Summary
Nucleosome assembly following DNA replication and gene transcription is important to maintain genome stability and epigenetic information. Newly synthesized histones H3-H4 first bind histone chaperone Asf1 and are then transferred to other chaperones for nucleosome assembly. However, it is unknown how H3-H4 is transferred from the Asf1-H3-H4 complex to other chaperones because Asf1 binds H3-H4 with high affinity. Here, we show that yeast Rtt101Mms1 E3 ubiquitin ligase preferentially binds and ubiquitylates new histone H3 acetylated at lysine 56. Inactivation of Rtt101 or mutating H3 lysine residues ubiquitylated by the Rtt101Mms1 ligase impairs nucleosome assembly and promotes Asf1-H3 interactions. Similar phenotypes occur in human cells in which the ortholog of Rtt101Mms1, Cul4ADDB1, is depleted. These results indicate that the transfer of H3-H4 from the Asf1-H3-H4 complex to other histone chaperones is regulated by a conserved E3 ligase and provide evidence for crosstalk between histone acetylation and ubiquitylation in nucleosome assembly.
Introduction
In eukaryotic cells, chromatin encodes epigenetic information and governs genome stability. The basic repeating unit of chromatin is the nucleosome, consisting of 146 base pairs of DNA wrapped around a histone octamer that contains one (H3-H4)2 tetramer and two H2A–H2B dimers. As nucleosomes are barriers for machinery involved in gene transcription and DNA replication, nucleosomes must be disassembled to allow gene transcription and DNA replication machinery access to DNA. Following completion of gene transcription and DNA replication, DNA must be reassembled into nucleosomes to maintain original chromatin states. Therefore, nucleosome assembly plays an important role in various processes related to DNA transaction including DNA replication, DNA repair, gene transcription and epigenetic memory (Burgess and Zhang, 2013; Groth et al., 2007; Nakano et al., 2011; Ransom et al., 2010; Stillman, 1986).
Deposition of H3-H4 molecules is the rate-limiting step of nucleosome formation (Luger, 2006). During DNA replication-coupled nucleosome assembly, replicated DNA is assembled into nucleosomes using both parental and newly synthesized H3-H4. While it remains an enigma how parental histones H3-H4 are deposited onto replicated DNA (Burgess and Zhang, 2013; Groth et al., 2007; Ransom et al., 2010), it is believed that newly synthesized histones H3-H4 form a heterotrimeric complex with histone chaperone Asf1, which presents new H3-H4 to the Rtt109-Vps75 lysine acetyltransferase complex for acetylation of histone H3K56 (H3K56ac) (Collins et al., 2007; Driscoll et al., 2007; Han et al., 2007). Asf1 binds the H3 interface involved in the formation of (H3-H4)2 tetramers (English et al., 2006); thus, H3-H4 of the Asf1-H3-H4 complex must be transferred to two other histone chaperones, CAF-1 and Rtt106, which will deposit (H3-H4)2 tetramers onto replicating DNA. In human cells, newly synthesized H3-H4 molecules also bind first to human Asf1a and Asf1b, two sequence homologs of yeast Asf1 (Campos et al., 2010), before being transferred to CAF-1 during replication-coupled nucleosome assembly.
Nucleosome assembly also occurs following gene transcription and histone exchange in a DNA replication-independent pathway (Burgess and Zhang, 2013). In budding yeast, histone chaperones Hir1, Asf1 and Rtt106 participate in this process (Kaufman et al., 1998; Silva et al., 2012). In human cells, HIRA (the sequence homolog of yeast Hir1) and Daxx, which shares limited sequence homology with yeast Rtt106, are two H3.3-H4 histone chaperones that deposit H3.3-H4 at distinct chromatin regions (Drane et al., 2010; Goldberg et al., 2010; Tagami et al., 2004) in a replication-independent process. H3.3 is a histone H3 variant that differs from canonical histone H3 (H3.1/H3.2) by four or five amino acids. Asf1a interacts specifically with HIRA and functions with HIRA during replication-independent nucleosome assembly (Tang et al., 2006). Thus, it is hypothesized that both yeast and human Asf1 deliver H3-H4 to others chaperones during both replication-coupled and replication-independent nucleosome assembly. Asf1 binds H3-H4 with high affinity, similar to that of CAF-1 or Rtt106 for H3-H4 (Donham et al., 2011; Winkler et al., 2012) in vitro. Therefore, it is unclear how H3-H4 can be transferred from Asf1 to other histone chaperones.
Rtt101 is a member of the cullin-RING finger E3 ubiquitin ligase family and functions to maintain genome stability. Rtt101 (the E3 core), Hrt1 (the RING finger protein) and Cdc34 (E2) are the core components for ubiquitylation reactions. It is proposed that Mms1 and Mms22 are adaptor proteins for substrate binding (Han et al., 2010; Zaidi et al., 2008). Mms1 shares sequence homology with human DDB1, which binds CUL4A and CUL4B to form the Cul4DDB1 E3 ligase (Havens and Walter, 2011; Jackson and Xiong, 2009; Lee and Zhou, 2007). In budding yeast, cells lacking Rtt101 are sensitive to DNA-damaging agents and are defective in replication through damaged DNA or natural pause sites (Zaidi et al., 2008). In human cells, the CUL4DDB1 ubiquitylates various proteins involved in DNA replication and chromatin dynamics (Abbas et al., 2010; Abbas et al., 2008; Nakagawa and Xiong, 2011; Terai et al., 2010; Wang et al., 2006). The expression of Cul4A is altered in a variety of cancers (Lee and Zhou, 2012). Therefore, determination of how the Rtt101Mms1 E3 ubiquitin ligase functions in the maintenance of genome stability may shed light on how alterations in human CUL4 contribute to diseases.
A genome wide study in budding yeast indicates that Rtt101 functions in the same genetic pathway as Mms1, Mms22, Asf1 and Rtt109 to maintain genome stability. In contrast to the asf1Δ and rtt109Δ mutations that abolish H3K56ac in cells, cells lacking Rtt101, Mms1 or Mms22 have normal levels of H3K56ac (Collins et al., 2007). Thus, it is not known how Rtt101, Mms1 and Mms22 function in the same genetic pathway as Rtt109 and Asf1. Here, we show that Rtt101Mms1 binds and ubiquitylates H3K56ac-H4 preferentially over unmodified H3-H4 in vivo and in vitro. Furthermore, we show that histone H3 lysines 121, 122 and 125, which are located adjacent to the H3 interface involved in Asf1 binding, are ubiquitylated by the Rtt101Mms1 E3 ubiquitin ligase. Cells lacking Rtt101 or expressing a H3K121,122,125R mutant are sensitive to DNA damaging agents and exhibit defects in the deposition of H3K56ac onto replicating DNA. Remarkably, the Asf1-H3 interaction is stabilized in these mutant cells. Defects in the deposition of new histone H3.1 and H3.3 and increased association of Asf1 with H3-H4 are also observed in human cells in which Cul4A or DDB1 is depleted. Together, our results reveal crosstalk between H3K56 acetylation and H3 ubiquitylation and a conserved mechanism whereby deposition of new H3 is regulated by an E3 ubiquitin ligase.
