Abstract
Patients with glioblastoma (GB) are known to have poor prognoses, and among these patients, those with poor neurological function have an even poorer prognosis. Consequently, aggressive surgeries and adjuvant therapies are often withheld because of this dismal outlook. The effects of aggressive therapies in this small subset of patients remain unknown. The goal of this study was to evaluate outcomes and factors associated with survival for poor functioning patients who underwent aggressive resection of their GB. Adult patients who underwent surgical resection of an intracranial primary GB at an academic tertiary-care institution between 1997 and 2007 were retrospectively reviewed. Patients with a Karnofsky Performance Scale (KPS) score of ≤60 were included. A total of 100 patients with primary GB met the inclusion criteria. The average age (±standard deviation) and KPS score of this cohort were 54 ± 15 years and 53 ± 12, respectively. No patient (0%) experienced perioperative mortality, and 0 (0%), 10 (10%), and 3 (3%) of patients incurred a new or increasing language, motor, and visual deficit, respectively. At last follow-up, 88 (88%) patients died with a median survival of 6.6 months. The factors associated with improved survival were age <65 year (p = 0.005), tumor size >2 cm (p = 0.01), radical tumor resection (p = 0.01), and temozolomide (p = 0.001). This study identifies a subset of patients with poor functional status who may benefit from aggressive surgical resection.
Keywords: Glioblastoma, Karnofsky Performance Scale score, Outcomes, Poor neurological status, Survival
1. Introduction
Patients diagnosed with glioblastoma (GB) have a dismal prognosis. 1,2 However, even though median survival is poor, individual survival is heterogeneous with some patients surviving for several years.1,3 This heterogeneity has motivated several studies to evaluate factors that predict prolonged survival for patients with GB.2,4,5 One factor most strongly associated with survival is preoperative functional status.2,4,5 Not surprisingly, higher functioning patients make up the majority of surgical studies, clinical trials, and outcome studies.6–9
Studies dedicated to poor functioning patients with GB are few and limited.6–8 This is primarily due to aggressive therapies being withheld from this patient population.6–8 As a result, factors that predict outcome for patients with poor preoperative neurological status who undergo surgical resection remain poorly understood. The goal of this paper was to therefore identify factors that predict outcome for patients with the worst prognoses.
2. Methods
2.1. Patient selection
A total of 701 patients underwent surgery for an intracranial primary GB at a single academic tertiary care institution between 1997 and 2007. Adult patients (age >18 years) with a Karnofsky Performance Scale (KPS) score ≤60 and who underwent non-biopsy, surgical resection of a primary GB were included in the analysis. A KPS score ≤60 was used because it is significantly associated with survival in several studies, and designates patients who are functionally dependent on others and require considerable assistance in their care.5,10,11 The pathology was determined by a senior neuropathologist in all instances, and the grading criteria were based on the World Health Organization (WHO) classification system.12 Patients with infratentorial gliomas, multicentric lesions, and prior lower grade gliomas were excluded from the analysis. This was to achieve a more uniform patient population with similar tumor types, tumor location, and treatment strategies.
2.2. Recorded variables
The clinical, operative, and hospital course records of the patients who met the inclusion criteria were reviewed retrospectively. The KPS score was used to classify patients’ preoperative functional status. 13 The KPS indices were assigned by two clinicians blinded by patient outcomes at the clinical visit prior to surgery during a chart review.
The MRI were obtained and reviewed. The characteristics that were recorded included: lesion size (largest diameter on T1-weighted gadolinium-enhanced images), specific lobe involvement, and adjacency to the lateral ventricles.2,14 Extent of resection was classified from MRI obtained <48 h after surgical resection as either radical (>95%) or subtotal resection (STR) (<95%) by an independent neuroradiologist blinded to patient outcomes. Recursive partitioning analysis (RPA) was assigned as described previously.15
Survival data for all patients were obtained from the social security index database.16 Time to death was defined as the time from surgery to death. For patients with recurrent GB, time to death was defined as time from repeat surgery to death. Patients whose deaths were unconfirmed were classified as lost to follow-up at the time of the last clinic visit.
