Abstract
We examined changes in intraepidermal nerve fibers (IENFs) to differentiate patients with diabetic neuropathy (DN) and neuropathic pain (DN-P) from those with DN without pain (DN-NOP). Punch skin biopsies were collected from the proximal thigh (PT) and distal leg (DL) of normal subjects (NS), patients with type 2 diabetes without evidence of DN (DM), or DN-P and DN-NOP patients. Protein gene product 9.5 (PGP) immunohistochemistry was used to quantify total IENF, and growth associated protein 43 (GAP43) for regenerating IENF. Compared to NS and DM, both DN-P and DN-NOP have reduced PGP+ IENF densities in DL and PT. Although GAP43+ IENF densities were also reduced in DL for both DN-P and DN-NOP, the GAP43+ IENF densities in PT of DN-P remained at the control levels. Higher GAP43/PGP ratios were detected in DN-P compared to DN-NOP in the DL and PT. In parallel, increased numbers of axonal swellings per PGP+ fiber (axonal swelling/PGP) were detected in DN-P compared to NS, DM, and DN-NOP in the DL. These axonal swellings were positive for tropomyosin-receptor-kinase (Trk) A and substance P, suggesting that they are associated with nociception.
Keywords: diabetic pain, intraepidermal nerve fiber, peripheral neuropathy
Introduction
Over 20 million Americans are affected by diabetes mellitus. Sixty percent of patients develop diabetic neuropathy (DN), which originates in the distal lower extremities and is frequently associated with pain (DN-P) early in the disease course 1-3. DN-P is frequently described as a continuously burning, tingling, electric-like, cramping, or aching pain which begins in the feet and progressively extends proximally over time. These neuropathic pain symptoms are generated from small caliber Aδ and C fibers which innervate the skin and are named intraepidermal nerve fibers (IENFs). Intraepidermal nerve fibers are identified as peptidergic or non-peptidergic based on their ability to express neuropeptides for nociception. Peptidergic, but not non-peptidergic, IENFs are positive for the high affinity NGF receptor, tropomyosin-receptor-kinase (Trk) A and express nociceptive neuropeptides such as substance P (SP) to mediate nociception.
Our previously published data suggest that NGF-mediated axonal regeneration in IENFs could be an important mechanism for the development of mechanical allodynia in diabetic mice 4. In the current study, we tested the hypothesis that there is more axonal regeneration in the skin of patients with DN-P compared to DN-NOP. First, we examined protein gene product 9.5-positive (PGP+) IENF density (IENFD) using a standard protocol for quantifying IENFD in patients. Results from patients with DN-P14 were compared to normal subjects (NS), patients with diabetes without neuropathy (DM), and patients with DN-NOP. We next examined the degree of axonal regeneration by measuring the ratio of growth-associated protein 43 (GAP43), a marker for axonal regeneration, to PGP+ IENFs. Finally, the density of axonal swelling, a morphological change associated with neuropathy, was measured using a previously published protocol by Lauria et. al 13. Our results suggest that ratios of GAP43/PGP and axonal swelling/PGP could be useful biomarkers for characterizing DN-P.
Methods
Patient populations
Skin samples were collected from NS, DM, DN-P, and DN-NOP patients (Table 1) participating in a longitudinal cohort study on DN (The Utah Diabetic Neuropathy Study – UDNS). Subjects fulfilling the pre-specified criteria for each group were randomly selected from the full UDNS cohort. All diabetic patients underwent a quantitative neurologic history and examination and a series of confirmatory neurophysiologic tests to diagnose and characterize neuropathy. Subjects were characterized based on six attributes: symptoms, signs (based on The Utah Early Neuropathy Scale >4), nerve conduction studies, quantitative sudomotor axon reflex testing (QSART), quantitative sensory testing (QST) for vibration detection and cold detection thresholds (VDT and CDT), and IENFD. Subjects with 0/6 abnormal attributes were classified as without, subjects with 3 or more abnormal attributes as probable, and subjects with 1-2 abnormal attributes as possible neuropathy 18. All DN patients in the current study had probable neuropathy for less than 5 years. Patients with a visual analog pain score (VAS) greater than 5 out of 100 were considered part of the DN-P group. All the DN-P patients have features of neuropathic pain (burning, tingling, electric-like, cramping, or aching pain) in both feet. Hemoglobin A1c (HbA1c) levels were measured for each patient. The study received approval by the Institutional Review Board of the University of Utah and written informed consent was obtained from all subjects.