Results
Rtt101 ubiquitylates and binds H3-H4 in an Mms1-dependent manner
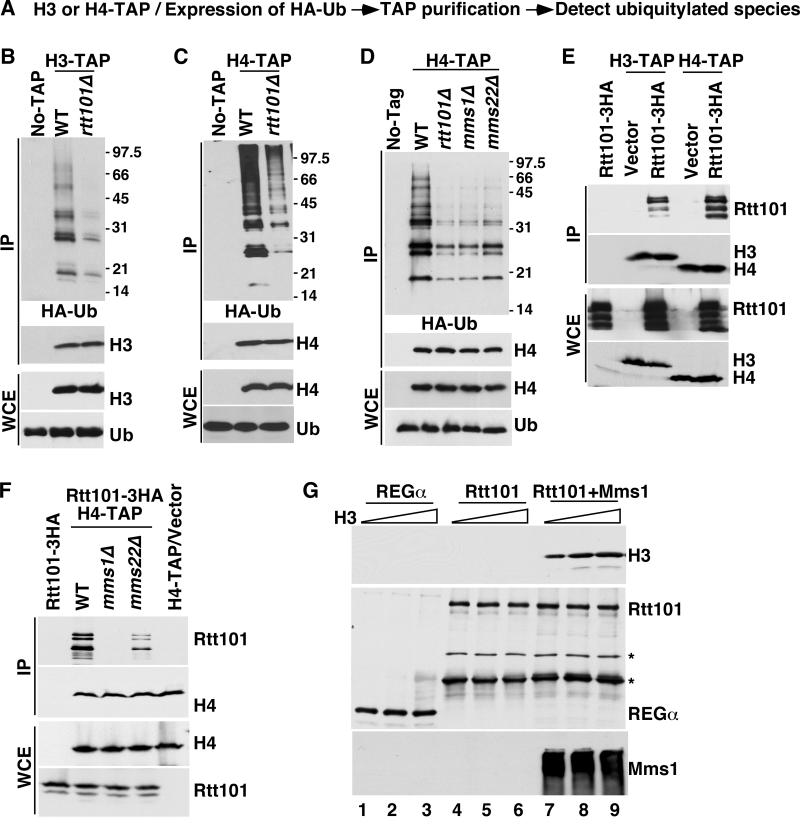
Genetic evidence indicates that Mms1, Mms22 and Rtt107 function in the same genetic pathway as Rtt101 to maintain genome stability (Collins et al., 2007). We have previously shown that Rtt101 ubiquitylates Spt16, a subunit of FACT, in an Mms1-independent manner (Han et al., 2010). Using a similar approach, we found that Rtt101, Mms1 and Mms22 were required for ubiquitylation of H3, H4 or their associated proteins. Briefly, H3 or H4, was purified from wild type and various mutant cells expressing HA-tagged ubiquitin. Co-purified ubiquitylated species were detected by Western blot using antibodies against the HA epitope (Figure 1A). Ubiquitylated species co-purifying with H3-TAP or H4-TAP were dramatically reduced in rtt101Δ and mms1Δ mutant cells compared to wild type cells (Figure 1B–D). Deletion of MMS22 also resulted in a reduction of ubiquitylated proteins co-purified with H4, but to a lesser degree than deletion of MMS1 or RTT101, whereas deletion of RTT107 had no apparent effect (Figure 1D and Figure S1A). This result combined with results presented later in Figure 2 and 3 indicates that H3 is ubiquitylated by an Rtt101-containing ubiquitin ligase in vivo and that unlike Rtt101-mediated Spt16 ubiquitylation, H3 ubiquitylation requires Mms1 and Mms22.
Figure 1. Rtt101 binds and ubiquitylates histone H3 in an Mms1-dependent manner.
A, An outline of the procedure for detecting ubiquitylated species co-purifying with H3 or H4. B–C, Ubiquitylated species co-purifying with H3 (B) or H4 (C) are reduced in rtt101Δ mutant cells. Histone H3 or H4, each tagged with the tandem affinity purification tag (TAP), was purified from wild type (WT) and rtt101Δ mutant cells expressing HA-ubiquitin (HA-Ub). Purified proteins (IP) and proteins in whole cell extracts (WCE) analyzed by Western blotting using antibodies against the HA epitope, H3 or H4. No-TAP: a control purification using a strain without the TAP tag. D, Ubiquitylated species co-purified with H4 are markedly reduced in mms1Δ and mms22Δ mutant cells. E–F, Rtt101 binds H3–H4 in an Mms1-dependent manner in vivo. H3-TAP or H4-TAP was purified from wild type, mms1Δ or mms22Δ cells expressing Rtt101-3HA proteins. Proteins in IP and in WCE were detected using Western blot. Strains with empty vector (vector) and without any TAP tag proteins were used as controls. G, Rtt101-Mms1, but not Rtt101 binds H3–H4 in vitro. GST pull-down assays using GST-Rtt101 or GST-REGα (negative control) were performed as described in Experimental Procedures. Protein bands marked by * may be degradation products. See also Figure S1.
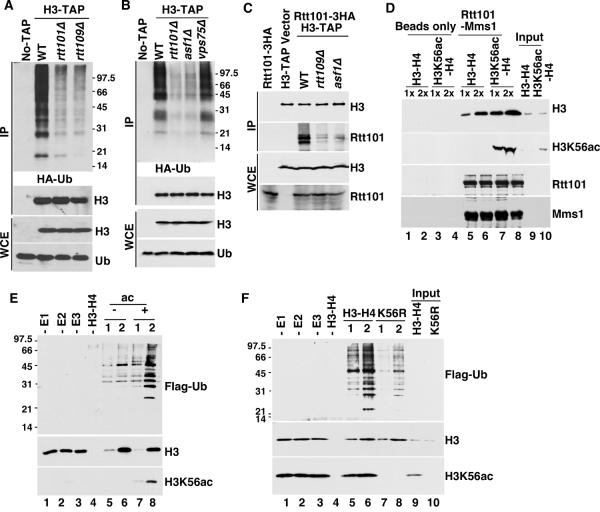
Figure 2. Rtt101-Mms1 binds and ubiquitylates H3K56ac-H4 preferentially over unmodified H3–H4.
A and B, Ubiquitylated species co-purifying with H3 were reduced in rtt109Δ (A) and asf1Δ (B), but not vps75Δ (B) mutant cells. C, The Rtt101-H3 interaction depends on Rtt109 and Asf1. The experiment was performed as described in Figure 1F except using H3-TAP rtt109Δ and H3-TAP asf1Δ mutant cells. D, The Rtt101-Mms1 complex preferentially binds H3K56ac-H4 over H3–H4 in vitro. The experiments were performed as described in Figure 1G excepting using H3–H4 with/without acetylation by the Rtt109-Vps75 complex. E, The Rtt101Mms1 E3 ligase ubiquitylates H3K56ac-H4 preferentially over unmodified H3–H4 in vitro. Two different amounts of recombinant H3–H4 (1× and 2×) with/without acetylation (ac) were used for in vitro ubiquitylation reactions containing Rtt101Mms1 E3 ligase and Flag-Ub. Reactions without E1 (−E1), E2 (−E2) or E3 (−E3) were assembled as negative controls. F, Rtt101Mms1 ubiquitylates H3K56ac-H4 more efficiently than mutant H3K56R-H4 in vitro. H3K56R: H3K56R-H4 tetramers. See also Figure S1C.
Figure 3. Lysine 121, 122 and 125 of H3 are three major ubiquitylation residues.
A, H3K125R mutation partially suppresses the phenotypes of hst3Δ hst4Δ mutant cells. Tenfold serial dilutions of yeast cells of the genotype indicated at the left were spotted onto YPD medium alone or YPD medium containing hydroxyurea (HU), camptothecin (CPT) or methylmethanesulfonate (MMS). B–C, Mutations at H3K121, H3K122 and H3K125 dramatically reduce H3 ubiquitylation in vivo. Cells expressing H3K125R (B), H3K121R, H3K122R or H3K121,122R (C) as the only copy of H3 were used to detect ubiquitylated species co-purified with H4-TAP. D, Rtt101Mms1 ubiquitylates H3 mutants less efficiently than wild type H3 in vitro. In vitro ubiquitylation assays were performed using wild type H3–H4 as well as H3–H4 mutants, each containing an indicated mutation at H3. E, The antibodies against H3K122ub specifically recognize H3K122ub peptide in vitro. Right panel: PonS staining of membrane spotted with a H3K122 (H3) peptide and a branch peptide (H3K122ub). Left panel: Western blot analysis using anti-H3K122ub antibody. F, H3K122 ubiquitylation was detected in vivo. Proteins co-purified with H4-TAP from wild type, rtt101Δ and H3K121,122,125R (H3 3KR) mutant cells were detected using antibodies against H3, H4 or H3K122ub. Proteins bands with apparent molecular weight corresponding to di- and hexa-ubiquitylated H3 were marked as H3-Ub2 and H3-Ub6, respectively. See also Table S1 and Figure S2.
