Abstract
Recent studies have shown that fingolimod (FTY720) is neuroprotective in CNS injury models of cerebral ischemia and spinal cord injury. The purpose of the study was to examine the effect of fingolimod in a mouse model of intracerebral hemorrhage. ICH was produced in adult CD1 mice by injecting collagenase VII-S (0.5 μL, 0.06 U) into the basal ganglia. Fingolimod (or saline) was given 30 min after surgery and once daily for two days. Three days after intracerebral hemorrhage, brain edema, hematoma volume and the number of apoptotic cells were quantified. In another cohort of mice, brain atrophy was evaluated 2 weeks following intracerebral hemorrhage. Neurobehavioral tests were performed at 3, 7 and 14 days. Fingolimod significantly decreased edema, apoptosis and brain atrophy. More importantly, fingolimod enhanced neurobehavioral recovery. Preliminary experiments showed no difference in the number of inflammatory (CD68-positive) cells between the two groups. In conclusion, fingolimod exerts protective effects in a mouse model of intracerebral hemorrhage; the mechanisms underlying this neuroprotective effects deserves further study.
Keywords: Intracerebral hemorrhage, stroke, FTY720, sphingosine 1-phosphate, neuroprotection
1. Introduction
Nontraumatic intracerebral hemorrhage (ICH) accounts for 15 to 20 % of all strokes; with only 38% of patients surviving the first year (Qureshi et al., 2001). Brain edema is an independent factor that determines the outcome, and therefore reducing brain edema is the most important method to treat ICH patients (review (Adeoye and Broderick, 2010; Kuramatsu et al., 2013)). In addition, apoptosis also represents a prominent form of cell death after ICH (Qureshi et al., 2003; Wang et al., 2011), suggesting that agents that would decrease both brain edema and apoptosis could be useful to decrease mortality and/morbidity associated with ICH.
Fingolimod (FTY720) is a sphingosine-1-phosphate (S1P) analogue, which is now used for the treatment of multiple sclerosis (Kappos et al., 2006). Although its mechanism of action in multiple sclerosis is thought to be peripheral and involve induction of lymphopenia, this agent does show protective effects in acute central nervous system injury models of cerebral ischemia (Hasegawa et al., 2010) and spinal cord injury (Lee et al., 2009a; Zhang et al., 2009). In a detailed study, we have found that fingolimod treatment decreases brain edema, apoptosis, and infarct volume and improves behavioral functions in rodents with ischemic stroke (Wei et al., 2011). More recently, our experiment showed that fingolimod reduces hemorrhagic transformation and prevents blood brain barrier breakdown following delayed tissue plasminogen activator treatment in a mouse thromboembolic model (Campos et al., 2013).
Based on these studies, our goal was to test the hypothesis that fingolimod would also be effective in a mouse model of ICH. We therefore compared brain edema, apoptosis, brain atrophy, inflammatory cells and neurobehavioral scores in fingolimod- and saline-treated mice. Our result show that fingolimod decreases brain edema, apoptosis and brain atrophy, as well as improves motor deficit, however it has no influence on the number of brain inflammatory cells.
2. Results
After the ICH surgery, the animals were randomly assigned to the fingolimod or saline treatment groups. In the short-term study, all the animals survived before they were euthanized on day 3. However, one mouse died (in the control group and on day 8) in the long-term experiment.
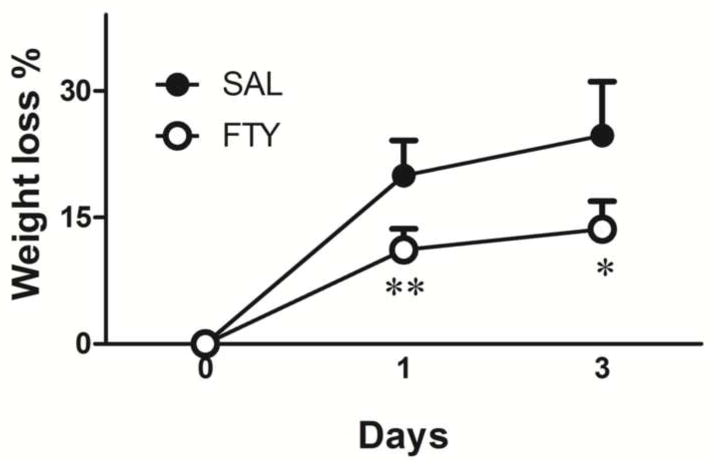
2.1. Fingolimod attenuated weight loss following ICH
Mice were weighed before and after surgery. All mice showed severe weight loss after brain hemorrhage. Fingolimod treatment significantly reduced weight loss, compared to controls (p=0.0001 for day 1 and p=0.02 for day 3, n=10 in each group) (Fig.1).