Table 1.
Demographic and clinical features of the study population
| NS | DM | DN-P | DN-NOP | |||||
|---|---|---|---|---|---|---|---|---|
| Median | 25-75% | Median | 25-75% | Median | 25-75% | Median | 25-75% | |
| Age | 61 | 53.5-65.5 | 57.5 | 52.3-64.8 | 58 | 48-61.5 | 59 | 52-61 |
| Female/Male | 9/8 | 9/11 | 6/10 | 10/9 | ||||
| Mean HbA1c (%) | N/A | 5.95 | 5.7-6.6 | 5.8 | 5.4-6.8 | 6.5$ | 6.0-8.0 | |
| Pain score (0-100) | 0 (0-0) | 0-0 | 0 | 0-0 | 50.0**** | 33.0-69.0 | 0 | 0-0 |
| Months of diabetes | N/A | 57 | 25-99.8 | 72 | 38-107.5 | 120$ | 60-156.0 | |
p < 0.0001 compared to DM and DN-NOP
p < 0.05 compare to DM and DN-P.
Human skin biopsy
All subjects underwent 3 mm punch skin biopsies at the distal leg (DL, 10 cm above the lateral malleolus, ankle) and the proximal lateral aspect of the thigh (PT, 20 cm below the anterior iliac spine) 11. Samples were immediately fixed in 0.75 M L-Lysine solution (pH 7.4) with 2% paraformaldehyde and 0.05 mM sodium periodate for 12-24 h at 4°C, cryoprotected in PBS with 20% glycerol, and sectioned with a sliding microtome into 50 μm thick free-floating sections before being processed for immunohistochemistry.
Intraepidermal nerve fiber analysis
Three randomly selected sections were stained with each marker as previously described 15. Sections were incubated at 4°C for 16-24 h with the following primary antibodies: PGP (1:1000, Millipore, Billerica, MA), Trk A (1:500, R&D Systems, Minneapolis, MN), SP (1:500, Abcam, Cambridge, MA), transient receptor potential cation channel subfamily V member 1 (TRPV1, 1:1000, Alomone labs, Jerusalem, Israel), and GAP43 (1:500, AbD Serotec, Raleigh, NC). Sections were then rinsed 3 times in PBS and incubated with secondary antiserum conjugated with appropriate fluorophores (AlexaFluor 594 or 647, Invitrogen, Carlsbad, CA). Finally, sections were rinsed and mounted with ProLong® Gold antifade reagent (Invitrogen). Fluorescent images were collected on an Olympus FluoView 500 confocal microscope using a 40 × 1.2 oil immersion objective at a resolution of 1040 × 1040 pixels. The optical section thickness was 0.5 μm. Forty images per stack were flattened using the MetaMorph (Molecular Devices, Sunnyvale, CA, version 6.14) arithmetic option. IENFD data were presented as the mean number of fibers crossing the dermal/epidermal junction per linear mm of epidermis from all six skin sections 5. Axonal swelling was defined as a PGP+ globular axonal structure with a diameter >2 times that of the diameter of the attached axons. Axonal swelling densities were determined by calculating the mean number of PGP+ axonal swellings in the epidermis and subepidermal plexus per linear mm of epidermis from all six skin sections 13. All studies for the quantification of IENF and axonal swelling densities were performed in a blinded fashion. Sections were incubated with primary antisera alone, and secondary antisera alone to ensure specificity. Nonspecific immunolabeling detected in either condition was eliminated during counting.