We hypothesized that Mms1 mediated the interaction between H3-H4 and Rtt101, which in turn promoted H3 ubiquitylation. To test this hypothesis, we first determined whether Rtt101 interacted with H3-H4 in vivo. In immunoprecipitation experiments using H3-TAP or H4-TAP, Rtt101 co-precipitated with either H3 or H4 in vivo (Figure 1E), and the amount of Rtt101 co-immunoprecipitated with H3 was dramatically reduced in mms1Δ and to a lesser extent in mms22Δ mutant cells compared to wild type cells (Figure 1F). Finally, recombinant Rtt101-Mms1, but not Rtt101 alone, bound to recombinant H3-H4 in vitro (Figure 1G). These results indicate that Rtt101 binds and ubiquitylates H3-H4 in an Mms1-dependent manner.
Rtt101-Mms1 binds and ubiquitylates H3K56ac-H4 preferentially over unmodified H3-H4 in vivo and in vitro
Genetic evidence suggests that Rtt101 functions downstream of the histone acetyltransferase Rtt109 and histone chaperone Asf1, two proteins required for H3K56ac (Collins et al., 2007). Thus, we determined whether Rtt109 and Asf1 were required for ubiquitylation of H3 using the same procedures described for Figure 1A. Ubiquitylated species co-purified with H3-TAP were greatly reduced in rtt109Δ and asf1Δ but not vps75Δ mutant cells compared to wild type cells (Figure 2A and 2B). In addition, ubiquitylated species co-purified with H4-TAP were also significantly reduced in cells expressing a H3K56R mutant (Figure S1B). Vps75 is not required for H3K56 acetylation (Han et al., 2007). These results strongly indicate that H3K56 acetylation is critical for Rtt101Mms1-mediated H3 ubiquitylation in vivo.
To understand how H3K56ac regulates H3 ubiquitylation in vivo, we performed two sets of experiments. First, we asked whether H3K56ac regulated the interaction of Rtt101-Mms1 with histone H3-H4. The interactions between Rtt101 and H3 were reduced in cells lacking Rtt109 or Asf1 (Figure 2C). In vitro, the recombinant Rtt101-Mms1 complex bound more H3K56ac-H4 than unacetylated H3-H4 (Figure 2D, compare lanes 7–8 to lanes 5–6 and Figure S1C). Thus, H3K56ac promotes interactions of H3-H4 with the Rtt101-Mms1 complex in vivo and in vitro.
Second, we asked whether the Rtt101Mms1 complex ubiquitylated H3K56ac-H4 preferentially over H3-H4. More ubiquitylated species were detected when H3K56ac-H4 was used as the substrate in comparison to unmodified H3-H4 (Figure 2E, compare lanes 7–8 to lanes 5–6). Mutating H3K56 to arginine abolished this effect (Figure 2F, compared lanes 7–8 to lanes 5–6). Together, these results are consistent with the idea that H3K56ac is critical for H3 ubiquitylation and provides a mechanism whereby Rtt109 and Asf1 are required for Rtt101-mediated H3 ubiquitylation in vivo.
Rtt101 ubiquitylates three lysine residues on H3
We utilized a genetic approach to identify histone lysine residues that were potentially ubiquitylated by Rtt101Mms1 E3 ligase. It is known that cells lacking Hst3 and Hst4, two H3K56ac deacetylases, grow poorly at 37 °C and are sensitive to DNA damaging agents (Celic et al., 2006; Maas et al., 2006). Deletion of Rtt101 or mutations at H3K56 suppresses these phenotypes (Collins et al., 2007). We hypothesized that mutations at the Rtt101Mms1 ubiquitylation site(s) of H3 or H4 might also suppress phenotypes of hst3Δ hst4Δ mutant cells. Therefore, we mutated each lysine residue on H3 or H4 alone, or in various combinations, in hst3Δ hst4Δ cells and tested the extent to which each of 22 mutants suppressed the temperature sensitivity (TS) and DNA damage sensitivity of hst3Δ hst4Δ cells. We found that mutations at five lysine residues (H3K79, H3K121, H3K122, H3K125, and H4K16) partially suppressed the phenotypes of hst3Δ hst4Δ (e. g. Figure 3A and Table S1) compared to rtt101Δ, suggesting that Rtt101Mms1 may ubiquitylate these lysine residues.
To test which lysine residues are ubiquitylated in yeast cells, we purified ubiquitylated species from cells expressing each of these mutants using the same procedures as in Figure 1. Cells expressing H3K79R, H4K16R or H3K79R-H4K16R had little effect on the amount of ubiquitylated species co-purifying with H3 or H4 (Figure S1B and S1D), suggesting that H3K79 and H4K16 are unlikely to be the predominant ubiquitylation sites. In contrast, mutations at H3K121, H3K122 and H3K125 dramatically reduced the amount of ubiquitylated species co-purifying with H4-TAP (Figure 3B–C), with the H3K121,122R double mutant exhibiting greater defects than either single mutant alone (data not shown). The reduction in the amount of H4-associated ubiquitylated species was not due to reduced levels of H3K56ac in these histone mutant cells (data not shown, also see Figure 6). In vitro, the Rtt101Mms1 E3 ligase ubiquitylated H3K122R-H4, H3K125R-H4 or H3K121,122,125R-H4 less efficiently than wild type H3-H4 (Figure 3D). Together, these results indicate that H3K121, H3K122 and H3K125 are the predominant ubiquitylation sites of the Rtt101Mms1 E3 ligase in vivo and in vitro.
Figure 6. Rtt101 and H3 ubiquitylation curb the Asf1-H3-H4 interaction.
A, Cells lacking Rtt101 or expressing H3 mutant forms exhibit an increased association of Asf1 with H3-H4. Asf1 was purified from wild type, rtt101Δ and indicated H3 mutant cells. Proteins in IP as well as in WCE were detected by Western blot. B, Recombinant Asf1 binds less H3-H4 molecules that were ubiquitylated by the Rtt101Mms1 E3 ligase. (His)6-tagged-Asf1 proteins were incubated with recombinant H3-H4 acetylated by Rtt109-Vps75 with or without ubiquitylation by Rtt101Mms1 in vitro. H3K121,122,125R-H4 mutant proteins were used as controls. Proteins that bound to Asf1 were detected by Western blot, and Asf1 with Ponceau S. C, The Rtt106-H3 interaction is reduced in cells lacking Rtt101 or expressing ubiquitylation-deficient H3 mutants. D, H3 ubiquitylation promotes transfer of H3-H4 from the Asf1-H3-H4 complex to Rtt106. Top panel outlines experimental procedures. Asf1-H3K56ac-H4 proteins were immobilized to Ni-NTA beads using the (His)6 tag on Asf1. Ubiquitylation reactions using immobilized Asf1-H3K56ac-H4 and Flag-Ub as substrates with (+) or without (−) E1 were performed. Half of the supernatants from each reaction were removed and precipitated with TCA, and the remaining reaction mixtures containing Ni-NTA beads were incubated overnight with addition of GST-Rtt106. After removal of Ni-NTA beads, GST-Rtt106 was pulled down from supernatants. Proteins in the TCA precipitates as well as bound to GST-Rtt106 were analyzed by Western blot (WB) using antibodies against Flag (bottom left), histone H3, Asf1 and Rtt106 (bottom right). See also Figures S4, S5 and S7.