2.3. Perioperative treatment
The general aim of each surgical resection was to achieve radical resection of the tumor when possible. STR primarily occurred when the tumor involved eloquent brain as confirmed by intraoperative mapping and/or monitoring (awake/speech language mapping, direct cortical motor stimulation, and motor-evoked or somatosensory-evoked potential). Patients not considered candidates for extensive surgical resection were excluded. The use of adjuvant therapies was typically determined by the patient and multidisciplinary team consisting of the surgeon, medical oncologist, and radiation oncologist.
2.4. Statistical analysis
All analyses were performed using JMP 9 (Statistical Analysis Software) (SAS Institute, Carey, NC, USA) unless otherwise noted. Summary data were presented as mean ± standard deviation for parametric data and as median (interquartile range [IQR]) for non-parametric data. Percentages were compared via Fisher’s exact test for categorical variables and the Student’s t-test for continuous variables. The Kaplan–Meier method was used to plot survival as a function of time, and the Log-rank analysis was used to compare Kaplan–Meier plots (GraphPad Prism 5, La Jolla, CA, USA). Stepwise multivariate proportional hazards regression analysis was used to identify factors associated with survival for patients with GB. Values with p < 0.05 in these analyses were considered statistically significant.
3. Results
3.1. Preoperative, perioperative, and postoperative characteristics of all patients
The preoperative characteristics of the 100 patients who met the inclusion criteria are summarized in Table 1. The average age was 54 ± 15 years at the time of surgery. The average preoperative KPS was 53 ± 12. The average size of the tumor was 4.7 ± 1.7 cm. The tumor was periventricular in 48% of patients and 33% had recurrent GB resections.
Table 1.
Preoperative characteristics of patients (n = 100) with Karnofsky Performance Scale score (KPS) ≤60 who underwent surgery for an intracranial glioblastoma (GB)
| Characteristics | Number (%) |
|---|---|
| Age (years)* | 54 ± 15 |
| Male | 51 (51%) |
| Presenting symptoms | |
| KPS* | 53 ± 12 |
| KPS 60 | 65 (65%) |
| KPS 40–50 | 23 (23%) |
| KPS 20–30 | 12 (12%) |
| RPA classes | |
| RPA IV | 31 (31%) |
| RPA V | 61 (61%) |
| RPA VI | 8 (8%) |
| Preoperative symptoms | |
| Seizures | 23 (23%) |
| Headaches/nausea/vomiting | 25 (25%) |
| Sensory deficit | 7 (7%) |
| Motor deficit | 54 (54%) |
| Language deficit | 26 (26%) |
| Visual deficit | 16 (16%) |
| Confusion/memory loss | 9 (9%) |
| Recurrent GB | 33 (33%) |
| Radiographics | |
| Tumor size (cm)* | 4.7 ± 1.7 |
| Location | |
| Frontal | 51 (51%) |
| Temporal | 15 (15%) |
| Parietal | 28 (28%) |
| Occipital | 6 (6%) |
| Hemorrhagic | 15 (15%) |
| Periventricular | 48 (48%) |
RPA = recursive partitioning analysis.
Mean ± standard deviation.
The perioperative and postoperative outcomes are summarized in Table 2. Radical resection was achieved in 55% and STR in 45%, respectively; and 23%, 22%, and 78% of patients had carmustine, temozolomide, and radiation therapy.
Table 2.
Perioperative and postoperative characteristics of patients with Karnofsky Performance Scale score (KPS) ≤60 who underwent surgery for an intracranial glioblastoma (GB)
| Characteristics | Number (%) |
|---|---|
| Surgical variables | |
| Radical resection (>95%) | 55 (55%) |
| Subtotal resection (<95%) | 45 (45%) |
| Perioperative | |
| Mortality | 0 (0%) |
| New motor deficit | 10 (10%) |
| New language deficit | 0 (0%) |
| New visual deficit | 3 (3%) |
| Hospital stay (days)* | 6 (5–10) |
| Adjuvant therapy | |
| Carmustine wafers | 23 (23%) |
| Temozolomide | 22 (22%) |
| Radiation therapy | 78 (78%) |
| Survival | |
| Died at last follow-up | 88 (88%) |
| Median survival (months) | 6.6 |
| 3-month survival rate | 73% |
| 6-month survival rate | 52% |
| 9-month survival rate | 41% |
| 12-month survival rate | 32% |
| 18-month survival rate | 14% |
| 24-month survival rate | 8% |
Median (interquartile range).
