. 3,3′-Diaminobenzidine (DAB) substrate staining kit was from Vector Laboratories. (RS)-3,5-Dihydroxyphenylglycine (DHPG) and anisomycin were obtained from Tocris Bioscience. Puromycin (P8833) and mouse anti-actin and anti-MAP2 antibodies were from Sigma.
Animals
Transgenic mice containing a forebrain-specific deletion of PERK (CamkIIα-Cre; PerkloxP/loxP) were generated, maintained, and genotyped as described previously (Trinh et al. 2012).
Immunohistochemistry and Nissl staining
Immunohistochemical and Nissl staining techniques were performed using standard procedures as described previously (Banko et al. 2006). For immunohistochemical studies, sections from 4-wk-old wild-type mice were incubated with rabbit anti-PERK (Rockland, 1:200) followed by secondary antibody incubation and detection using the DAB staining method. For immunofluorescence studies, mouse primary hippocampal neurons (DIV 9) were incubated with rabbit anti-PERK (Rockland, 1:200) and either mouse anti-MAP2 (1:500) or mouse anti-eIF2α (1:500) antibodies followed by an incubation with Alexa Fluor 488-coupled goat anti-rabbit and Alexa Fluor 568-coupled goat anti-mouse antibodies (1:500).
Hippocampal slice preparations and treatment
Transverse hippocampal slices were prepared and CA1 regions were dissected as described previously (Banko et al. 2005). Briefly, for mGluR-LTD experiments, slices from 4- to 5-wk old mice were equilibrated in oxygenated artificial CSF (ACSF) and subsequently incubated with DHPG (50 µM) for 10 min prior to being snap-frozen over dry ice. For LTP studies, slices were isolated and prepared from 3- to 4-mo-old adult mice. Upon stimulation of E-LTP and L-LTP (see Electrophysiology), slices were prepared in a fashion identical to that used for mGluR-LTD experiments. For NMDA receptor-dependent LTD studies, slices were isolated and prepared from 3-mo-old mice.
Electrophysiology
Field excitatory postsynaptic potentials (fEPSPs) were recorded from hippocampal slices as described previously (Banko et al. 2005). For mGluR-LTD studies, slices were incubated with DHPG (50 µM) for 10 min in the presence of either vehicle or anisomycin (20 µM). For PP-LFS studies, mGluR-LTD was induced in the presence of D-(–)-2-amino-5-phosphonopentanoic acid (D-APV) (50 µM, Sigma) by using 900 paired-pulse stimuli with a 50-msec interstimulus interval delivered at 1 Hz for 20 min. NMDA receptor-dependent LTD was induced by 900 paired-pulse stimulations with 50-msec interstimulus interval delivered at 1 Hz for 15 min (Jiang et al. 2007). For LTP studies, LTP was induced with either one train (E-LTP) or four trains (L-LTP) (5-min intertrain interval [ITI]) of a 100-Hz high-frequency stimulation (HFS) for 1 sec.
Western blotting
Western blots were performed using standard procedures as described previously (Banko et al. 2005). Notably, for optimal detection of PERK in mouse brain samples, a minimum of 40 μg of protein was needed. Primary antibodies of interest include: PERK (C33E10, 1:500), phospho-eIF2αS52 (1:1000), eIF2α (1:1000), ATF4 (1:2000), mGluR1 (1:1000), mGluR5 (1:200), and actin (1:10000).
Measurement of de novo protein synthesis
Four-hundred-micrometer transverse hippocampal slices were obtained from 3-mo-old wild-type and PERK cKO mice. Puromycin labeling of the slices was adapted from procedures described previously (Schmidt et al. 2009). Briefly, slices were allowed to recover in ACSF at 32°C for 2 h and subsequently treated with puromycin (5 μg/mL) for 35 min and incubated with either DHPG (100 μM) or ACSF vehicle (control) for 10 min. Reactions were stopped by flash freezing the slices on dry ice. Then, proteins were prepared, blotted, and quantified by standard Western blotting procedures. Fifty micrograms of puromycin-labeled protein were resolved on 4%–12% gradient gels and visualized using an antibody specific to puromycin. Protein synthesis levels were determined by taking the total lane signal from 30 kDa to 250 kDa and subtracting the signal from a control lane that was not labeled by puromycin. Comparisons of protein synthesis levels between both genotypes and treatment were made by normalizing to the average wild-type control signal obtained from different experimental replicates.
Statistical analysis
All data are presented as the mean ± SEM. Intergroup differences were evaluated by a one-way ANOVA. For electrophysiological data analysis, two-way ANOVA and post-hoc tests were used where appropriate. When only two means were analyzed, a two-tailed Student's t-test analysis was performed. Values of P < 0.05 were considered to represent statistically significant differences.
Acknowledgments
This work was supported by National Institutes of Health grants 1F31NS063686 (M.A.T.), NS034007 and NS047384 (E.K.), the FRAXA Research Foundation (E.K.), the Bill & Melinda Gates Foundation (M.A.T.), the BrightFocus Foundation (T.M.), and the Charles H. Revson Foundation (A.B.). We thank Dr. Susumu Tonegawa for providing the CamkIIα-Cre mice, Dr. Philippe Pierre for providing us with the anti-puromycin antibody, and Dr. Ronald Wek for providing us with the anti-ATF4 antibody and for helpful comments on this manuscript. We also thank Heather Bowling for providing mouse hippocampal neuron cultures and Maggie Mamcarz for maintaining and genotyping the PERK mouse colony for this study.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.032219.113.