We made an antibody recognizing a branch peptide with four residues at the C-terminus of Ub (GGRL) attached to H3K122 (H3K122ub). This antibody specifically recognized H3K122ub peptide over unmodified peptide in vitro (Figure 3E), and could detect two proteins co-purified with H4 from wild type but not from H3K121,122,125R mutant cells. The amounts of these two proteins were dramatically reduced in rtt101Δ cells compared to wild type cells (Figure 3F). The apparent molecular weight of these two proteins corresponds to those of one histone H3 conjugated with two and six ubiquitin molecules (H3-Ub2 and H3-Ub6), respectively. In addition, this antibody could detect a protein with apparent molecular weight of H3-Ub6 from protein mixtures purified using acid extraction from wild type, but not from rtt101Δ and H3K121,122,125R mutant cells (Figure 2SA). This pattern of H3 ubiquitylation may prevent recognition by the 26S proteasome. Consistent with this idea, the half-life of newly synthesized H3-H4 was not altered in rtt101Δ mutant cells (Figure S2B–C). Together, These results indicate that Rtt101Mms1-catalyzed H3 ubiquitylation of lysine 121, 122 and 125 mediates a function other than H3 degradation.
Cells expressing H3 ubiquitylation-deficient H3 mutants are sensitive to DNA damaging agents and exhibit spontaneous chromosome breaks
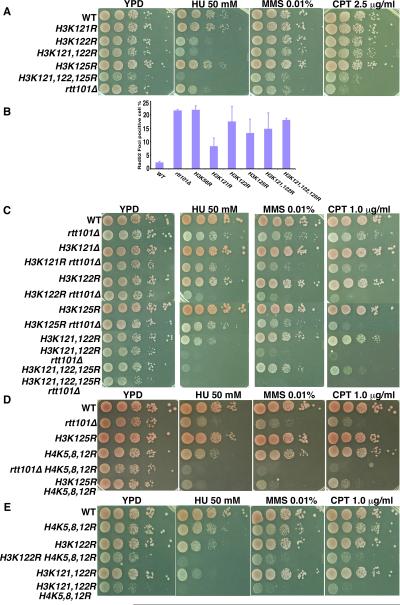
Cells lacking Rtt101 are sensitive to DNA damaging agents and exhibit increased spontaneous chromosome breaks (Collins et al., 2007; Zaidi et al., 2008). We determined whether cells harboring mutations at H3K121, 122 or 125 exhibited these phenotypes. Cells expressing each single mutant were slightly sensitive to three DNA damaging agents (HU, MMS and CPT), with H3K122R mutant cells showing the greatest defects. The H3K121,122,125R triple mutant cells, which grow slowly, appeared to be more sensitive to DNA damaging agents than either single mutant alone (Figure 4A), consistent with the idea that these three lysine residues are ubiquitylated in vivo.
Figure 4. Cells expressing ubiquitylation-deficient H3 mutants are sensitive to DNA damaging agents and exhibit spontaneous chromosome breaks.
A, Cells expressing H3K121, 122, 125R mutant are sensitive to DNA damaging agents. B, Cells expressing an ubiquitylation-compromised H3 mutant show marked increases in cells containing Rad52 foci. Rad52-YFP in yeast cells with the indicated genome type were detected using a fluorescence microscope and the percentage of cells with Rad52-foci was reported (mean ±SEM). C, Deletion of RTT101 in H3K122R, H3K125R, H3K121,122R or H3K121,122,125R mutant cells results in an increased sensitivity towards DNA damaging agents. D–E, Mutations at RTT101, H3K121, H3K122 or H3K125 worsen the DNA damage sensitivity of H4K5,8,12R mutant cells. See also Figure S2D.
Rad52 is a protein involved in homologous recombination and forms foci at chromosome breaks (Lisby et al., 2003). Like rtt101Δ mutant cells, cells expressing each ubiquitylation-deficient mutant exhibited a marked increase in the number of cells containing Rad52 foci (Figure 4B), an indication of increased spontaneous chromosome breaks. These results suggest that Rtt101 and H3 ubiquitylation are required for the maintenance of genome stability under normal as well as genotoxic stress conditions.
We also performed epistasis analysis of rtt101Δ and H3K121R, H3K122R and H3K125R alone and in combination. The rtt101Δ H3K121R double mutant cells were only slightly more sensitive to DNA damaging agents (HU, MMS and CPT) than either rtt101Δ or H3K121R mutant alone. In contrast, deletion of RTT101 in H3K122R, H3K125R, H3K121,122R or H3K121,122,125R mutant cells resulted in a synergistic increase in sensitivity towards genotoxic agents compared to rtt101Δ cells or each corresponding H3 mutant alone (Figure 4C and Figure S2D). These results are consistent with the fact that Rtt101 has other substrates such as Spt16 in addition to histone H3 (Han et al., 2010) and that H3K122 is also known to be acetylated in human cells and fission yeast (Tropberger et al., 2013).
Rtt101 and H3 ubiquitylation are required for replication-coupled nucleosome assembly
Rtt109 and H3K56ac are involved in replication-coupled nucleosome assembly (Li et al., 2008). We asked whether Rtt101 and H3 ubiquitylation also participated in DNA replication-coupled nucleosome assembly using four sets of experiments. First, we determined whether RTT101 genetically interacted with mutations at lysine residues of H4 (H4K5,8,12R). Acetylation of H4K5, H4K8 and H4K12 of newly synthesized H4 is implicated in replication-coupled nucleosome assembly (Verreault et al., 1996). The rtt101Δ H4K5,8,12R mutant cells were more sensitive to each of the three DNA damage agents (HU, MMS and CPT) than either the rtt101Δ or H4K5,8,12R mutant alone (Figure 4D). When combined with the H4K5,8,12R mutant, H3K125R-H4K5,8,12R, H3K122R-H4K5,8,12R and H3K121, 122R-H4K5,8,12R mutant cells were also more sensitive to DNA damaging agents than the H3K122R, H3K125R or H4K5,8,12R mutant alone (Figure 4D–E). These results suggest that Rtt101 and ubiquitylation at H3K121, K122 and K125 are genetically linked to DNA replication-coupled nucleosome assembly.
Second, we determined whether H3K122ub could be detected at replicating DNA using sequential chromatin immunoprecipitation (ChIP) assays. Briefly, wild type and rtt101Δ, H3K121,122,125R mutant cells were synchronized at G1 and then released into fresh media containing HU. While having no effect on initiation of DNA replication at early replication origins such as ARS305 and ARS607, HU slows down replication forks originating from early replication origins and prevents them from reaching distal sites (12kb distal to ARS305, ARS305+12kb or 14kb distal to ARS607, ARS607+14kb) (Tercero and Diffley, 2001). ChIP assays were performed at G1 phase (0 min) and early S phase (30 and 45 min following release) first using antibodies against H4-TAP tag. After elution, the H4-ChIP DNA was immunoprecipitated using antibodies against H3K122ub (Figure 5A). As expected, H4 was detected at both replicating DNA (ARS607) and non-replicating DNA (ARS607+14kb) in both G1 and early S phase cells (Figure S3A), and deletion of RTT101 or mutations at H3K121,122,125 had no apparent effect on H4 occupancy at these chromatin regions (Figure S3A). In contrast, H3K122ub was detected at replicating DNA (ARS607), but not non-replicating DNA (ARS607+14kb) in wild type cells. Furthermore, H3K122ub ChIP signals at replicating DNA were dramatically reduced in rtt101Δ or H3K121,122,125R mutant cells compared to wild type cells (Figure 5B). These results indicate that H3K122ub is deposited onto replicating DNA during S phase of the cell cycle.