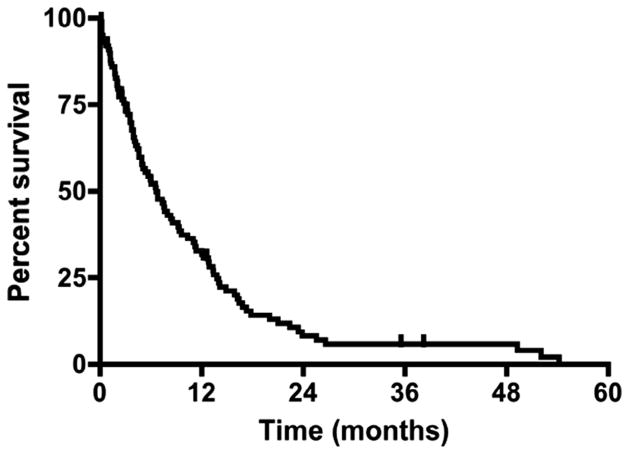
At last follow-up, 88% patients had died. The patients who were not confirmed as deceased had a median [IQR] follow-up time of 6.4 [2.4–9.3] months. The median survival of the entire cohort was 6.6 months from the time of GB diagnosis, where the 6-month and 12-month survival rates were 52% and 32%, respectively (Fig. 1). The median survival times for patients who had a preoperative KPS score of 60, 40–50, and 20–30 were 6.6, 7.9, and 3.7 months, respectively (Fig. 2) (p > 0.05).
Fig. 1.
Percentage survival for patients with poor preoperative Karnofsky Performance Scale scores (KPS). Kaplan–Meier curve for patients with KPS ≤60 prior to glioblastoma resection. Median survival was 6.6 months, where the 3-, 6-, 12-, and 18-month survival rates were 73%, 52%, 32%, and 14%, respectively.
Fig. 2.
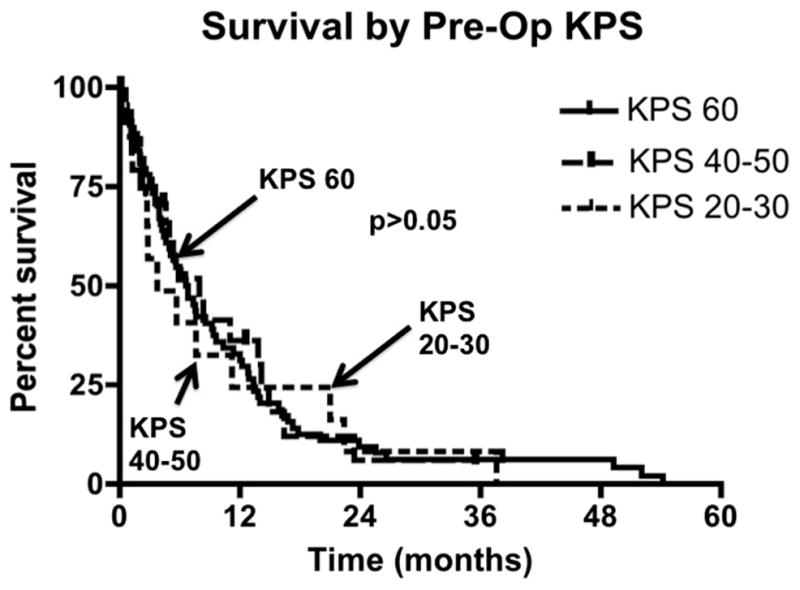
Survival for patients with poor preoperative Karnofsky Performance Scale score (KPS) stratified by their preoperative KPS score. Median survival for patients with preoperative KPS score 60 was 6.6 months, where the 6-, 12-, and 18-month survival rates were 35%, 21%, and 9%, respectively. Median survival for patients with preoperative KPS score of 40–50 was 7.9 months, where the 6-, 12-, and 18-month survival rates were 11%, 8%, and 3%, respectively. Median survival for patients with preoperative KPS score of 20–30 was 3.7 months, where the 6-, 12-, and 18-month survival rates were 6%, 4%, and 4%, respectively. In Log-rank analysis, there was no statistical significance in survival between the cohorts (p > 0.05).
