Abstract
We investigated physiological responses and changes in circulating immune cells following exercise in cold and thermoneutral conditions. Participants were short track skaters (n=9) who were acclimatized to cold conditions, and inline skaters (n=10) who were not acclimatized. All skaters were young, and skating at a recreational level three days per week for at least one year. Using a cross-over design, study variables were measured during 60 min of submaximal cycling (65% O2max) in cold (ambient temperature: 5±1°C, relative humidity: 41±9%) and thermoneutral conditions (ambient temperature: 21±1°C, relative humidity: 35±5%). Heart rate, blood lactate and tympanic temperature were measured at rest, during exercise and recovery. Plasma cortisol, calprotectin and circulating blood cell numbers were measured before and after 60 min of cold or thermoneutral conditions, and during recovery from exercise. Heart rate was lower in both groups during exercise in cold versus thermoneutral conditions (P<0.05). The increase in total leukocytes during recovery was primarily due to an increase in neutrophils in both groups. The cold-acclimatized group activated neutrophils after exercise in cold exposure, whereas the non-acclimatized group activated lymphocyte and cortisol after exercise in cold exposure. Lymphocyte subsets significantly changed in both groups over time during recovery as compared to rest. Immediately after exercise in both groups, CD16+ and CD69+ cells were elevated compared to rest or before exercise in both conditions. Acclimatization to exercise in the cold does not appear to influence exercise-induced immune changes in cold conditions, with the possible exception of neutrophils, lymphocytes and cortisol concentration.
Keywords: exercise, cold, neutrophils, lymphocyte subset, cortisol
INTRODUCTION
Cold exposure affects physiological responses at rest and during exercise. The two major physiological adjustments that occur during cold exposure are increased thermogenesis and peripheral vasoconstriction [1]. Cold exposure stimulates intense sympatho-adrenal activity, eliciting secretion of catecholamine and cortisol [39]. Exercise can cause changes in immune function. Furthermore, because shivering-induced muscle contractions are similar to volitional exercise, cold conditions could affect immune responses. Alterations in the immune system during cold exposure result from physiological changes in blood flow distribution, plasma volume and cardiac output [31]. Kozyreva and Eliseeva [21] have proposed that the thermal factor of the environment and long-term adaptation to cold are key factors influencing the function of immunocompetent cells.
Humans adapt to cold environments in order to maintain homeostasis. Factors affecting physiological responses to cold exposure are body composition, gender, and age. Training status and cold acclimatization are also important determinants of physiological responses to exercise in the cold. Kollias et al. [20] found that cold-exposed, endurance-trained subjects had a low thermogenic threshold. Conversely, others have reported that trained athletes have improved cold tolerance [15] and greater thermoregulatory sensitivity [2]. Cold acclimation may interfere with training adaptations, as it reduces sympathetic responses to the cold and therefore reduces the rates of glycogenolysis and lipolysis, as well as promoting vasoconstriction [17].
It is commonly believed that exposure to cold environmental temperatures suppresses immune function and increases the risk of infection [33]. However, Castellani et al. [5] do not support the concept that cold exposure depresses immune function. Jansky et al. [16] have suggested that improvements in cold tolerance may actually be based on enhanced activity of the immune system. During moderate exercise, several positive changes occur in the immune system [26], and acute bouts of endurance exercise lead to transient but significant changes in immunity and host defence [28]. The effects of cold acclimation on immune changes during exercise in cold conditions are not well understood because it is often difficult to separate the effects of exercise from cold exposure per se. Long-term adaptation to cold conditions affects neuroendocrine function, as well as neuro-immunomodulatory properties. We hypothesized that cold acclimation would alter the magnitude of physiological responses and stimulatory responses of circulating immune cells following exercise in cold conditions. To test this hypothesis, we compared physiological and immunological responses both at rest and in response to exercise in cold and thermoneutral conditions in cold-acclimatized and non-acclimatized skaters.