Figure 5. Cells lacking Rtt101 or expressing an ubiquitylation-deficient H3 mutant are defective in deposition of H3K56ac onto replicating DNA.
A, An outline of sequential ChIP assays to detect the occupancy of H3 ubiquitylated at lysine 122 (H3K122ub) on replicating DNA. B, H3K122ub is enriched at replicating DNA. ChIP assays were performed using wild type, rtt101Δ and H3K121,122,125R (3KR) mutant cells when these cells were arrested at G1 and released into S phase in the presence of HU. All ChIP DNAs were analyzed using two different primer pairs amplifying ARS607 and ARS607+14Kb by a real-time PCR and the results from the first H4-TAP ChIP were shown in Figure S3A. The percentage of immunoprecipitated DNA over the total input DNA was reported. No-TAP strain was used as negative control. C–F, The deposition of new H3K56ac is reduced in cells lacking Rtt101 or cells expressing H3K121,122,125R mutant. Cells were synchronized at G1 using α-factor and then released into fresh medium with 0.2M HU (C–D) or without HU (E–F) for indicated time. ChIP assays using antibodies against H3K56ac were performed and ChIP DNAs were analyzed using four different primer pairs as indicated in figures using a quantitative PCR (±SD). Each ChIP experiment was repeated at least three times, with results from on representative experiment shown). See also Figure S3.
Third, we asked whether Rtt101 and H3 ubiquitylation were required for efficient deposition of new H3 marked by H3K56ac onto replicating DNA when cells were released from G1 to early S phase in the presence of HU. Consistent with previous reports (Burgess et al., 2010; Li et al., 2008), more H3K56ac were detected at ARS305 and ARS607 than the corresponding distal sites at early S phase when cells were released from G1 (Figure 5C–D). Importantly, deposition of H3K56ac at ARS305 and ARS607 was dramatically reduced in rtt101Δ, H3K121,122,125R (Figure 5C–D) and mms1Δ (Figure S3B–C) mutant cells compared to wild type cells. This reduction in H3K56ac in rtt101Δ or H3K121,122,125R cells was not due to reduced levels of H3K56ac in cell extracts (Figure S3D and Figure 6). Finally, we observed that deposition of H3K56ac was also reduced at replicating DNA (ARS607 and ARS607+14kb, 40 min) after cells were released into S phase without HU (Figure 5E–F). These results indicate Rtt101 and H3 ubiquitylation are required for efficient deposition of new H3 onto replicating DNA in budding yeast.
Rtt101 and H3 ubiquitylation regulate the association of H3-H4 with histone chaperones Asf1 and Rtt106 in budding yeast
Since the loss of Rtt101 had no apparent effect on the half-life of new H3 or H3K56ac (Figure S2B–C), we tested whether Rtt101 and H3 ubiquitylation regulated the association of H3-H4 with each of three histone chaperones involved in deposition of H3K56ac: Asf1, CAF-1 and Rtt106 (Li et al., 2008; Ransom et al., 2010). Deletion of RTT101 and expression of H3K125R, H3K121,122R or H3K121,122,125R resulted in marked increases in the association of H3 with Asf1 (Figure 6A and Figure S4A). In addition, recombinant Asf1 bound less H3-H4 molecules that were ubiquitylated by the Rtt101Mms1 E3 ligase than unmodified H3-H4 in vitro (Figure 6B, compare lanes 5–6 to lanes 3–4 and Figure S4B). Together, these results indicate that H3 ubiquitylation weaken the interaction between Asf1 and H3-H4. H3K121, K122 and K125 are located close to the H3 interface involved in Asf1 binding (Figure S5), and it is conceivable that attachment of one or more ubiquitin molecules to these lysine residues can disrupt the contact between Asf1 and H3-H4.
The association of H3 with Rtt106 was dramatically reduced in each of the mutant cells (rtt101Δ, H3K125R, H3K121,122R and H3K121,122,125R) tested (Figure 6C and Figure S4C). Interestingly, the association of CAF-1 with H3-H4 was not affected to a detectable degree in each of the mutant cells tested (Figure S4D). These results indicate that Rtt101 and H3 ubiquitylation weaken the H3-Asf1 interaction, which facilitates the transfer of H3-H4 from Asf1-H3-H4 complex to other histone chaperones including Rtt106. Consistent with this interpretation, more H3 molecules were disassociated from the (His)6-Asf1-H3-H4 complex immobilized to the Ni-NTA beads when H3 was ubiquitylated by Rtt101Mms1 E3 ligase. In addition, more H3 from the immobilized Asf1-H3-H4 complex were transferred to Rtt106 when H3 was ubiquitylated (Figure 6D). These results support the idea that H3 ubiquitylation by Rtt101Mms1 E3 ligase weakens the Asf1-H3 interaction, facilitating the transfer of H3-H4 from the Asf1-H3-H4 complex to other histone chaperones.
The Cul4A-DDB1 E3 ligase regulates both replication-coupled and replication-independent nucleosome assembly in human cells
Mms1 shares sequence homology to DDB1, an adaptor protein for Cul4A and Cul4B E3 ubiquitin ligases (Zaidi et al., 2008). Cul4A-DDB1 are known to ubiquitylate H3 and H4 in human cells (Wang et al., 2006). Therefore, we tested whether the Cul4ADDB1 E3 ligase has any role in nucleosome assembly of H3-H4 in human cells. In addition to canonical histone H3 (H3.1/H3.2) that are deposited by CAF-1 in a replication-coupled nucleosome assembly, human cells express histone H3 variant H3.3 that is deposited by HIRA and Daxx in a replication-independent process (Goldberg et al., 2010; Tagami et al., 2004). To monitor the deposition of newly synthesized H3.1 and H3.3, we utilized cell lines exogenously expressing H3.1 or H3.3, each tagged with the SNAP tag, and performed the “quench-chase” experiment as outlined in Figure S6 (Ray-Gallet et al., 2011) to monitor deposition of new H3.1 and H3.3. SNAP tag is a mutant form of O6-guanine nucleotide alkyltransferase that covalently reacts with benzyl-guanine (BG). Depletion of Cul4A and DDB1 resulted in a marked reduction in new H3.1 on chromatin compared to non-targeting control cells by counting H3.1-positive cells under a fluorescence microscope (Figure 7A) and by using a chromatin fractionation assay (Figure S6A–B), suggesting that deposition of new H3.1 is compromised in Cul4A- and DDB1-depleted cells. In addition, depletion of Cul4A and DDB1 also compromised the deposition of H3.3 compared to controls cells (Figure 7B and Figure S6C). Thus, Cul4A and DDB1 are required for efficient deposition of both new H3.1 and H3.3 in human cells.
Figure 7. Cul4A and DDB1 regulate deposition of new H3.1 and H3.3 in human cells.
A–B, Depletion of Cul4A or DDB1 results in reduced deposition of new H3.1 (A) and H3.3 (B). DDB1 (shDDB1) and Cul4A (shCul4A) were depleted from HeLa cells expressing H3.1- or H3.3-SNAP and DDB1 and Cul4A levels were analyzed by Western blot (left). Old H3.1- or H3.3-SNAP was blocked using a non-fluorescent blocker, and new H3-SNAP was marked with TMR-STAR and visualized using fluorescence microscopy (middle: representative images). EdU (5-ethynyl-2'-deoxyuridine) marks S phase cells. Cells with H3.1-SNAP- or EdU-positive staining were counted. The percentage of H3.1-SNAP-over EdU-positive cells (A) or the fluorescence intensity of H3.3-SNAP (B) from three independent experiments was calculated and reported (mean±SEM). C–D, Depletion of Cul4A (C) and DDB1 (D) results in increased H3 binding to Asf1a and Asf1b. Exogenously expressed Asf1a (e-Asf1a) or Asf1b (e-Asf1b) were immunoprecipitated (IP) from cells treated with non-target shRNA (NT) or Cul4A (C), DDB1 (D) depleted cells. Proteins in the input and IP samples were detected by Western blot. Con: control IP using cells without expressing e-Asf1a and e-Asf1b. E–F, Depletion of Cul4A (E) and DDB1 (F) results in reduced binding of H3.1 and H3.3 with their corresponding chaperones. e-H3.1 or e-H3.3 were IPed from controls cells (−), Cul4A-(E) and DDB1-depleted cells (F). Proteins in input and IP samples were detected by Western blots. Con, control IP using 293T cells. Bands marked with (*) were non-specific. See also Figures S6 and S7.