3.2. Factors independently associated with survival
3.2.1. Univariate analysis
In a univariate proportional hazards regression analysis, the factors associated with survival were: age, ataxia, tumor size, STR, temozolomide, and radiation therapy. No other clinical or imaging variable was found to be associated with survival. There was no association between preoperative KPS and survival [KPS of 60 (p = 0.89), ≥50 (p = 0.49), ≥40 (p = 0.61), ≥30 (p = 0.59), and ≥20 (p = 0.89)] (Table 3).
Table 3.
Multivariate associations with survival for patients with preoperative Karnofsky Performance Scale score (KPS) ≤60 who underwent surgery for an intracranial glioblastoma (GB)
| Multivariate associations with survival | ||
|---|---|---|
| Variables | Relative risk (95% CI) | p value |
| Factors associated with improved survival | ||
| Decreasing age | 0.985 (0.969–0.995) | 0.05 |
| Age <65 years | 0.455 (0.275–0.779) | 0.005 |
| Tumor size >2 cm | 0.269 (0.119–0.722) | 0.01 |
| Radical resection | 0.415 (0.226–0.798) | 0.01 |
| Temozolomide | 0.465 (0.274–0.756) | 0.001 |
| Factors notably not associated with survival | ||
| Preoperative KPS score | 0.996 (0.980–1.014) | 0.66 |
| RPA IV | 0.860 (0.544–1.325) | 0.50 |
| RPA V | 1.136 (0.752–1.741) | 0.55 |
| RPA VI | 1.125 (0.501–2.186) | 0.75 |
| Motor deficit | 0.846 (0.546–1.270) | 0.42 |
| Language deficit | 0.733 (0.447–1.156) | 0.19 |
| Recurrent GB | 0.921 (0.584–1.424) | 0.71 |
CI = confidence interval, RPA = recursive partitioning analysis. Values in bold are statistically significant.
3.2.2. Multivariate analysis
In stepwise multivariate proportional hazards regression analysis (Table 3), the factors that remained significantly associated with improved survival were: decreasing age (relative risk [RR] 95% confidence interval [CI]; 0.985 [0.969–0.995], p = 0.05), tumor size >2 cm (RR [95% CI]; 0.269 [0.119–0.722], p = 0.01), radical resection (RR [95% CI]; 0.415 [0.226–0.798], p = 0.01), and temozolomide (RR [95% CI]; 0.465 [0.274–0.756], p = 0.001). Among the different ages, patients younger than 65 years (RR [95% CI]; 0.985 [0.969–0.995], p = 0.005) had the greatest statistical association with improved survival. There were 20 (20%) patients with a tumor size <2 cm.
3.2.3. Subgroup analysis
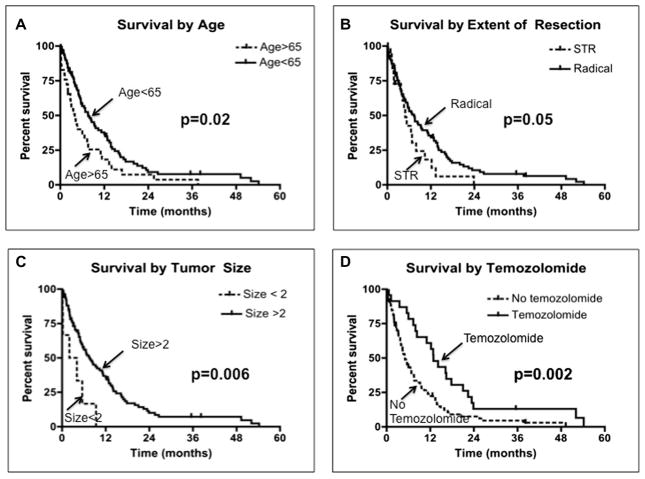
Subgroup analysis was performed on the factors identified in multivariate analysis. When evaluating age (Fig. 3A), patients younger than 65 years had a median survival of 7.6 months as compared to 3.9 months for patients older than 65 years (p = 0.02). When evaluating extent of resection (Fig. 3B), patients who underwent radical resection had a median survival of 7.3 months as compared to 4.3 months for patients who underwent STR (p = 0.05). When evaluating tumor size (Fig. 3C), patients with tumors >2 cm had a median survival of 7.6 months as compared to 3.1 months for patients with smaller tumors (p = 0.006). When evaluating temozolomide therapy (Fig. 3D), patients who received this adjuvant therapy had a median survival of 12.9 months as compared to 5.1 months for patients who did not receive this adjuvant therapy (p = 0.002).