Data presentation and statistical analyses
All data are presented as group medians with interquartile ranges (25 to 75 percentile) and analyzed with a Mann-Whitney test. A p-value of less than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
The demographic and clinical features of the study population are demonstrated in Table 1. Gender and age distribution were similar between groups. When comparing the clinical features between groups, theDN-NOP group had higher HbA1c levels than DM and DN-P (p < 0.05 for both pairs). The mean pain score in the DN-P group was 50.0, which was significantly higher than pain scores of 0 in the NS controls, DM , and DN-NOP groups (p < 0.0001 for all pairs). Typically, DN-P patients presented with burning, tingling, prickling, and/or sharp stabbing foot pain. Additionally, the duration of diabetes was significantly longer in DN-NOP, compared to DM and DN-P (p < 0.05, Table 1).
Neurophysiologic tests, including the measurements of peroneal motor response proximal conduction velocity (PMCV), peroneal motor response amplitude (PMA), sural sensory amplitude (SSA), QST for cold and vibration detection thresholds (CDT and VDT), quantitative sudomotor axon reflex testing (QSART), and the Utah Early Neuropathy Scale (UENS) were performed for the diagnosis of DN. All measurements were consistent with the presence of neuropathy (Table 2). There was no significant difference in these parameters between the DN-P and DN-NOP groups.
Table 2.
Parameters of neuropathy
| NS | DM | DN-P | DN-NOP | |||||
|---|---|---|---|---|---|---|---|---|
| Median | 25-75% | Median | 25-75% | Median | 25-75% | Median | 25-75% | |
| Electrophysiology | ||||||||
| PMCV (m/s) | N/A | 46.4 | 42.5-50.3 | 40.5*** | 378.0-42.2 | 41.8*** | 38.1-45.8 | |
| PMA | N/A | 5.8 | 3.6-7.2 | 2.9** | 2.3-4.0 | 3.0* | 1.8-3.5 | |
| SSA | N/A | 11.4 | 8.2-15.0 | 4.6** | 0.0-9.2 | 3.0** | 0.0-5.1 | |
| QST | ||||||||
| CDT (%) | N/A | 27.0 | 11.8-48.5 | 97.0**** | 72.0-98.0 | 97.0**** | 82.0-98.0 | |
| VDT (%) | N/A | 62.5 | 41.8-78.5 | 90.0** | 62.0-95.0 | 95.0**** | 88.0-96.0 | |
| QSART | ||||||||
| Foot | N/A | 1.19 | 1.03-1.68 | 0.38* | 0.33-1.53 | 0.72 | 0.18-1.89 | |
| Distal leg | N/A | 1.22 | 0.66-1.63 | 0.17**** | 0.06-0.47 | 0.42** | 0.12-0.74 | |
| Proximal thigh | N/A | 0.73 | 0.48-1.05 | 0.21 | 0.08-0.78 | 1.05 | 0.46-1.37 | |
| UENS | 0 | 0.0-2.0 | 9.0**** | 7.0-15.0 | 8.0** | 2.0-11.5 | ||
PMCV, peroneal motor response proximal conduction velocity; PMA, peroneal motor response amplitude; SSA, sural sensory amplitude; CDT and VDT, cold and vibration detection thresholds; QSART, quantitative sudomotor axon reflex testing; QST, quantitative sensory testing; UENS, Utah Early Neuropathy Scale.
p < 0.05
p < 0.01
p < 0.001
p < 0.0001 compare to DM.
Measurements from distal leg skin
Although the DN-P subjects have pain in the feet, the current protocol for measuring IENFD collects skin biopsies from 10 cm above the lateral malleolus (defined as DL) and 20 cm below the anterior iliac spine (defined as PT) as described previously14. To evaluate the length-dependent changes in IENFs for patients with neuropathy, we performed immunohistochemistry for PGP and GAP43 on skin biopsies from both the DL (Table 3) and PT (Table 4) of patients from each group.
Table 3.