Fig. 1. Weight loss after ICH.
Compared to pre-surgery, animals lost weight in both treatment groups after collagenase injection. However, fingolimod-treated mice lost less weight than vehicle-treated mice. *p<0.05, **p<0.01, compared to control group, n=10 in each group.
2.2. Fingolimod reduced brain edema
Brain water content was measured 3 days after ICH. After euthanasia and decapitation, the brains were immediately placed into a brain-cutting matrix. Each hemisphere was separated into two halves: section 1, surrounding the hematoma, and section 2, comprising the rest of the forebrain. Compared to the saline-treated group, fingolimod significantly reduced brain edema in section 1 (P=0.037) and in the hemisphere as a whole (P=0.029, n=5 in each group) (Fig.2).
Fig. 2. Fingolimod reduces brain edema.
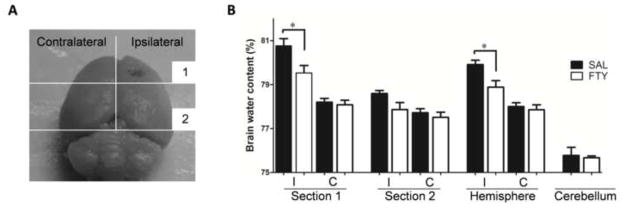
Brain water content was measured 3 days after ICH in the ipsilateral (I) and contralateral (C) hemispheres. A) Each hemisphere was separated into two sections as shown in Panel A. B) The percentage of water content was expressed as (wet − dry weight) / wet weight. Compared to vehicle-treated mice (SAL), fingolimod (FTY) significantly reduced brain edema. *p<0.05, compared to control group, n=5.
2.3. Fingolimod decreased apoptotic cells
Fingolimod has previously been shown to decrease the number of apoptotic neurons in a mouse stroke model (Wei et al., 2011). To examine the effect of fingolimod on apoptosis in this model of ICH, we used a TUNEL detection kit for the detection of the endonucleolytic cleavage of chromatin, characteristic of apoptosis. All the cell nuclei were counterstained with DAPI. The ratios of apoptotic to normal cell nuclei were compared in the fingolimod and saline groups. Three days after ICH, fingolimod treatment significantly reduced the proportion of apoptotic cells in the core (p=0.024, n=10 in each group), but not at the edge of the hematoma (Fig.3 and S2).
Fig. 3. Fingolimod decreased apoptotic cells.
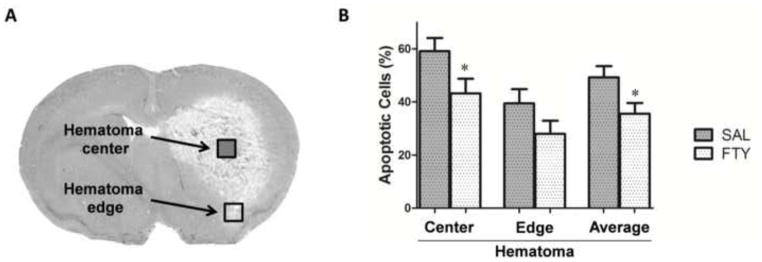
The apoptotic cells were counted at 3 days after ICH. The ratios of apoptosis were compared between FTY720 and saline group. A) Cells were counted independently in the core of the hemorrhage (open square) and in the peripheral region (filled square). B) Fingolimod significantly reduced the number of apoptotic cells located in the center of the hematoma. Data are presented as mean ± SEM, *p< 0.05, compared to control group, n=10 in each group.