References
- Abel T, Martin KC, Bartsch D, Kandel ER 1998. Memory suppressor genes: Inhibitory constraints on the storage of long-term memory. Science 279: 338–341 [DOI] [PubMed] [Google Scholar]
- Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW 2005. The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc Natl Acad Sci 102: 2180–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E 2005. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci 25: 9581–9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E 2006. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci 26: 2167–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER 1995. Aplysia CREB2 represses long-term facilitation: Relief of repression converts transient facilitation into long-term functional and structural change. Cell 83: 979–992 [DOI] [PubMed] [Google Scholar]
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, Yonan AL, Vronskaya S, Grody MB, Cepeda I, et al. 2003. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron 39: 655–669 [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, et al. 2005. Translational control of hippocampal synaptic plasticity and memory by the eIF2α kinase GCN2. Nature 436: 1166–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, et al. 2007. eIF2α phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129: 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N 2009. Translational control of long-lasting synaptic plasticity and memory. Neuron 61: 10–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C 2000. EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott–Rallison syndrome. Nat Genet 25: 406–409 [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM 2004. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci 24: 4859–4864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D 2000. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D 2001. Diabetes mellitus and exocrine pancreatic dysfunction in perk–/– mice reveals a role for translational control in secretory cell survival. Mol Cell 7: 1153–1163 [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E 2004. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci 24: 6352–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E 2006. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 51: 441–454 [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF 2000. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288: 1254–1257 [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF 2002. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci 99: 7746–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Trevino M, Kirkwood A 2007. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J Neurosci 27: 9648–9652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Belforte JE, Lu Y, Yabe Y, Pickel J, Smith CB, Je HS, Lu B, Nakazawa K 2010. eIF2α phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation. J Neurosci 30: 2582–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julier C, Nicolino M 2010. Wolcott–Rallison syndrome. Orphanet J Rare Dis 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ III, Govindarajan A, Tonegawa S 2004. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron 44: 59–73 [DOI] [PubMed] [Google Scholar]
- Kim TK, Sul JY, Helmfors H, Langel U, Kim J, Eberwine J 2013. Dendritic glutamate receptor mRNAs show contingent local hotspot-dependent translational dynamics. Cell Rep 5: 114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM 2006. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol 95: 3291–3295 [DOI] [PubMed] [Google Scholar]
- Reis AF, Kannengiesser C, Jennane F, Manna TD, Cheurfa N, Oudin C, Savoldelli RD, Oliveira C, Grandchamp B, Kok F, et al. 2011. Two novel mutations in the EIF2AK3 gene in children with Wolcott–Rallison syndrome. Pediatr Diabetes 12: 187–191 [DOI] [PubMed] [Google Scholar]
- Richter JD, Klann E 2009. Making synaptic plasticity and memory last: Mechanisms of translational regulation. Genes Dev 23: 1–11 [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM 2008. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci 28: 543–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Cabezas O, Patch AM, Minton JA, Flanagan SE, Edghill EL, Hussain K, Balafrej A, Deeb A, Buchanan CR, Jefferson IG, et al. 2009. Wolcott–Rallison syndrome is the most common genetic cause of permanent neonatal diabetes in consanguineous families. J Clin Endocrinol Metab 94: 4162–4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EK, Clavarino G, Ceppi M, Pierre P 2009. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 6: 275–277 [DOI] [PubMed] [Google Scholar]
- Senee V, Vattem KM, Delepine M, Rainbow LA, Haton C, Lecoq A, Shaw NJ, Robert JJ, Rooman R, Diatloff-Zito C, et al. 2004. Wolcott–Rallison syndrome: Clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes 53: 1876–1883 [DOI] [PubMed] [Google Scholar]
- Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS 2010. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci 30: 694–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC 1998. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol Cell Biol 18: 7499–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N, Dever TE 2003. Eukaryotic translation initiation factors and regulators. Curr Opin Struct Biol 13: 56–63 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM 2006. Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127: 49–58 [DOI] [PubMed] [Google Scholar]
- Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H 2001. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: Comparison with the effects of insulin. J Biol Chem 276: 42818–42825 [DOI] [PubMed] [Google Scholar]
- Thornton CM, Carson DJ, Stewart FJ 1997. Autopsy findings in the Wolcott–Rallison syndrome. Pediatr Pathol Lab Med 17: 487–496 [PubMed] [Google Scholar]
- Trinh MA, Kaphzan H, Wek RC, Pierre P, Cavener DR, Klann E 2012. Brain-specific disruption of the eIF2α kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep 1: 676–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem KM, Wek RC 2004. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci 101: 11269–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Huber KM 2009. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Curr Opin Neurobiol 19: 319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Sheng X, Feng D, McGrath B, Cavener DR 2008. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J Cell Physiol 217: 693–707 [DOI] [PubMed] [Google Scholar]
- Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR 2002. The PERK eukaryotic initiation factor 2 α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22: 3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Huang W, Kalikulov D, Yoo JW, Placzek AN, Stoica L, Zhou H, Bell JC, Friedlander MJ, Krnjevic K, et al. 2011. Suppression of PKR promotes network excitability and enhanced cognition by interferon-γ-mediated disinhibition. Cell 147: 1384–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]