Measurements of IENF and axonal swelling densities in DL skin biopsies.
| NS | DM | DN-P | DN-NOP | |||||
|---|---|---|---|---|---|---|---|---|
| Median | 25-75% | Median | 25-75% | Median | 25-75% | Median | 25-75% | |
| PGP+ IENFD (fiber/mm) | 9.76 | 6.08-13.15 | 10.7 | 6.87-15.5 | 2.62**$$$ | 1.07-8.83 | 2.16****$$$$ | 1.53-4.64 |
| GAP43+ IENFD (fiber/mm) | 3.97 | 2.26-6.09 | 5.12 | 3.68-7.99 | 1.64*$$$ | 0.34-4.08 | 0.35****$$$$# | 0.25-0.50 |
| GAP43/PGP | 0.43 | 0.33-0.54 | 0.5 | 0.40-0.76 | 0.34$ | 0.27-0.47 | 0.15***$$$$### | 0.09-0.20 |
| Axonal swelling/PGP | 0.39 | 0.00-2.96 | 0.26 | 0.05-1.75 | 2.57*$$ | 0.96-4.41 | 0.80# | 0.23-2.63 |
p < 0.05
p < 0.01
p < 0.001
p < 0.0001, compare to NS.
p < 0.05
p < 0.01
p < 0.001
p < 0.0001, compare to DM.
p < 0.05
p < 0.001, compare to DN-P.
Table 4.
Measurements of IENF and axonal swelling densities in PT skin biopsies.
| NS | DM | DN-P | DN-NOP | |||||
|---|---|---|---|---|---|---|---|---|
| Median | 25-75% | Median | 25-75% | Median | 25-75% | Median | 25-75% | |
| PGP9.5+ IENFD (fiber/mm) | 13.90 | 11.56-19.84 | 16.69 | 11.97-28.53 | 12.17$ | 7.20-16.3 | 11.01$ | 7.52-13.99 |
| GAP43+ IENFD (fiber/mm) | 11.22 | 7.90-15.21 | 11.73 | 5.43-14.60 | 9.99 | 5.32-14.05 | 0.41****$$$$#### | 0.32-0.58 |
| GAP43/PGP | 0.74 | 0.66-0.84 | 0.67 | 0.41-0.83 | 0.82$ | 0.75-0.88 | 0.03***$$$$#### | 0.03-0.06 |
| Axonal swelling/PGP | 1.20 | 0.46-2.65 | 4.29** | 1.15-7.63 | 5.62*** | 2.55-8.07 | 2.16### | 1.22-3.16 |
p < 0.01
p < 0.001
p < 0.0001; compared to NS.
p < 0.05
p < 0.0001 compare to DM.
p < 0.001
p < 0.0001 compare to DN-P.
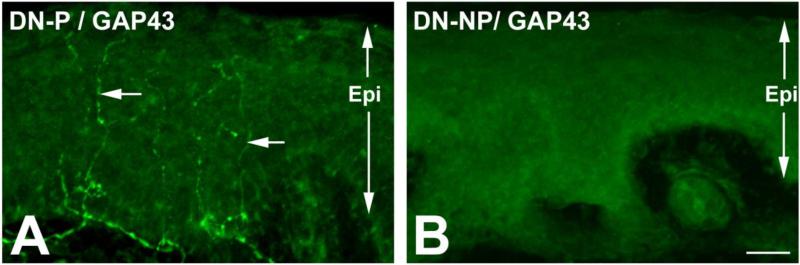
In the DL (Table 3), PGP+ IENFDs were significantly reduced in DN-P and DN-NOP, compared to NS and DM groups. There was no significant difference between the two DN groups. To examine the degree of axonal regeneration, GAP43 immunohistochemistry was performed (Fig. 1). Although the GAP43+ IENFDs were significantly lower in both DN groups compared to NS and DM, those of the DN-P group were higher than the DN-NOP group (p < 0.05). We then calculated the GAP43/PGP ratios to reflect the degree of axonal regeneration per IENF. Using this approach, GAP43/PGP ratios in DN-P were similar to the NS group and were significantly higher than DN-NOP (p < 0.001).