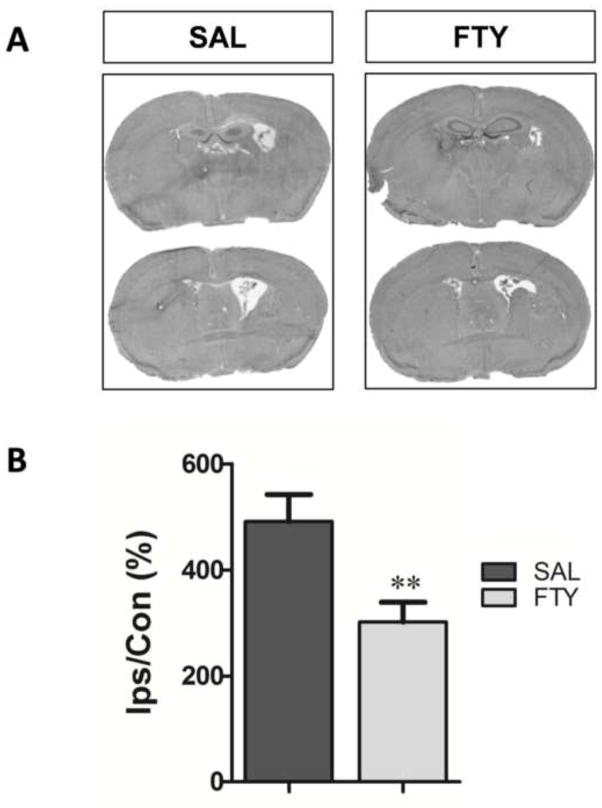
2.4. Fingolimod alleviated brain atrophy
As an indirect measure of brain atrophy, the ventricle sizes were compared between the two groups (Sun et al., 2011). The ipsilateral ventricle size was expressed as a percentage of the contralateral ventricle. Two weeks after ICH, fingolimod significantly decreased the volume of the ipsilateral dilated ventricle, compared to control group (p=0.001, n=10 in FTY720 group and n=9 in saline group) (Fig.4).
Fig. 4. Fingolimod decreases brain atrophy.
Ventricle sizes were used as indirect measure of brain atrophy 2 weeks after ICH. A) Representative eosin-hematoxylin-stained brain sections in saline (SAL) and fingolimod-treated mice (FTY). B. The ipsilateral (Ips) ventricle size was expressed as a percentage of that of the contralateral (Con). **p< 0.01, compared to control group; n=10 in FTY720 group and n=9 in saline group.
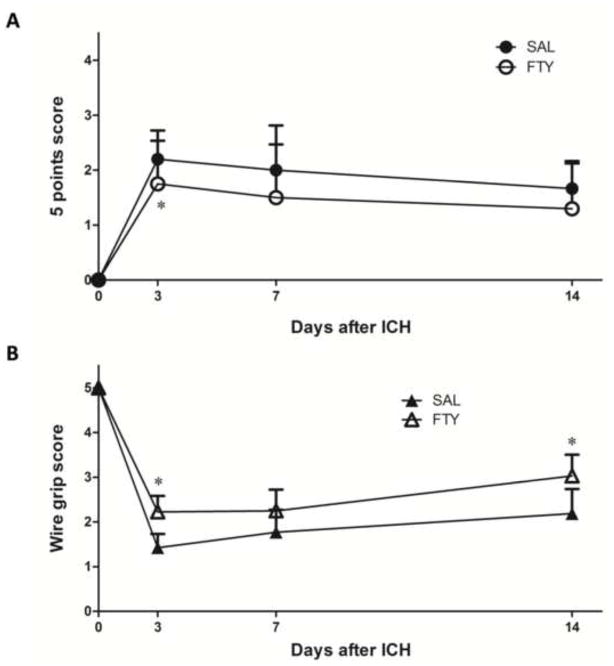
2.5. Fingolimod improved neurobehavioral functions
Neurobehavioral outcome was evaluated 3 days, 1 week and 2 weeks after ICH. We used a gross 5-point neurological assessment and a “wire grip” test to evaluate motor functions. All the tests were performed in a blinded manner. Compared to the saline-treated group, fingolimod significantly reduced behavioral deficits in both tests (p<0.05, n=10 in FTY720 group and n=9 in saline group) (Fig.5).
Fig. 5. Fingolimod improves neurobehavioral functions.
Neurobehavioral outcomes were evaluated 3 days, 1 week and 2 weeks after ICH. A) The 5-point scale evaluation was implemented as indicated in the text; higher scores correspond to larger deficits. B) The wire grip performance was quantified as described in Methods; lower scores correspond to larger deficits. FTY=fingolimod, SAL=Saline. *p< 0.05, compared to control group (at 3 days: n=20 in the fingolimod group and n=19 in the saline group; at 1 and 2 weeks, n=10 in the fingolimod group and n=9 in the saline group).
2.6. Fingolimod did not change hematoma volume and inflammatory cells
Since S1P is known to attenuate blood-brain barrier dysfunction (van Doorn et al., 2012) and fingolimod decreases hemorrhagic transformation associated with delayed administration of Tissue Plasminogen Activator in a mouse stroke model (Campos et al., 2013), we examined whether fingolimod affected the extent of collagenase-induced bleeding, using DAB staining to assess the volume of hemorrhagic brain tissue. Compared to saline, fingolimod did not affect hemorrhage volume (p=0.46, n=10 in FTY720 group and n=9 in saline group) (Fig.6A and S3A). In addition, because a previous study has found that fingolimod-induced neuroprotection was accompanied by decreased inflammation (Wei et al., 2011), we assessed the number of inflammatory cells stained with antibodies against CD68 (monocytes/macrophages). Fingolimod did not impact the number of cells labeled with CD68 antibody (p=0.74, n=10 in FTY720 group and n=9 in saline group) (Fig.6B and S3B for CD68).