Next, we determined whether Cul4A and DDB1 regulated interactions between histone H3.1 and H3.3 with their associated histone chaperones including Asf1a and Asf1b, which bind both H3.1 and H3.3. Depletion of Cul4A and DDB1 resulted in a dramatic increase in the amounts of histone H3 co-purified with Asf1a and Asf1b (Figure 7C–D and Figure S6D–E). In contrast, depletion of Cul4A (Figure 7E and Figure S6F) and DDB1 (Figure 7F and Figure S6G) led to a marked reduction in the interaction between H3.1 and CAF-1 as well as the association of H3.3 with its specific chaperones Daxx and HIRA. Thus, Cul4A-DDB1 E3 ubiquitin ligase also curbs the Asf1a/Asf1b-histone interactions in human cells, which in turn may regulate the interactions between histones H3.1 and H3.3 with their corresponding histone chaperones and subsequent deposition onto DNA in human cells.
Discussion
Histone acetylation promotes histone ubiquitylation
Genetic studies suggest that RTT101 and MMS1 function in the same genetic pathway as RTT109 and ASF1 in maintenance of genome stability (Collins et al., 2007). Here, we present the following lines of evidence supporting the idea that the Rtt101Mms1 E3 ubiquitin ligase ubiquitylates H3K56ac-H4 preferentially over unmodified H3-H4, revealing mechanistic insights into the function of Rtt101Mms1 in the maintenance of genome stability. First, H3 is ubiquitylated in yeast cells, and this ubiquitylation depends on Rtt101 and Mms1. Second, H3 ubiquitylation depends on Asf1 and Rtt109, but not Vps75, suggesting that H3K56ac is required for Rtt101Mms1-mediated H3 ubiquitylation in vivo. Consistent with this interpretation, we show that Rtt101 binds H3-H4, and this association depends on Mms1, Asf1 and Rtt109 in vivo. In vitro, Rtt101-Mms1 binds and ubiquitylates recombinant H3K56ac-H4 preferentially over unmodified H3-H4. Together, these results strongly support a model by which H3 is ubiquitylated: following Rtt109-Vps75 catalyzed acetylation of H3K56, Mms1 binds H3K56ac-H4 and presents new H3-H4 for ubiquitylation by the Rtt101Mms1 E3 ligase (Figure S7).
A large number of posttranslational modifications (PTM) have been detected on histone proteins and some of these PTMs are functionally linked to specific cellular processes (Suganuma and Workman, 2008). For instance, in S. cerevisiae, H2B ubiquitylation by Rad6 is essential for H3K4 methylation (Lee et al., 2007; Sun and Allis, 2002). Our results indicate that H3K56ac promotes H3 ubiquitylation by Rtt101Mms1 ubiquitin ligase, revealing crosstalk between histone acetylation and histone ubiquitylation. This finding may also have a general implication on the function of protein acetylation and ubiquitylation. Acetylation has been detected on many proteins in addition to histones, and it is as abundant as protein phosphorylation (Choudhary et al., 2009; Zhao et al., 2010). It is well documented that protein phosphorylation is important for its ubiquitylation by the family of SCF ubiquitin ligases (Skowyra et al., 1997). It is unknown whether acetylation of internal lysine residues can promote protein ubiquitylation. Therefore, our studies provide evidence for a paradigm that acetylation of internal lysine residues can serve as a signal for protein ubiquitylation, which in turn regulates protein stability and/or other cellular functions.
The Rtt101Mms1 and Cul4DDB1 E3 ubiquitin ligase regulate nucleosome assembly in yeast and in human cells
Our studies reveal a conserved role of Rtt101Mms1 and its human ortholog Cul4ADDB1 in nucleosome assembly. First, we show that yeast cells lacking Rtt101 or expressing H3 ubiquitylation-defective mutants exhibit defects in deposition of new H3 onto replicating DNA during the S phase of the cell cycle. In human cells, depletion of Cul4A and DDB1 results in reduced deposition of both new H3.1 and histone H3 variant H3.3 onto chromatin. Together, these results indicate that both yeast Rtt101Mms1 and human Cul4ADDB1 are required for efficient deposition of new H3 onto DNA.
Mechanistically, Rtt101Mms1 and Cul4ADDB1 impact nucleosome assembly through weakening the interactions between histone chaperone Asf1 and H3-H4. Yeast cells lacking Rtt101 and expressing ubiquitylation-defective H3 mutant show an increased association of Asf1 with H3-H4. In human cells, depletion of Cul4A or DDB1 results in increased association of Asf1a/Asf1b with H3-H4. Thus, both Rtt101Mms1 and Cul4ADDB1 are negative regulators of the Asf1-histone interactions.
Asf1 binds the H3 interface involved in formation of (H3-H4)2 tetramers (English et al., 2006). It is proposed that H3-H4 in the Asf1-H3-H4 complex must be transferred to histone chaperone CAF-1 during DNA replication-coupled nucleosome assembly and histone chaperone HIRA during DNA replication-independent nucleosome assembly (Burgess and Zhang, 2013; Groth et al., 2005; Groth et al., 2007; Li et al., 2008; Ransom et al., 2010). In vitro, Asf1 binds H3-H4 with high affinity (Donham et al., 2011), similar to or higher than that of the binding of H3-H4 to CAF-1 or Rtt106 (Su et al., 2012; Winkler et al., 2012). In principle, the interaction between Asf1 and H3-H4 should be weakened to facilitating the transfer of H3-H4 from the Asf1-H3-H4 complex to other histone chaperones. We propose that by negatively regulating Asf1-H3 interactions, Rtt101Mms1 and Cul4ADDB1 facilitate the dissociation of H3-H4 from the Asf1-H3-H4 complex, regulating a key step in replication-coupled and replication-independent nucleosome assembly. Supporting this interpretation, we show that more histone H3 is transferred to Rtt106 after ubiquitylation of H3-H4 by Rtt101Mms1 in vitro. In addition, three histone H3 lysine residues (K121, K122 and K125) that are ubiquitylated by Rtt101Mms1 in budding yeast are located close to the Asf1-H3 binding interface consisting of C-terminal α3 helix (residues 122–134, Figure S5) (English et al., 2006). It is possible that addition of an ubiquitin(s) moiety at the three lysine residues of yeast histone H3 could change the conformation of the α3 helix and/or obstruct the Asf1-H3 interaction due to the large size of ubiquitin. Of the three H3 lysine residues (K121, K122 and K125) identified affecting H3 ubiquitylation in yeast cells, H3K122 is highly conserved in human histone H3.1/H3.2 and H3.3. Also H3K122 ubiquitylation of both H3.1 and H3.3 has been detected by mass spectrometry (Kim et al., 2011). We propose that Cul4ADDB1 regulates the association of Asf1 with H3-H4 through a mechanism similar to that of Rtt101Mms1.
Deletion of RTT101 did not have a detectable effect on the CAF-1-histone interaction in yeast cells. In human cells, while depletion of Cul4A and DDB1 leads to a consistent reduction in binding of CAF-1 to histone H3.1-H4, the extent of the reduction is smaller than that of HIRA or Daxx for H3.3-H4. These results suggest that in addition to H3 ubiquitylation, the transfer of H3-H4 from Asf1 to CAF-1 is likely also regulated via other mechanisms. In budding yeast, the Asf1-H3 interaction is also regulated by the checkpoint kinase Rad53 (Hu et al., 2001). Future studies are needed to address whether Rad53 or other factors function with Rtt101Mms1 and Cul4ADDB1 to regulate this important step of nucleosome assembly.