Fig. 1. GAP43+ IENFs in DN-P.
A: Representative images of GAP43 immunohistochemistry on skin biopsies from DN-P (A) and DN-NOP (B) patients. Prominent GAP43+ fibers (arrows) were detected in DN-P, but not in DN-NOP. Bar = 10 μm. Epi: Epidermis.
An axonal swelling is defined as an enlargement of an axonal segment with a diameter greater than 2 times that of the axons attached to it13. The IENF axonal swellings were PGP+ globular structures that were distributed along the axons (Fig. 2). Using the method described by Lauria and colleagues13, we calculated the number of PGP+ axonal swellings per IENF (axonal swelling/PGP ratio), and observed a significant increase in axonal swelling/PGP ratio in DN-P patients compared to NS, DM, and DN-NOP patients in the DL (Table 3).
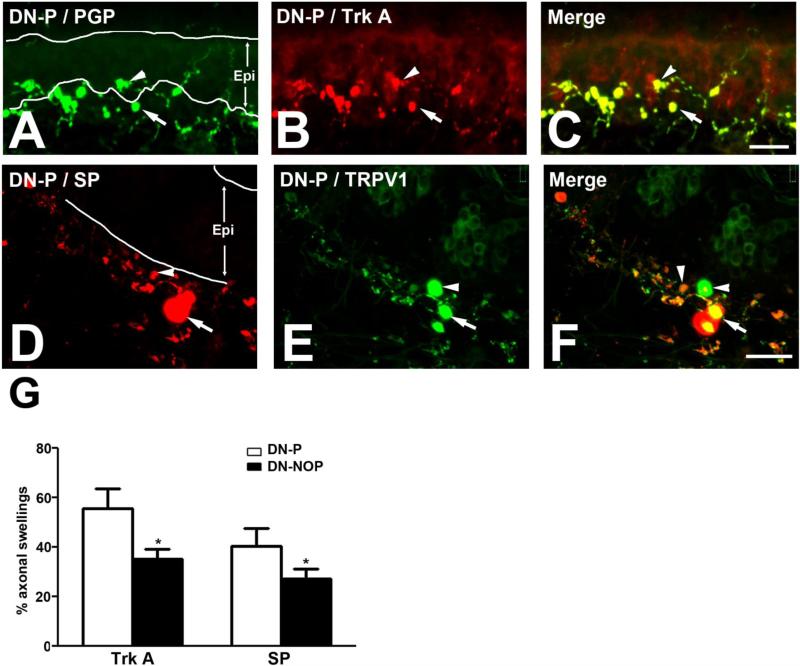
Fig. 2. Immunohistochemical characterization of axonal swellings from DN-P skin samples.
A-C: Axonal swellings (arrows and arrowheads) were identified along IENFs (arrowheads) and in the subepidermal plexus (arrows). Most of these structures were positive for both PGP (A, C) and Trk A (B, C). Bar = 50 μm. D-F: Some axonal swellings in DN-P were positive for both SP (arrow, D) and TRPV1 (arrow, E), or either one of these pain markers (D-F, arrowheads). Bar = 25 μm. Epi: Epidermis. G: Percentages of PGP+ axonal swellings that contain Trk A and SP immunoreactivity in DN-P and DN-NOP. p < 0.05.
Measurements from proximal thigh skin
In contrast to the DL, PGP+ IENFDs were not significantly different among the NS, DN-P, and DN-NOP groups in the PT (Table 4). GAP43+ IENFDs were lower in the DN-NOP group compared to NS, DM, and DN-P groups. GAP43+ IENFD of DN-P patients remained at the NS and DM levels. The DN-NOP group presented with lower GAP43 IENFD (p<0.0001) compared to the other three groups. In addition, GAP43/PGP levels of DN-NOP were significantly reduced compared to NS (p < 0.0001), DM (p < 0.001), and DN-P (p < 0.0001). The DN-P group had a higher ratio of axonal swellings/PGP in the PT compared to NS (p < 0.001) and DN-NOP (p < 0.001) groups.