Fig. 6. Fingolimod does not alter hematoma volume or the number of CD68+ positive cells.
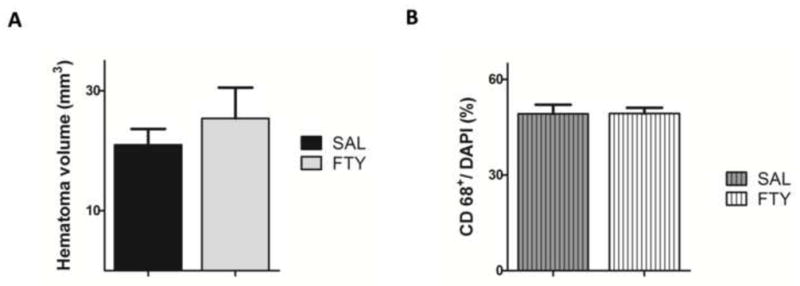
A) The volume of hemorrhage was determined using DAB-stained sections. B) The number of CD68 positive cells was compared between the fingolimod and the control groups. Data were presented as mean ± SEM, n=10 in the fingolimod group and n=9 in the saline group.
3. Discussion
The present study was designed to test the efficacy of fingolimod in a mouse model of ICH. Our results show that fingolimod decreased brain edema and atrophy and inhibited apoptosis, improved neurological functions, but did not seem to influence brain inflammation. These data, taken together with the results of another study showing that fingolimod reduced sensorimotor deficits and brain edema in experimental ICH in mice (Rolland et al., 2013), strengthen the notion that fingolimod could eventually be used clinically to reduce brain damage and enhance recovery after ICH.
Drugs acting on the immune system, although useful in their ability to reduce inflammation, are often limited by their toxicity profile. We therefore first assessed the safety of fingolimod in mice with ICH, using body weight as an indirect indicator of general toxicity. Previous studies have shown that fingolimod had either no effect or induced a slight decrease in body weight (Lee et al., 2009b; Quesniaux et al., 2000). In the present study, all mice lost weight following ICH. However, mice treated by fingolimod had a less pronounced weight loss, compared to the animals in the saline group, suggesting either that fingolimod had no toxicity, or that any adverse effects were more than compensated for by reversing the consequences of intracranial hemorrhage. The effect of fingolimod on body weight may indeed have been a sign that it was neuroprotective, considering studies in stroke, showing that changes in body weight are often proportional to cerebral infarct sizes and an indirect index of ischemic brain damage (Reglodi et al., 2003; Rogers et al., 1997; Yamamoto et al., 1988).
Brain edema develops in most patients with ICH and has been linked to early neurological deterioration. It is a predictor of poor functional outcome (Adeoye and Broderick, 2010; Kuramatsu et al., 2013). In the current study, fingolimod significantly decreased brain water content, which is consistent with the results of a recently published study (Rolland et al., 2013). Fingolimod has been shown to promote adherent junction assembly and decreased permeability of the blood brain barrier via S1P1 receptors (Brinkmann et al., 2004; Lee et al., 2006). It is therefore possible that fingolimod prevents blood brain barrier breakdown after ICH, a contention supported by our recent observation that fingolimod inhibited Evans blue leakage caused by delayed tissue plasminogen activator (tPA) administration in a mouse model of hemorrhagic transformation and thromboembolic stroke (Campos et al., 2013).
Histological analyses performed in tissue from patients with ICH indicate that apoptosis represents a prominent form of cell death after ICH (Qureshi et al., 2003; Wang et al., 2011). Several pathways leading to apoptosis have been identified and agents targeting these pathways are now undergoing clinical trial (Keep et al., 2012). Fingolimod had previously been found to decrease apoptotic cell death following middle cerebral artery occlusion in mice (Wei et al., 2011), but its effect in an ICH model had not been studied. We now report that fingolimod significantly reduced apoptotic cells. It is unclear whether this effect is related to a direct neuroprotective action on neurons, or is accounted for by a decrease in deleterious mediators or conditions surrounding neurons in vivo. The fact that we were unable to observe neuroprotective effect of fingolimod using primary neurons (Wei et al., 2011) suggests that the latter hypothesis is more likely.