Fig. 3.
Survival for patients with poor preoperative Karnofsky Performance Scale scores stratified by independent predictors of outcome. (A) Survival by age. Patients older than 65 years had a median survival of 3.9 months as compared to 7.6 months for patients younger than 65 years (p = 0.02). (B) Survival by extent of resection. Patients who underwent subtotal resection (STR) had a median survival of 4.3 months as compared to 7.3 months for patients who had radical resection (p = 0.05). (C) Survival by tumor size. Patients who had tumors <2 cm in size had a median survival of 3.1 months as compared to 7.6 months for patients with larger tumors (p = 0.006). (D) Survival by temozolomide therapy. Patients who did not receive temozolomide had a median survival of 5.1 months as compared to 12.9 months for patients who received adjuvant therapy (p = 0.002).
4. Discussion
Patients with GB have a median survival of approximately 12 months, but individual survival is heterogeneous.1,3 Age and preoperative neurological function are the two factors most consistently associated with survival in several studies.1,3,17,18 In addition to older patients, patients with poor neurological function are often excluded from clinical trials and withheld aggressive therapies because of their poor prognoses.6–8,15,19 An understanding of outcomes for poor functioning patients with GB therefore remains unclear. This lack of clarity will continue to prevent these patients from being offered surgical and medical therapies, which are typically offered to higher functioning patients. Among poor functioning patients with GB, there may be a subset of patients who benefit from surgical resection.
Previous studies on patients with GB with poor neurological function are few and limited (Table 4).6–8,19 Marina et al. studied 74 patients with GB and KPS ≤50, where 38 patients underwent needle biopsy and 36 patients underwent surgical resection.7 They found that patients with lower RPA class, patients who received resection as compared to biopsy, and patients who underwent radiation therapy as compared to no radiation had improved survival.7 Likewise, Jeremic et al. studied 47 elderly patients with GB, where 31 patients had a KPS score ≤70.6 Among this group of patients, in which 21 underwent needle biopsy, they found that the extent of surgery and tumor size were the only factors associated with survival.6 Mohan et al. studied 102 patients with GB, where 53 patients underwent needle biopsy and only 28 patients had a KPS ≤60. They found that KPS, RPA class, and optimal treatment (radical resection or STR with radiation) were associated with improved outcomes.8
Table 4.
Outcomes for patients with poor Karnofsky Performance Scale scores (KPS) with glioblastoma (GB) reported in the literature
Patients with younger age were more likely to have prolonged survival regardless of the magnitude of their poor preoperative neurological status. This finding has also been documented in prior studies, which primarily consisted of high functioning patients.2,5 Older age may lead to decreased abilities to withstand neurological insults caused by the tumor, surgery, and/or adjuvant therapy.5,20 Furthermore, older patients may harbor tumors with different molecular profiles and resistance genes that confer a more aggressive-behaving tumor.21 These features may explain why older patients with GB tend to have poorer outcomes.
Patients with smaller tumors surprisingly had poorer survival as compared to patients with larger tumors. This is contradictory to prior studies, which have shown that larger tumors are associated with poorer prognoses.22 These studies, however, are primarily comprised of high functioning patients.22 Poor functioning patients with smaller tumors may have less benefit from surgery. Their functional status might have less to do with mass effect caused by smaller tumors, and therefore may have less benefit from surgical resection. These smaller tumors may exert their effect on function by being either deep seated or located in more eloquent areas.
Extent of resection was associated with survival for poor functioning patients in this study. This advantage in survival may be as a result of extensive resection, which may decrease the tumor burden to a point that makes adjuvant therapy (radiotherapy and chemotherapy) more effective. A lower tumor load has increased the efficacy of adjuvant chemotherapy and radiotherapy in killing remaining cells and increasing survival.23 This surgical resection goal is more plausible as a result of advances in surgical adjuncts including functional MRI, cortical mapping, and intraoperative MRI.24
The use of temozolomide therapy independently predicted longer survival for patients in this study. This has been also seen in prior studies.25 Most patients in these studies, however, had high preoperative function.25,26 Patients with poor function are often withheld aggressive chemotherapeutic regimens.6–8,15,19,27 The present study shows that poor functioning patients with GB may also have improved survival similar to their higher functioning counterparts.