MATERIALS AND METHODS
Subjects
The participants were young male skaters participating at a recreational level in either short track (cold-acclimatized group) (n=9) or inline skating (non-acclimatized group) (n=10). All participants had been skating three days per week for at least one year. Short track skaters of the cold-acclimatized group performed 2 hours of skating per day in cold conditions (ambient temperature: 5±1°C), whereas inline skaters of the non-acclimatized group performed 2 hours of skating per day in thermoneutral conditions (ambient temperature: 21±1°C). These participants were regarded as moderate trained individuals. The characteristics of the skaters are shown in Table 1. There were no significant differences between the groups in cardiopulmonary endurance capacity or body composition. The study protocol was approved by the Institutional Review Board of Keimyung University. Informed consent was obtained from all study participants.
TABLE 1.
CHARACTERISTICS OF ACCLIMATIZED AND NON-ACCLIMATIZED SUBJECTS
| Variables | Acclimatized (n=9) | Non-acclimatized (n=10) | P value |
|---|---|---|---|
| Age (yr) | 24.00 ± 1.03 | 25.30 ± 1.42 | 0.478 |
| Height (cm) | 179.44 ± 1.52 | 170.80 ± 2.64 | 0.013* |
| Body weight (kg) | 70.94 ± 1.88 | 69.74 ± 2.89 | 0.737 |
| % Fat (%) | 15.00 ± 1.68 | 18.13 ± 2.26 | 0.291 |
| O2 (mL · kg-1 · min-1) | 48.20 ± 2.79 | 46.24 ± 2.11 | 0.578 |
| HRrest (beats · min-1) | 72.33 ± 4.67 | 67.50 ± 3.48 | 0.412 |
| HRmax (beats · min-1) | 185.78 ± 2.74 | 184.20 ± 3.24 | 0.718 |
| LApeak (mmol · L-1) | 8.42 ± 0.48 | 7.53 ± 0.21 | 0.098 |
| EXtime (sec) | 1147.56 ± 60.78 | 1076.50 ± 65.09 | 0.439 |
| Peak KP | 5.00 ± 0.26 | 4.75 ± 0.24 | 0.490 |
| 65% O2max (mL · kg-1 · min-1) | 31.33 ± 1.81 | 30.07 ± 1.37 | 0.535 |
| HR on 65% O2max (beats · min-1) | 146.22 ± 8.18 | 145.60 ± 8.17 | 0.870 |
| LA on 65% O2max (mmol · L-1) | 2.83 ± 0.25 | 2.38 ± 0.12 | 0.128 |
| KP on 65% O2max | 2.78 ± 0.21 | 2.55 ± 0.16 | 0.386 |
| RPE on 65% O2max | 14.56 ± 0.47 | 13.70 ± 0.40 | 0.181 |
| Career (month) | 52.67 ± 14.36 | 28.40 ± 7.78 | 0.145 |
Note: Values are mean and SE, P<0.05, HR: heart rate, LA: blood lactate, KP: kilopond, RPE: ratings of perceived exertion, EXtime: duration of exercise in maximal exercise test
Exercise testing
In preliminary testing, a graded maximal exercise test was used in thermoneutral conditions to determine O2max. Skaters started cycling at 60 rpm at 0.5 kp for 2 min. Thereafter, the intensity increased 0.5 kp every 2 min until the skaters were exhausted. Once O2max was established, all skaters cycled for 60 min at 65% O2max in cold (ambient temperature: 5±1°C, relative humidity 41±9%) and thermoneutral conditions (ambient temperature: 21±1°C, relative humidity 35±5%). To minimize the effects of circadian rhythm, all exercise bouts including preliminary testing were performed between 2:00 and 8:00 pm, and at the same time of day for each trial in the cool season. Trials were separated by 1 week to ensure complete recovery between trials, and were randomized in a counterbalanced order, with each subject serving as his own control. Except for the last 48 hours before each trial, when exercise was regulated by the study protocol, the skaters completed their regular training programme and usual daily activity without any interruption during the study period. During each trial, the skaters were served their usual meals, but they did not consume any food within 2 hours before exercise testing. Physiological variables were measured at rest before and after 1 hour of bed rest preceding exercise. We included measurements under resting conditions before exercise, so that we could separate the effects of ambient temperature from the effects of exercise. A flexible intravenous catheter (Venflon 1.2, BOC health care, Helsing, Sweden) was inserted for blood collection. After the first blood sample was drawn at rest, the skaters rested in bed for 60 min in either thermoneutral (ambient temperature: 21±1°C, relative humidity 35±5%) or cold conditions (ambient temperature: 5±1°C, relative humidity 41±9%). After the second blood sampling (after 60 min bed rest and before exercise), the skaters performed a 5-min warm-up or the familiarization, cycling at 30-45% of O2max, immediately followed by 60 min at the skaters’ predetermined kilopond (KP) of 65% O2max in cold or thermoneutral conditions, respectively. The skaters had a 120-min bed rest recovery phase in thermoneutral conditions after exercise (Figure 1). The same clothing was worn in each exercise testing session; they wore three layers of clothes during rest or one layer during exercise. The skaters were permitted to drink a maximum of 300 mL of tap water during exercise testing.