Protective effects of fingolimod have now been observed in a large number of acute brain injury models, including cerebral ischemia (Liu et al., 2013), spinal cord injury (Lee et al., 2009a; Zhang et al., 2009), traumatic brain injury (Zhang et al., 2007), intracerebral hemorrhage (Rolland et al., 2013), and t-PA-induced hemorrhagic transformation (Campos et al., 2013) et al. Some studies indicate that this protection is associated with decreased macrophage infiltration (Kaneider et al., 2004; Rausch et al., 2004; Wei et al., 2011; Zhang et al., 2007) and microglial activation (Gao et al., 2012; Jackson et al., 2011; Miron et al., 2010; Noda et al., 2013). After ICH, the number of cells positive for CD68 (a marker for macrophage and microglia) increased 7–8 fold in the cerebral parenchyma adjacent to the hematoma (Dahnovici et al., 2011), and numerous CD68-positive cells were also seen in infarcted tissue after brain ischemia (Krupinski et al., 1996). In our study, the number of CD68 positive cells was not altered by fingolimod. This lack of effect was also reported in another study using demyelination models (Hu et al., 2011); it suggests that the protective effects of fingolimod may involve mechanisms other than inflammation. In agreement with this hypothesis, several studies hint at direct CNS effects of fingolimod in experimental autoimmune encephalomyelitis (EAE) (Miron et al., 2008), in particular on astrocytes (Choi et al., 2011).
In conclusion, this study documents the safety and potential protective effects of fingolimod in a mouse model of ICH. Further studies are needed to determine the possible mechanisms underlying this protection.
4. Experimental procedures
4.1. Animals
Male CD1 mice (35 to 45 g, Charles River Laboratory, Wilmington, MA, USA) were maintained on a 12/12 hours light/dark cycle and fed ad libitum. Experiments were conducted according to protocols approved by the Animal Research Committee of Massachusetts General Hospital and NIH guide for the Care and Use of Laboratory Animals. Group sizes were predetermined based on the results of preliminary experiments. Mice were randomly allocated to each treatment group.
4.2. Experimental design
The project included both a short-term and a long-term study (Fig.S1). Mice received saline or 0.5 mg/kg fingolimod (i.p.) 30 min after surgery and once daily on the following two days. Fingolimod was dissolved in saline at a concentration of 0.5mg/ml immediately before administration. In the short-term study, mice were euthanized 3 days after ICH to measure inflammatory cells, brain edema, hematoma volume and apoptosis. In the long-term experiment, mice were euthanized 2 weeks following surgery to quantify brain atrophy. Behavioral tests were performed 3 days, 1 week and 2 weeks after induction of ICH. All assessments were performed by investigators blinded to the treatment group.
4.3. Intracerebral hemorrhage model
ICH was induced by injecting collagenase into the striatum as previously described (Rodrigues et al., 2003; Rosenberg et al., 1990). Mice were anesthetized with isoflurane (1.8%) and placed in a stereotaxic frame. A burr hole was made and a 30-gauge needle was inserted into the striatum (location 2.5 mm lateral to the midline, 0 mm to bregma, 3.5 mm in depth below the skull). Half a microliter of saline containing 0.06 IU of collagenase (Type VII; Sigma) was injected over 5 min using a micropump. After infusion, the needle was left in place for 25 min, and slowly removed; the skin incision was sutured. Rectal temperature was maintained at 37.5 °C with a heating pad during the surgery. Mice were kept in an incubator to maintain body temperature for 3 h after the surgery.
4.4. Brain edema
After euthanasia by decapitation, the brains were immediately removed and placed into a brain-cutting matrix. Hemispheres were separated into two sections based on the position of the needle track: the rostral first section comprised brain tissue around the core of the hematoma, while the caudal second section comprised unaffected brain tissue (Fig.2A). The cerebellum was used as internal control for brain water content. Tissue samples were weighed to determine the wet weight (WW), and then dried at 100 °C for 24h to obtain dry weight (DW). The percentage of water content was calculated using the formula, [(WW − DW) / WW] × 100%.