Immunological characterization of axonal swellings
To further characterize the axonal swellings detected in IENFs in our DN-P skin samples, we performed double immunofluorescent studies for PGP and common pain markers, including Trk A, SP, TRPV1, and GAP43 (Fig. 2). As demonstrated in Fig. 2, most of these axonal swellings were positive for PGP (Fig. 2A, arrow and arrowhead), Trk A (Fig. 2B, arrow and arrowhead), SP (Fig. 2D, arrow and arrowhead), and TRPV1 (Fig. 2E, arrow and arrowhead). The percentages of PGP+ axonal swellings that contained Trk A and SP immunoreactivity were quantified for DN-P and DN-NOP as demonstrated in Fig. 2G. DN-P had higher percentages of Trk A+ and SP+ axonal swellings compared to those of DN-NOP (p < 0.05).
Discussion
Neuropathic pain from distal polyneuropathy is a common symptom that affects at least 40-50% of patients who suffer from diabetic neuropathy 21. However, only a subgroup of patients develop painful phenotypes (DN-P), while others with neuropathy have no pain (DN-NOP). Here, we present data to support the use of skin biopsy to study the distinct pathomechanisms of DN-P and DN-NOP. Our results indicate that PGP+ IENFD measurement, the most commonly used methodology for analyzing IENFs, may not be useful for specifically identifying DN-P among patients with DN. Instead, analysis of GAP43+ fibers and axonal swellings could provide further understanding of the underlying pathomechanisms that are specific to DN-P.
Our sample size was determined by the amount of available skin samples at the time of the study. Even with randomization, we detected higher HbA1C values and longer duration of diabetes in the DN-NOP group compared to DN-P. These finding suggest that increased severity and duration of diabetes could be confounding factors that contribute to the development of insensate neuropathy. This finding is supported by our animal data that suggests mechanical allodynia in db/db mice is developed in the early phase while loss of sensation occurs at later stages of diabetic neuropathy 3. However, follow-up studies are necessary to determine if DN-P patients develop an insensate phenotype overtime.
Our data suggest that measurement of PGP+ IENFDs, the most widely used method in current practice for small fiber neuropathy14, is not a useful method for the diagnosis of DN-P. In support of our current data, loss of PGP+ IENFs in animals and humans with DN-P from both type 1 and type 2 diabetes have been reported in the literature10, 15, 17, 19, 20. Sorensen and colleagues specifically studied the relationship among pain severity and loss of PGP+ IENFs in the DL of patients with both types of diabetes19, 20. They concluded that the severity of PGP+ IENF loss is associated only in patients with neuropathic pain with no objective signs of neuropathy, suggesting that this information could be useful to acknowledge early stages of DN-P. In our study, both DN-P and DN-NOP patients had significant features of neuropathy. However, we did not detect a significant correlation between IENFD loss and the severity of pain (data not shown).
GAP43 is a neuronal membrane protein involved in axonal growth and regeneration. Our data demonstrated an increased GAP43/PGP ratio in DN-P, suggesting increased regeneration of IENFs in DN-P skin. Axonal regeneration is a well-known feature of DN16, 22; however, the link between axonal regeneration and pain is still unclear. Previously, we reported increased NGF-mediated nerve regeneration in IENFs of db/db mice during the period of mechanical allodynia. In that study, anti-NGF antiserum treatment reduced axonal regeneration and pain behavior, suggesting that axonal regeneration mediates painful phenotypes4. In support of our hypothesis, Britland et al reported that increased axonal regeneration is detected in painful phenotypes in sural nerve biopsies of DN1. Fantini and colleagues reported co-localization of GAP43 and Trk A in human skin, suggesting the regeneration of nociceptive nerve fibers7. The current study confirms our previous findings and supports our hypothesis that targeting NGF-mediated nerve regeneration could be a viable therapeutic strategy for treating DN-P associated with type 2 diabetes.