FIG. 1.
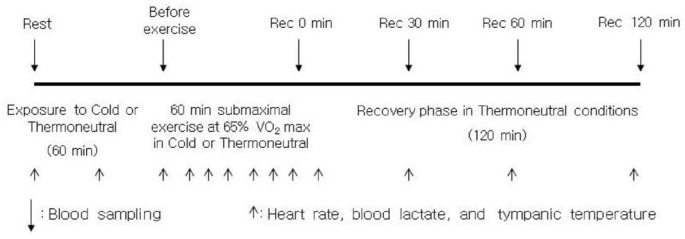
SCHEMATIC DIAGRAM OF EXPERIMENTAL DESIGN
Physiological variables
For the comparison of basic physiological responses, heart rate, blood lactate, and tympanic temperature were measured at the following times (Figure 1): at rest before exposure to cold or thermoneutral conditions (rest 0 min); after 30 or 60 min exposure to cold or thermoneutral conditions (rest 30 min or 60 min); at 10-min intervals during exercise in cold or thermoneutral conditions; at 10, 30, 60 and 120 min during the recovery phase in thermoneutral conditions after submaximal exercise. Heart rate was measured using a heart rate monitor (Polar Electro OY PE3000, Kempele, Finland), and blood lactate was measured from the fingertips by a lactate analyser (1500-L, YSI, Yellow Springs, USA). Tympanic temperature was measured using a ThermoScan thermometer (IRT 4520, Braun Co., Kronberg, Germany) without additional thermal sensor implementation for ear canal insulation. During exercise, heart rate and RPE (ratings of perceived exertion: 6-20 scale) were continuously monitored.
Circulating cortisol, calprotectin and blood cell counts
Blood samples for determination of circulating blood cell numbers and plasma concentrations of cortisol and calprotectin were collected at the following times (Figure 1): before and after 60 min rest in cold or thermoneutral conditions; before and after 60 min submaximal exercise in cold or thermoneutral conditions (before exercise); at 0, 30, 60 and 120 min during the recovery phase in thermoneutral conditions after exercise. All blood samples were drawn from an antecubital vein during bed rest, except for the sample taken immediately after exercise, which was obtained with the skaters seated on the cycle ergometer. At each sampling, blood was collected in a 5 mL EDTA tube for haematological analyses. Blood for lymphocyte subsets was collected in a 5 mL EDTA tube (Becton-Dickinson Vacutainer system), and analysed later on the same day. Plasma samples were separated from whole blood using EDTA as an anticoagulant immediately after blood sampling, followed by centrifugation at 1,000 g for 15 min. These samples were stored at -80°C until assayed. Plasma concentrations of cortisol and calprotectin were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Cortisol ELISA, IBL, Hamburg, Germany; Human Calprotectin ELISA, HyCult Biotechnology, Uden, Netherlands), according to the manufacturer 's instructions. The absorbance was measured spectrophotometrically with a microplate reader (VersaMax, Molecular Devices, California, USA). Whole blood (2 mL) treated with EDTA was analysed for total leukocytes, neutrophils, lymphocytes, monocytes, and haematocrit with an automated Coulter counter (XE-2100D, Sysmex, Kobe, Japan) by the clinical haematology group at Keimyung University.