4.5. Behavioral testing
For behavioral tests, 5-point assessment and wire grip tests were performed in a blinded manner to evaluate outcomes at day 3, week 1 and week 2. The 5-point scale evaluation was implemented as follows (Foerch et al., 2008): 0 = no apparent deficit; 1 = slight deficit, extension deficit of left forepaw or slight instability during walking, but no circling; 2 = circling to the right with at least some straight movements and some covering of distance; 3 = heavy circling to the right without straight movements or no movements at all; 4 = moribund. For the hanging wire test, mice were picked up by the tail and placed on a taut metal wire suspended between two upright bars 50 cm above a padded table (Bermpohl et al., 2006). The wire grip scores were quantitated as follows: 0, if the mouse was unable to remain on the wire for more than 30 sec; 1, if the mouse failed to hold onto the wire with both forepaws and hindpaws; 2, if the mouse held onto the wire with both forepaws and hindpaws but not the tail; 3, if the mouse used its tail along with both forepaws and both hindpaws; 4, if the mouse moved along the wire on all four paws plus tail; 5, if mouse that scored 4 points also ambulated down one of the posts supporting the wire.
4.6. Tissue preparation
After behavioral tests, the animals were over anesthetized and euthanized. Brains were frozen immediately, and cut into 20 μm sections with a cryostat. Brain sections were taken every 500 μm, starting at + 1.5 mm and extending to −3.5 mm with respect to bregma. The slides were stored at − 80 °C until used for assessment of apoptosis, hematoma volume, lesion size and brain atrophy.
4.6. Analysis of apoptosis
The extent of cell death was assessed using a Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) kit (12156792910, Roche, USA) (Matsushita et al., 2011). Nuclei were counterstained by 4′,6-diamidino-2-phenylindole (DAPI). Images were acquired from 4 randomly selected fields (40X) at both the edge and center of the hematoma region (Fig.3 and S2). The total number of nuclei and TUNEL positive cells were counted in each field of view, and the ratio of apoptotic cells to nuclei was calculated.
4.7. Hematoma volume
Hematoma volume was assessed by 3,33-diaminobenzidine tetrahydrochloride (DAB) staining after ICH (Wasserman et al., 2008). The DAB kit contains a metal enhancer (Sigma; D0426, USA), which reacts with hemoglobin to form a blue–black product while blood-free tissue remains almost clear (Fig.S3A). After DAB staining, the area of hematoma was circled in each section and the volume of hematoma was integrated for all the sections with blood (MCID Elite, InterFocus Imaging, UK) (Campos et al., 2013).
4.8. Inflammatory cells
In order to count the number of inflammatory cells in brain sections, we performed immunohistochemical staining with antibodies against CD68, a marker for macrophages (Dahnovici et al., 2011). Fresh frozen sections were fixed with 4% PFA for 10 min, and blocked with 10% normal donkey serum in PBS. The primary antibody mouse monoclonal against CD68 (KP1, 1:2000, ab955, Abcam, USA) was then added and incubated for 1h at room temperature. After washing with PBS, the sections were incubated with a donkey anti-mouse IgG secondary antibody (1:300, 715165150, Jackson, USA), and nuclei were counterstained with DAPI (Fig.S3B). Using a wide-field fluorescence microscope, 4 fields were randomly selected (20X) and the number of positive cells was counted by an investigator blinded to the treatment groups.
4.9. Brain atrophy
Frozen brain sections were stained using hematoxylin and eosin (H & E). Enlargement of the lateral ventricle was used as a proxy measure for brain tissue loss in the injured hemisphere as described (Sun et al., 2011) Lateral ventricular areas were measured in consecutive sections with the software MCID. The volume of ipsilateral ventricle size was expressed as percent of that of the contralateral ventricle (Fig 4).
4.10. Statistical Analysis
Data (except for behavior) are shown as mean ± SEM. The distribution of all data was normal, as assessed by Normal Quantile Plots analysis. Differences between two groups were compared by Student’s t test. Neurological scores are given as median, and were analyzed by Mann-Whitney rank sum test. All statistical analyses (except Normal Quantile Plots) were performed using SPSS 16.0. Differences were considered statistically significant at p < 0.05.
Supplementary Material
Highlights.
We examined the effect of fingolimod in a mouse model of intracerebral hemorrhage.
Intracerebral hemorrhage was produced by injecting collagenase into the striatum.
Fingolimod significantly decreased edema and apoptosis (3 days after hemorrhage).
Long term reductions in brain atrophy and neurobehavioral deficit were also seen.
In conclusion, fingolimod exerts protective effects in a mouse model of ICH.
Acknowledgments
Funding for this study was provided by NIH grants (NS049263 and NS055104 to Dr. Christian Waeber). We thank Prof. Wolfgang Eisert and Boehringer Ingelheim Pharma GmbH & Co. KG for providing the BI Stroke Award fellowship to Dr. Lei Lu. We thank Prof. Eng H. Lo for his critical advice and support during the study.