4.1. Clinical implications/treatment recommendations
Patients with GB with poor neurological function are considered to have dismal prognoses. Their seemingly inevitable poor survival make them non-ideal candidates for aggressive interventions. Extensive surgical resection can be achieved in this patient population with minimal morbidity and mortality. Patients with younger age and larger tumors may benefit the most from non-biopsy surgical resection. Older patients and patients with smaller tumors may not have the same benefits. When surgery is pursued, the aim of surgery should be to achieve at least near total resection, if safe to do so, to provide a survival advantage. Postoperatively, patients should be offered temozolomide and radiation. These features may help prolong survival for patients who are typically considered to have the worst prognoses.
4.2. Strengths and limitations
We believe this study provides several useful insights. First, the role of non-biopsy, surgical resection in poor functioning patients is poorly understood. This study shows that surgery can be performed in these patients with minimal morbidity and mortality, and certain patients can experience prolonged survival similar to high functioning patients with GB. Second, studies attempting to ascertain factors associated with surgical resection for patients with poor functional status are few and limited. The present study is the largest study to date and uses multivariate analyses to identify independent predictors of survival in this cohort with presumed poor prognoses. Additionally, prior studies on poor functioning patients with GB are limited by small patient numbers, lack of multivariate analyses, inclusion of patients who underwent biopsy, and failure to separate high functioning from low functioning patients.6–8,19 Outcomes for poor functioning patients therefore remains unclear. Last, this study may provide useful information that may help guide treatment strategies aimed at prolonging survival for the small subset of patients with GB with poor function.
This study, however, has some limitations. One limitation is that these findings apply only to poor functioning patients undergoing a craniotomy for tumor resection, and are not necessarily applicable to high functioning patients and patients undergoing needle biopsies of their lesion, or those undergoing conservative management of their tumors. This study also does not evaluate patients with previously diagnosed lower grade gliomas, but evaluates only those patients who underwent surgery for a primary GB. This study also includes patients with recurrent GB. An additional limitation is that this study does not analyze the prognostic implication of molecular markers and genotypes. Furthermore, the patients in this study underwent disparate treatment regimens. Most patients in this study did not undergo gross total resection and/or receive aggressive triple combinatorial adjuvant therapy (carmustine wafer, temozolomide, radiation). As a result, the relevance of the findings in this study may be altered in the context of those patients receiving the most aggressive of treatment regimens. Finally, this study is inherently limited by its retrospective design, and, as a result, it is not appropriate to infer direct causal relationships. However, we tried to create a uniform patient population by utilizing strict inclusion and exclusion criteria, thus providing more relevant information for patients with low KPS scores. We included only patients who underwent non-biopsy surgical resection and excluded patients with incomplete medical records, prior history of lower grade gliomas, and pediatric patients. Furthermore, we performed multivariate analyses to control for potentially confounding variables, which included disparate treatment regimens. Given these statistical controls and a relatively precise outcome measure, we believe our findings offer useful insights into the role of surgical resection for poor functioning patients with GB. However, prospective studies are needed to provide better data to guide clinical decision-making.
5. Conclusion
A small subset of patients with GB has poor neurological function at presentation and aggressive therapies are often withheld from them of because of their poor prognosis. The present study shows that surgical resection can be achieved safely in these patients. Age less than 65 years, tumors >2 cm, gross or near total resection, and temozolomide therapy are factors that independently predicted prolonged survival for patients with poor neurological function. Patients with one or more of these factors may have similar survival times as their higher functioning counterparts. The findings of this study may help guide treatment paradigms, prognosticate survival, and provide more information for poor functioning patients who are often withheld aggressive treatments.