In the current study, we detected increased axonal swellings in IENFs, previously reported as early axonal changes of sensory neuropathy9, 12, 13. These axonal swellings could be associated with an increased risk of developing sensory symptoms in suspected small fiber neuropathy8. Similar to our current results, Lauria and colleagues reported higher axonal swelling/PGP ratios in the DL of patients with painful neuropathy from a variety of causes, including diabetes, in comparison to healthy controls13. Nonetheless, we observe similar findings at both the PT and DL in patients with type 2 diabetes in the DN-P group.
The current results demonstrate that axonal swellings in DN-P are positive for Trk A, SP, and TRPV1. Previously, axonal swellings from neuropathy were characterized by Lauria and colleagues, whose results indicated that the swellings contain components of microtubules and ubiquitin–associated proteins13. In addition, Ebenezer and colleagues studied the microstructure of axonal swellings using electron microscopy and reported that axonal swellings contain an accumulation of mitochondria, vesicular organelles, and neurofilaments6. These reports suggest that axonal swelling could be a result of defective axonal transport, a common feature of sensory neuropathy2. Our current findings suggest the accumulation of nociceptive molecules, including Trk A, SP, and TRPV1, in axonal swellings. The increased percentages of Trk A+ and SP+ axonal swellings in DN-P could suggest these nociceptive molecules could enhance the axonal sensitivity for mechanical and thermal stimuli and result in allodynia and hyperalgesia. However, further study is necessary to clarify the functional roles for axonal swellings in DN-P.
Skin samples from both PT and DL were characterized in the current study. In general, the IENFD is higher in the PT than in the DL, consistent with the length-dependent features of DN and other published reports14, 17. In addition, we detected increased levels of axonal regeneration in the PT compared to the DL. At any given time, the IENFD and morphological changes observed in the PT may demonstrate earlier stages of DN, while those of the DL may reflect the current phase of the disease in the same patient. Our findings suggest that skin from the PT could also provide useful data for differentiating DN-P from DN-NOP.
In the current study, we evaluated potential indicators for DN-P in punch skin biopsies. Our results do not support that PGP+ IENFD measurement is useful for identifying DN-P. Instead, our data suggest that the measurement of GAP43/PGP and axonal swelling/PGP ratios could be useful methods to differentiate between DN-P and DN-NOP patients.
Perspective.
Among patients with DN, the ratios of GAP43/PGP and axonal swelling/PGP are likely to differentiate painful from painless phenotypes.
Acknowledgments
The authors thank Dr. Brian Callaghan and Dr. Junguk Hur for statistical analysis; John Hayes and Chelsea Lindblad for technical assistance. This work utilized the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center funded by NIH (5P60 DK20572) from the National Institute of Diabetes & Digestive & Kidney Diseases.
This study is supported by National Institutes of Health [UO1-DK60994 (ELF); 1K08NS061039 (HTC); R01DK064814 (AGS, JRS); M01RR0064 (AGS, JRS)], the American Diabetes Association [ADA08CR52 (AGS, JRS)], and the Juvenile Diabetes Research Foundation Center for the Study of Complications in Diabetes.
Footnotes
Disclosures
The authors report no other disclosures.