Footnotes
Disclosures
The authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat Rev Neurol. 2010;6:593–601. doi: 10.1038/nrneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- Bermpohl D, You Z, Korsmeyer SJ, Moskowitz MA, Whalen MJ. Traumatic brain injury in mice deficient in Bid: effects on histopathology and functional outcome. J Cereb Blood Flow Metab. 2006;26:625–33. doi: 10.1038/sj.jcbfm.9600258. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Cyster JG, Hla T. FTY720: Sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Campos F, Qin T, Castillo J, Seo JH, Arai K, Lo EH, Waeber C. Fingolimod reduces hemorrhagic transformation associated with delayed tissue plasminogen activator treatment in a mouse thromboembolic model. Stroke. 2013;44:505–11. doi: 10.1161/STROKEAHA.112.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A. 2011;108:751–6. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahnovici RM, Pintea IL, Malaescu DG, Busuioc CJ, Predescu A, Mogoanta L. Microscopic aspects of macrophage system cells in hemorrhagic stroke in humans. Rom J Morphol Embryol. 2011;52:1249–53. [PubMed] [Google Scholar]
- Foerch C, Arai K, Jin G, Park KP, Pallast S, van Leyen K, Lo EH. Experimental model of warfarin associated intracerebral hemorrhage. Stroke. 2008;39:3397–404. doi: 10.1161/STROKEAHA.108.517482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Liu Y, Li X, Wang Y, Wei D, Jiang W. Fingolimod (FTY720) inhibits neuroinflammation and attenuates spontaneous convulsions in lithium-pilocarpine induced status epilepticus in rat model. Pharmacol Biochem Behav. 2012;103:187–96. doi: 10.1016/j.pbb.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–74. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YH, Lee XH, Ji BX, Guckian K, Apicco D, Pepinsky RB, Miller RH, Mi S. Sphingosine 1-phosphate receptor modulator fingolimod (FTY720) does not promote remyelination in vivo. Molecular and Cellular Neuroscience. 2011;48:72–81. doi: 10.1016/j.mcn.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Jackson SJ, Giovannoni G, Baker D. Fingolimod modulates microglial activation to augment markers of remyelination. J Neuroinflammation. 2011;8:76. doi: 10.1186/1742-2094-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneider NC, Lindner J, Feistritzer C, Sturn DH, Mosheimer BA, Djanani AM, Wiedermann CJ. The immune modulator FTY720 targets spingosine-kinse-depent migration of human monocytes in response to amyloid beta-protein and its precurson. FASEB J. 2004;18:1309–11. doi: 10.1096/fj.03-1050fje. [DOI] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–40. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–31. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S. Immunocytochemical studies of cellular reaction in human ischemic brain stroke. MAB anti-CD68 stains macrophages, astrocytes and microglial cells in infarcted area. Folia Neuropathol. 1996;34:17–24. [PubMed] [Google Scholar]
- Kuramatsu JB, Huttner HB, Schwab S. Advances in the management of intracerebral hemorrhage. J Neural Transm. 2013 doi: 10.1007/s00702-013-1040-y. [DOI] [PubMed] [Google Scholar]
- Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, Hla T, Wang E, Lee MJ. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281:29190–200. doi: 10.1074/jbc.M604310200. [DOI] [PubMed] [Google Scholar]
- Lee KD, Chow WN, Sato-Bigbee C, Graf MR, Graham RS, Colello RJ, Young HF, Mathern BE. FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J Neurotrauma. 2009a;26:2335–44. doi: 10.1089/neu.2008.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KD, Chow WN, Sato-Bigbee C, Graf MR, Graham RS, Colello RJ, Young HF, Mathern BE. FTY720 Reduces Inflammation and Promotes Functional Recovery after Spinal Cord Injury. J Neurotrauma. 2009b;26:2335–2344. doi: 10.1089/neu.2008.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang C, Tao W, Liu M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. Int J Neurosci. 2013;123:163–9. doi: 10.3109/00207454.2012.749255. [DOI] [PubMed] [Google Scholar]
- Matsushita H, Hijioka M, Hisatsune A, Isohama Y, Shudo K, Katsuki H. A retinoic acid receptor agonist Am80 rescues neurons, attenuates inflammatory reactions, and improves behavioral recovery after intracerebral hemorrhage in mice. J Cereb Blood Flow Metab. 2011;31:222–34. doi: 10.1038/jcbfm.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, Antel JP. Fingolimod (FTY720) Enhances Remyelination Following Demyelination of Organatypic Cerebellar Slices. American Journal of Pathology. 2010;176:2682–2694. doi: 10.2353/ajpath.2010.091234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Schubart A, Antel JP. Central nervous system directed effects of FTY720 (fingolimod) J Neurol Sci. 2008;274:13–7. doi: 10.1016/j.jns.2008.06.031. [DOI] [PubMed] [Google Scholar]