Footnotes
Conflicts of interest/disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- 1.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30:10–4. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 2.Chaichana K, Parker S, Olivi A, et al. A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. J Neurosurg. 2010;112:997–1004. doi: 10.3171/2009.9.JNS09805. [DOI] [PubMed] [Google Scholar]
- 3.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–23. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 4.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 5.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro oncol. 2004;6:227–35. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeremic B, Shibamoto Y, Grujicic D, et al. Short-course radiotherapy in elderly and frail patients with glioblastoma multiforme. A phase II study. J Neurooncol. 1999;44:85–90. doi: 10.1023/a:1006356021734. [DOI] [PubMed] [Google Scholar]
- 7.Marina O, Suh JH, Reddy CA, et al. Treatment outcomes for patients with glioblastoma multiforme and a low Karnofsky Performance Scale score on presentation to a tertiary care institution. Clinical article. J Neurosurg. 2011;115:220–9. doi: 10.3171/2011.3.JNS10495. [DOI] [PubMed] [Google Scholar]
- 8.Mohan DS, Suh JH, Phan JL, et al. Outcome in elderly patients undergoing definitive surgery and radiation therapy for supratentorial glioblastoma multiforme at a tertiary care institution. Int J Radiat Oncol Biol Phys. 1998;42:981–7. doi: 10.1016/s0360-3016(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 9.Thomas R, James N, Guerrero D, et al. Hypofractionated radiotherapy as palliative treatment in poor prognosis patients with high grade glioma. Radiother Oncol. 1994;33:113–6. doi: 10.1016/0167-8140(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 10.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–93. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 11.Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–73. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 12.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl) 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta D, Vanere P, Gupta T, et al. Factors influencing activities of daily living using FIM-FAM scoring system before starting adjuvant treatment in patients with brain tumors: results from a prospective study. J Neurooncol. 2009;94:103–10. doi: 10.1007/s11060-009-9810-y. [DOI] [PubMed] [Google Scholar]
- 14.Chaichana KL, McGirt MJ, Frazier J, et al. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89:219–24. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 15.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–10. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 16.Social Security Death Index Database. http://searches.rootsweb.ancestry.com.
- 17.Chaichana KL, Chaichana KK, Olivi A, et al. Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival. Clinical article. J Neurosurg. 2011;114:587–94. doi: 10.3171/2010.8.JNS1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaichana KL, Halthore AN, Parker SL, et al. Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection. Clinical article. J Neurosurg. 2011;114:604–12. doi: 10.3171/2010.4.JNS091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang EL, Yi W, Allen PK, et al. Hypofractionated radiotherapy for elderly or younger low-performance status glioblastoma patients: outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 2003;56:519–28. doi: 10.1016/s0360-3016(02)04522-4. [DOI] [PubMed] [Google Scholar]
- 20.Krex D, Klink B, Hartmann C, et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 21.Alonso M, Hamelin R, Kim M, et al. Microsatellite instability occurs in distinct subtypes of pediatric but not adult central nervous system tumors. Cancer Res. 2001;61:2124–8. [PubMed] [Google Scholar]
- 22.Kreth FW, Faist M, Rossner R, et al. Supratentorial World Health Organization grade 2 astrocytomas and oligoastrocytomas. A new pattern of prognostic factors. Cancer. 1997;79:370–9. doi: 10.1002/(sici)1097-0142(19970115)79:2<370::aid-cncr21>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 23.Ng WH, Wan GQ, Too HP. Higher glioblastoma tumour burden reduces efficacy of chemotherapeutic agents: in vitro evidence. J Clin Neurosci. 2007;14:261–6. doi: 10.1016/j.jocn.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Muragaki Y, Iseki H, Maruyama T, et al. Usefulness of intraoperative magnetic resonance imaging for glioma surgery. Acta Neurochir Suppl. 2006;98:67–75. doi: 10.1007/978-3-211-33303-7_10. [DOI] [PubMed] [Google Scholar]
- 25.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 26.McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583–8. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez JC, Maruyama Y, Yaes R, et al. Accelerated fractionation radiotherapy for hospitalized glioblastoma multiforme patients with poor prognostic factors. J Neurooncol. 1990;9:41–5. doi: 10.1007/BF00167067. [DOI] [PubMed] [Google Scholar]
- 28.Bauman GS, Gaspar LE, Fisher BJ, et al. A prospective study of short-course radiotherapy in poor prognosis glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1994;29:835–9. doi: 10.1016/0360-3016(94)90573-8. [DOI] [PubMed] [Google Scholar]