Both Rtt101Mms1 and Cul4ADDB1 E3 ligase are important to maintain genome stability. We suggest that the ability of Rtt101Mms1 and Cul4ADDB1 in maintenance of genome stability is due, in part, to their roles in nucleosome assembly, a process that is important for maintaining genome integrity and epigenetic memory (Burgess and Zhang, 2013; Groth et al., 2007; Ransom et al., 2010). Overexpression of Cul4A has been documented in a variety of cancers (Lee and Zhou, 2010). Asf1b is overexpressed in breast cancer and is predictive for the outcome of this disease (Corpet et al., 2011). In addition, histone chaperone Daxx is mutated in pancreatic neuroendocrine tumors and pediatric brain tumors (Heaphy et al., 2011; Jiao et al., 2011). Therefore, we propose that cancer cells may have evolved a mechanism to deregulate nucleosome assembly pathways, which in turn alter genome stability and/or epigenetic states to facilitate tumorigenesis. Future challenges would be to translate molecular insights into the regulations of nucleosome assembly pathways for a better understanding of human diseases including cancer.
Experimental procedures
Methods and Materials
Yeast Strains
The yeast strains are listed as Table S2. Standard yeast media and manipulations were used.
Detection of Ubiquitylated Species
We followed a published procedure to detect ubiquitylated species in yeast cells (Han et al., 2010). Briefly, yeast strains containing the TAP-tagged H3 or H4 were transformed with the plasmid pGPD416-Ub expressing HA-ubiquitin. A standard TAP purification procedure was then followed to purify TAP-tagged proteins first using IgG sepharose beads (GE Healthcare) and then using calmodulin sepharose beads. Purified ubiquitylated species were detected by Western blot using antibodies against the HA epitope and H3 or H4 was detected using antibodies against CBP that was fused to H3 or H4. Because ubiquitylated species are labile, it is important to perform experiments at the same time when comparing the effect of two mutants on co-purified ubiquitylated species.
In vitro Ubiquitylation Assay
To test whether Rtt101 ubiquitylates histone H3-H4 in vitro, a 200 μl reaction mixture including 25 ng yeast E1, 155 ng Cdc34 (E2), 1 μg Rtt101 (E3), 2 μgDrosophila or yeast H3-H4 tetramer (substrate), 25 ng FLAG-Ub and 5 μM ubiquitin aldehyde was assembled in a buffer containing 50 mM Tris-HCl pH 7.5, 5 mM MgCl2, 0.6 mM DTT, 2 mM ATP, and 200 μM Na3VO4. Control reactions without E1, E2, E3 or substrates were also assembled similarly. Following incubation at 30 °C for 1h, ubiquitin or ubiquitylated proteins were either precipitated using M2 anti-FLAG beads (Sigma), or total proteins by TCA. Ubiquitylated proteins were detected by Western blot using antibodies against the FLAG epitope. Recombinant Cdc34 was purified from E. coli. The Rtt101Mms1 E3 ligase was purified from yeast cells using tandem affinity purification using either an Rtt101- or Mms1-TAP strain.
In vitro Histone Binding Assays
Recombinant Drosophila H3-H4 proteins (0.1, 0.25 and 0.50 μg) were incubated with GST-Rtt101 fusion protein (2 μg) purified from E. coli or GST-Rtt101-Mms1-Flag complex purified from Sf9 cells in a buffer containing 25 mM Tris-HCl pH 7.6, 150 mM NaCl, 10 mM imidazole and 0.2% Triton X-100 at 4 °C for 2hs. Glutathione agarose (GE Healthcare) or M2 beads (Sigma) were added. After incubation at 4 °C for 2hs, the beads were washed extensively with a buffer containing 25 mM Tris-HCl pH 7.6, 500 mM NaCl, 20 mM imidazole and 0.2% Triton X-100. The bound proteins were eluted using 1X SDS sample buffer, resolved by SDS-PAGE and detected by Western blot using the relevant antibodies indicated in figures. GST-REGα, a mammalian proteasome binding protein, was used as a negative control. Recombinant H3-H4 were acetylated by Rtt109-Vps75 complex in the presence or absence of acetyl-CoA as described (Li et al., 2008) and used for the in vitro binding assays as well as in vitro ubiquitylation assays described above.
Chromatin immunoprecipitation assay (ChIP) and DNA damage sensitivity assays were performed as previously described (Li et al., 2008). Other procedures including H3.1 and H3.3-SNAP staining, H3K122ub antibody production were described in Supplemental information, which include seven supplemental figures, two supplemental tables experimental procedures, and references.
Supplementary Material
Highlights
-
1)
Histone H3K56 acetylation promotes Rtt101Mms1-catalyzed H3 ubiquitylation
-
2)
Rtt101Mms1 and H3 ubiquitylation function in nucleosome assembly in yeast cells
-
3)
Cul4ADDB1 E3 ligase regulates deposition of new H3.1 and H3.3 in human cells
-
4)
Rtt101Mms1 and Cul4ADDB1 curb the interaction of histone chaperone Asf1 with H3-H4
Acknowledgements
We thank Drs. Burgess, Lou, Katzmann, van Deursen, Ekker and Wu and Mr. Dahlin for critical reading of this manuscript. We thank Dr. Kamura for plasmids and Dr. Verreault for a yeast histone purification protocol. This work is supported by NIH grants (GM72719 and GM81838).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas T, Shibata E, Park J, Jha S, Karnani N, Dutta A. CRL4(Cdt2) regulates cell proliferation and histone gene expression by targeting PR-Set7/Set8 for degradation. Mol Cell. 2010;40:9–21. doi: 10.1016/j.molcel.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 2008;22:2496–2506. doi: 10.1101/gad.1676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RJ, Zhang Z. Histone chaperones in nucleosome assembly and human disease. Nat Struct Mol Biol. 2013;20:14–22. doi: 10.1038/nsmb.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RJ, Zhou H, Han J, Zhang Z. A role for Gcn5 in replication-coupled nucleosome assembly. Mol Cell. 2010;37:469–480. doi: 10.1016/j.molcel.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos EI, Fillingham J, Li G, Zheng H, Voigt P, Kuo WH, Seepany H, Gao Z, Day LA, Greenblatt JF, et al. The program for processing newly synthesized histones H3.1 and H4. Nat Struct Mol Biol. 2010;17:1343–1351. doi: 10.1038/nsmb.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celic I, Masumoto H, Griffith WP, Meluh P, Cotter RJ, Boeke JD, Verreault A. The sirtuins hst3 and hst4p preserve genome integrity by controlling histone h3 lysine 56 deacetylation. Curr Biol. 2006;16:1280–1289. doi: 10.1016/j.cub.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Collins SR, Miller KM, Maas NL, Roguev A, Fillingham J, Chu CS, Schuldiner M, Gebbia M, Recht J, Shales M, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- Corpet A, De Koning L, Toedling J, Savignoni A, Berger F, Lemaitre C, O'Sullivan RJ, Karlseder J, Barillot E, Asselain B, et al. Asf1b, the necessary Asf1 isoform for proliferation, is predictive of outcome in breast cancer. EMBO J. 2011;30:480–493. doi: 10.1038/emboj.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donham DC, 2nd, Scorgie JK, Churchill ME. The activity of the histone chaperone yeast Asf1 in the assembly and disassembly of histone H3/H4-DNA complexes. Nucleic Acids Res. 2011;39:5449–5458. doi: 10.1093/nar/gkr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane P, Ouararhni K, Depaux A, Shuaib M, Hamiche A. The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev. 2010;24:1253–1265. doi: 10.1101/gad.566910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll R, Hudson A, Jackson SP. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science. 2007;315:649–652. doi: 10.1126/science.1135862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English CM, Adkins MW, Carson JJ, Churchill ME, Tyler JK. Structural basis for the histone chaperone activity of Asf1. Cell. 2006;127:495–508. doi: 10.1016/j.cell.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Banaszynski LA, Noh KM, Lewis PW, Elsaesser SJ, Stadler S, Dewell S, Law M, Guo X, Li X, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140:678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth A, Ray-Gallet D, Quivy JP, Lukas J, Bartek J, Almouzni G. Human Asf1 regulates the flow of S phase histones during replicational stress. Mol Cell. 2005;17:301–311. doi: 10.1016/j.molcel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Han J, Li Q, McCullough L, Kettelkamp C, Formosa T, Zhang Z. Ubiquitylation of FACT by the cullin-E3 ligase Rtt101 connects FACT to DNA replication. Genes Dev. 2010;24:1485–1490. doi: 10.1101/gad.1887310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Zhou H, Horazdovsky B, Zhang K, Xu RM, Zhang Z. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science. 2007;315:653–655. doi: 10.1126/science.1133234. [DOI] [PubMed] [Google Scholar]