References
- 1.Britland ST, Young RJ, Sharma AK, Clarke BF. Association of painful and painless diabetic polyneuropathy with different patterns of nerve fiber degeneration and regeneration. Diabetes. 1990;39:898–908. doi: 10.2337/diab.39.8.898. [DOI] [PubMed] [Google Scholar]
- 2.Cheng HT CB, Dauch JR, Feldman EL. Cytoskeleton, axonal transport, and mechanisms of axonal neuropathy. In: Cytoskeleton of the nervous system. In: R. A. Nixon AY, editor. Springer; 2011. pp. 657–78. [Google Scholar]
- 3.Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol. 2009;68:1229–43. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng HT, Dauch JR, Hayes JM, Yanik BM, Feldman EL. Nerve growth factor/p38 signaling increases intraepidermal nerve fiber densities in painful neuropathy of type 2 diabetes. Neurobiol Dis. 2012;45:280–7. doi: 10.1016/j.nbd.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christianson JA, Riekhof JT, Wright DE. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp Neurol. 2003;179:188–99. doi: 10.1016/s0014-4886(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 6.Ebenezer GJ, McArthur JC, Thomas D, Murinson B, Hauer P, Polydefkis M, Griffin JW. Denervation of skin in neuropathies: the sequence of axonal and Schwann cell changes in skin biopsies. Brain. 2007;130:2703–14. doi: 10.1093/brain/awm199. [DOI] [PubMed] [Google Scholar]
- 7.Fantini F, Johansson O: Expression of growth-associated protein 43 and nerve growth factor receptor in human skin: a comparative immunohistochemical investigation. J Invest Dermatol. 1992;99:734–42. doi: 10.1111/1523-1747.ep12614465. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons CH, Griffin JW, Polydefkis M, Bonyhay I, Brown A, Hauer PE, McArthur JC. The utility of skin biopsy for prediction of progression in suspected small fiber neuropathy. Neurology. 2006;66:256–8. doi: 10.1212/01.wnl.0000194314.86486.a2. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann DN, McDermott MP, Henderson D, Chen L, Akowuah K, Schifitto G: Epidermal nerve fiber density, axonal swellings and QST as predictors of HIV distal sensory neuropathy. Muscle Nerve. 2004;29:420–7. doi: 10.1002/mus.10567. [DOI] [PubMed] [Google Scholar]
- 10.Holland NR, Stocks A, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Intraepidermal nerve fiber density in patients with painful sensory neuropathy. Neurology. 1997;48:708–11. doi: 10.1212/wnl.48.3.708. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy WR, Nolano M, Wendelschafer-Crabb G, Johnson TL, Tamura E: A skin blister method to study epidermal nerves in peripheral nerve disease. Muscle Nerve. 1999;22:360–71. doi: 10.1002/(sici)1097-4598(199903)22:3<360::aid-mus9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Lauria G, Lombardi R, Camozzi F, Devigili G: Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology. 2009;54:273–85. doi: 10.1111/j.1365-2559.2008.03096.x. [DOI] [PubMed] [Google Scholar]
- 13.Lauria G, Morbin M, Lombardi R, Borgna M, Mazzoleni G, Sghirlanzoni A, Pareyson D: Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology. 2003;61:631–6. doi: 10.1212/01.wnl.0000070781.92512.a4. [DOI] [PubMed] [Google Scholar]
- 14.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998;55:1513–20. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- 15.Polydefkis M, Hauer P, Griffin JW, McArthur JC. Skin biopsy as a tool to assess distal small fiber innervation in diabetic neuropathy. Diabetes Technol Ther. 2001;3:23–8. doi: 10.1089/152091501750219994. [DOI] [PubMed] [Google Scholar]
- 16.Said G, Slama G, Selva J: Progressive centripetal degeneration of axons in small fibre diabetic polyneuropathy. Brain. 1983;106(Pt 4):791–807. doi: 10.1093/brain/106.4.791. [DOI] [PubMed] [Google Scholar]
- 17.Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, Tai TY, Hsieh ST. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. 2004;127:1593–605. doi: 10.1093/brain/awh180. [DOI] [PubMed] [Google Scholar]
- 18.Singleton JR, Bixby B, Russell JW, Feldman EL, Peltier A, Goldstein J, Howard J, Smith AG. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst. 2008;13:218–27. doi: 10.1111/j.1529-8027.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen L, Molyneaux L, Yue DK. The level of small nerve fiber dysfunction does not predict pain in diabetic Neuropathy: a study using quantitative sensory testing. Clin J Pain. 2006;22:261–5. doi: 10.1097/01.ajp.0000169670.47653.fb. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care. 2006;29:883–7. doi: 10.2337/diacare.29.04.06.dc05-2180. [DOI] [PubMed] [Google Scholar]
- 21.Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9:660–74. doi: 10.1111/j.1526-4637.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, Kashiwagi A, Kikkawa R: Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69:229–85. doi: 10.1016/s0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]