- Noda H, Takeuchi H, Mizuno T, Suzumura A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J Neuroimmunol. 2013;256:13–8. doi: 10.1016/j.jneuroim.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Quesniaux VF, Menninger K, Kunkler A, Vedrine C, Bernhard M, Hedinger R, Kraus G, Schuurman HJ. The novel immunosuppressant FTY720 induces peripheral lymphodepletion of both T- and B-cells in cynomolgus monkeys when given alone, with Cyclosporine Neoral or with RAD. Transpl Immunol. 2000;8:177–87. doi: 10.1016/s0966-3274(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–60. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Ostrow PT, Kim SH, Ali Z, Shatla AA, Guterman LR, Hopkins LN. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery. 2003;52:1041–7. discussion 1047–8. [PubMed] [Google Scholar]
- Rausch M, Hiestand P, Foster CA, Baumann DR, Cannet C, Rudin M. Predictability of FTY720 efficacy in experimental autoimmune encephalomyelitis by in vivo macrophage tracking: Clinical implications for ultrasmall superparamegnetic iron oxide-enhanced magnetic resonance imaging. Journal of Magnetic Resonance Imaging. 2004;20:16–24. doi: 10.1002/jmri.20057. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Tamas A, Lengvari I. Examination of sensorimotor performance following middle cerebral artery occlusion in rats. Brain Res Bull. 2003;59:459–66. doi: 10.1016/s0361-9230(02)00962-0. [DOI] [PubMed] [Google Scholar]
- Rodrigues CM, Sola S, Nan Z, Castro RE, Ribeiro PS, Low WC, Steer CJ. Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc Natl Acad Sci U S A. 2003;100:6087–92. doi: 10.1073/pnas.1031632100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- Rolland WB, Lekic T, Krafft PR, Hasegawa Y, Altay O, Hartman R, Ostrowski R, Manaenko A, Tang J, Zhang JH. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp Neurol. 2013;241:45–55. doi: 10.1016/j.expneurol.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–7. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- Sun Y, Dai M, Wang Y, Wang W, Sun Q, Yang GY, Bian L. Neuroprotection and sensorimotor functional improvement by curcumin after intracerebral hemorrhage in mice. J Neurotrauma. 2011;28:2513–21. doi: 10.1089/neu.2011.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn R, Nijland PG, Dekker N, Witte ME, Lopes-Pinheiro MA, van het Hof B, Kooij G, Reijerkerk A, Dijkstra C, van van der Valk P, van Horssen J, de Vries HE. Fingolimod attenuates ceramide-induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes. Acta Neuropathol. 2012;124:397–410. doi: 10.1007/s00401-012-1014-4. [DOI] [PubMed] [Google Scholar]
- Wang YX, Yan A, Ma ZH, Wang Z, Zhang B, Ping JL, Zhu JS, Zhou Y, Dai L. Nuclear factor-kappaB and apoptosis in patients with intracerebral hemorrhage. J Clin Neurosci. 2011;18:1392–5. doi: 10.1016/j.jocn.2010.11.039. [DOI] [PubMed] [Google Scholar]
- Wasserman JK, Yang H, Schlichter LC. Glial responses, neuron death and lesion resolution after intracerebral hemorrhage in young vs. aged rats. Eur J Neurosci. 2008;28:1316–28. doi: 10.1111/j.1460-9568.2008.06442.x. [DOI] [PubMed] [Google Scholar]
- Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, Guo S, Qin T, Alsharif N, Brinkmann V, Liao JK, Lo EH, Waeber C. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69:119–29. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Tamura A, Kirino T, Shimizu M, Sano K. Behavioral changes after focal cerebral ischemia by left middle cerebral artery occlusion in rats. Brain Res. 1988;452:323–8. doi: 10.1016/0006-8993(88)90036-4. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang A, Sun Y, Cao X, Zhang N. Treatment with immunosuppressants FTY720 and tacrolimus promotes functional recovery after spinal cord injury in rats. Tohoku J Exp Med. 2009;219:295–302. doi: 10.1620/tjem.219.295. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Fauser U, Artelt M, Burnet M, Schluesener HJ. FTY720 attenuates accumulation of EMAP-II+ and MHC-II+ monocytes in early lesions of rat traumatic brain injury. J Cell Mol Med. 2007;11:307–14. doi: 10.1111/j.1582-4934.2007.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.