- Havens CG, Walter JC. Mechanism of CRL4(Cdt2), a PCNA-dependent E3 ubiquitin ligase. Genes Dev. 2011;25:1568–1582. doi: 10.1101/gad.2068611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Alcasabas AA, Elledge SJ. Asf1 links Rad53 to control of chromatin assembly. Genes Dev. 2001;15:1061–1066. doi: 10.1101/gad.873201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Xiong Y. CRL4s: the CUL4-RING E3 ubiquitin ligases. Trends Biochem Sci. 2009;34:562–570. doi: 10.1016/j.tibs.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PD, Cohen JL, Osley MA. Hir proteins are required for position-dependent gene silencing in Saccharomyces cerevisiae in the absence of chromatin assembly factor I. Mol Cell Biol. 1998;18:4793–4806. doi: 10.1128/mcb.18.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell. 2007;26:775–780. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Lee J, Zhou P. Cullins and cancer. Genes Cancer. 2010;1:690–699. doi: 10.1177/1947601910382899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Zhou P. Pathogenic Role of the CRL4 Ubiquitin Ligase in Human Disease. Front Oncol. 2012;2:21. doi: 10.3389/fonc.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Shukla A, Schneider J, Swanson SK, Washburn MP, Florens L, Bhaumik SR, Shilatifard A. Histone crosstalk between H2B monoubiquitination and H3 methylation mediated by COMPASS. Cell. 2007;131:1084–1096. doi: 10.1016/j.cell.2007.09.046. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhou H, Wurtele H, Davies B, Horazdovsky B, Verreault A, Zhang Z. Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell. 2008;134:244–255. doi: 10.1016/j.cell.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M, Mortensen UH, Rothstein R. Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat Cell Biol. 2003;5:572–577. doi: 10.1038/ncb997. [DOI] [PubMed] [Google Scholar]
- Luger K. Dynamic nucleosomes. Chromosome Res. 2006;14:5–16. doi: 10.1007/s10577-005-1026-1. [DOI] [PubMed] [Google Scholar]
- Maas NL, Miller KM, Defazio LG, Toczyski DP. Cell cycle and checkpoint regulation of histone h3 k56 acetylation by hst3 and hst4. Mol Cell. 2006;23:109–119. doi: 10.1016/j.molcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Xiong Y. X-linked mental retardation gene CUL4B targets ubiquitylation of H3K4 methyltransferase component WDR5 and regulates neuronal gene expression. Mol Cell. 2011;43:381–391. doi: 10.1016/j.molcel.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S, Stillman B, Horvitz HR. Replication-coupled chromatin assembly generates a neuronal bilateral asymmetry in C. elegans. Cell. 2011;147:1525–1536. doi: 10.1016/j.cell.2011.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom M, Dennehey BK, Tyler JK. Chaperoning histones during DNA replication and repair. Cell. 2010;140:183–195. doi: 10.1016/j.cell.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray-Gallet D, Woolfe A, Vassias I, Pellentz C, Lacoste N, Puri A, Schultz DC, Pchelintsev NA, Adams PD, Jansen LE, et al. Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol Cell. 2011;44:928–941. doi: 10.1016/j.molcel.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Silva AC, Xu X, Kim HS, Fillingham J, Kislinger T, Mennella TA, Keogh MC. The replication-independent histone H3-H4 chaperones HIR, ASF1, and RTT106 co-operate to maintain promoter fidelity. J Biol Chem. 2012;287:1709–1718. doi: 10.1074/jbc.M111.316489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- Stillman B. Chromatin assembly during SV40 DNA replication in vitro. Cell. 1986;45:555–565. doi: 10.1016/0092-8674(86)90287-4. [DOI] [PubMed] [Google Scholar]
- Su D, Hu Q, Li Q, Thompson JR, Cui G, Fazly A, Davies BA, Botuyan MV, Zhang Z, Mer G. Structural basis for recognition of H3K56-acetylated histone H3-H4 by the chaperone Rtt106. Nature. 2012;483:104–107. doi: 10.1038/nature10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma T, Workman JL. Crosstalk among Histone Modifications. Cell. 2008;135:604–607. doi: 10.1016/j.cell.2008.10.036. [DOI] [PubMed] [Google Scholar]
- Sun ZW, Allis CD. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature. 2002;418:104–108. doi: 10.1038/nature00883. [DOI] [PubMed] [Google Scholar]
- Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone h3.1 and h3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116:51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Poustovoitov MV, Zhao K, Garfinkel M, Canutescu A, Dunbrack R, Adams PD, Marmorstein R. Structure of a human ASF1a-HIRA complex and insights into specificity of histone chaperone complex assembly. Nat Struct Mol Biol. 2006;13:921–929. doi: 10.1038/nsmb1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai K, Abbas T, Jazaeri AA, Dutta A. CRL4(Cdt2) E3 ubiquitin ligase monoubiquitinates PCNA to promote translesion DNA synthesis. Mol Cell. 2010;37:143–149. doi: 10.1016/j.molcel.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercero JA, Diffley JF. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature. 2001;412:553–557. doi: 10.1038/35087607. [DOI] [PubMed] [Google Scholar]
- Tropberger P, Pott S, Keller C, Kamieniarz-Gdula K, Caron M, Richter F, Li G, Mittler G, Liu ET, Buhler M, et al. Regulation of transcription through acetylation of H3K122 on the lateral surface of the histone octamer. Cell. 2013;152:859–872. doi: 10.1016/j.cell.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Verreault A, Kaufman PD, Kobayashi R, Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell. 1996;87:95–104. doi: 10.1016/s0092-8674(00)81326-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhai L, Xu J, Joo HY, Jackson S, Erdjument-Bromage H, Tempst P, Xiong Y, Zhang Y. Histone H3 and H4 ubiquitylation by the CUL4-DDB ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Mol Cell. 2006;22:383–394. doi: 10.1016/j.molcel.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Winkler DD, Zhou H, Dar MA, Zhang Z, Luger K. Yeast CAF-1 assembles histone (H3-H4)2 tetramers prior to DNA deposition. Nucleic Acids Res. 2012;40:10139–10149. doi: 10.1093/nar/gks812. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zaidi IW, Rabut G, Poveda A, Scheel H, Malmstrom J, Ulrich H, Hofmann K, Pasero P, Peter M, Luke B. Rtt101 and Mms1 in budding yeast form a CUL4(DDB1)-like ubiquitin ligase that promotes replication through damaged DNA. EMBO Rep. 2008 doi: 10.1038/embor.2008.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.