Lymphocyte subsets were assessed by measuring the concentrations of T cells (CD3+), B cells (CD19+), natural killer (NK) cells (CD16+) and activated T-cells (CD69+). Two-colour immunophenotyping of EDTA-treated whole blood was used to determine T cell subsets by CD3 [fluorescein isothiocyanate (FITC)] and CD16 [phycoerythrin (PE)] mixed (10 µl each) with 100 µl of whole blood. An additional 100 µl of blood was mixed with 10 µl each of CD19 (FITC), and CD69 (PE). All reagents were purchased from BD Biosciences (San Jose, CA, USA) and samples were stained as per the manufacturer's instructions within 6 hours of collection. Briefly, mixed samples were vortexed and incubated for 20 min at room temperature (RT) in the dark. After adding 2 mL of FACS Lysing Solution to lyse red blood cells, samples were vortexed and incubated for a further 10 min at RT. Samples were centrifuged (300 g for 5 min at RT), washed with 2 mL of phosphate-buffered saline (PBS) containing 0.1% sodium azide, centrifuged (200 g for 5 min at RT), and fixed with 0.5 mL PBS (1% paraformaldehyde). Samples were stored at 3°C for no more than 48 h before analysis by a FACScan flow cytometer (Becton Dickson, San Jose, CA, USA) and CELLQuest software (BD Biosciences). The lymphocyte population was gated using forward-scatter versus side-scatter characteristics and 10,000 events were counted per sample. Cell concentrations of each lymphocyte subset were calculated by multiplying the percentage of cells with appropriate fluorescence by the absolute number of lymphocytes obtained from the blood tests. Whole blood cell concentrations, plasma concentrations of cortisol and calprotectin were corrected for exercise-induced changes in blood volume according to Dill and Costill [11].
Statistical analyses
Values are reported as the mean ± standard error (SE). All statistical analyses were performed using SPSS for Windows (Version 13.0). Significant differences of dependent variables were analysed using a three-way (time × ambient temperature × group) repeated-measures ANOVA and Bonferroni adjustments were used for post hoc analysis. P<0.05 was considered statistically significant.
RESULTS
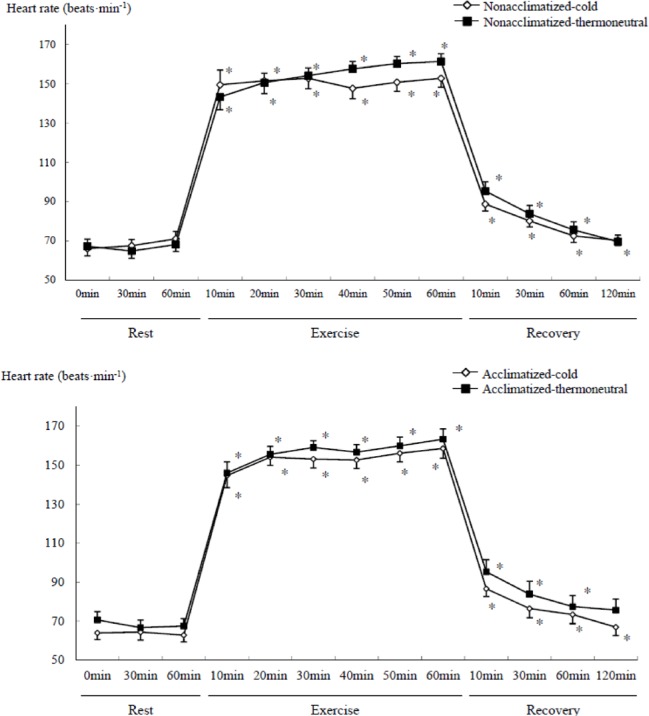
Heart rate increased during exercise (Figure 2), and was significantly lower in the cold than the thermoneutral conditions (temperature main effect, F=5.075, P<0.05) in both groups. Heart rate responses also demonstrated significant interactions between time and temperature (F=5.913, P<0.001) or time × temperature × group (F=2.119, P<0.01) without a significant difference between groups. Heart rate significantly changed in both groups over time (P<0.05, Figure 2) during exercise and recovery as compared to rest. During recovery from exercise in the cold, heart rate remained elevated 120 min after exercise in both groups.
FIG. 2.
COMPARISON OF HEART RATE DURING SUBMAXIMAL EXERCISE BETWEEN COLD AND THERMONEUTRAL CONDITIONS IN COLD-ACCLIMATIZED AND NON-ACCLIMATIZED GROUPS
Note: * P <0.05 compared to rest (0 min), Significant main effect by 3-way ANOVA : Temperature (P =0.038), Time (P <0.001), Significant interaction time × temperature (P <0.001), time × group × temperature (P <0.001)
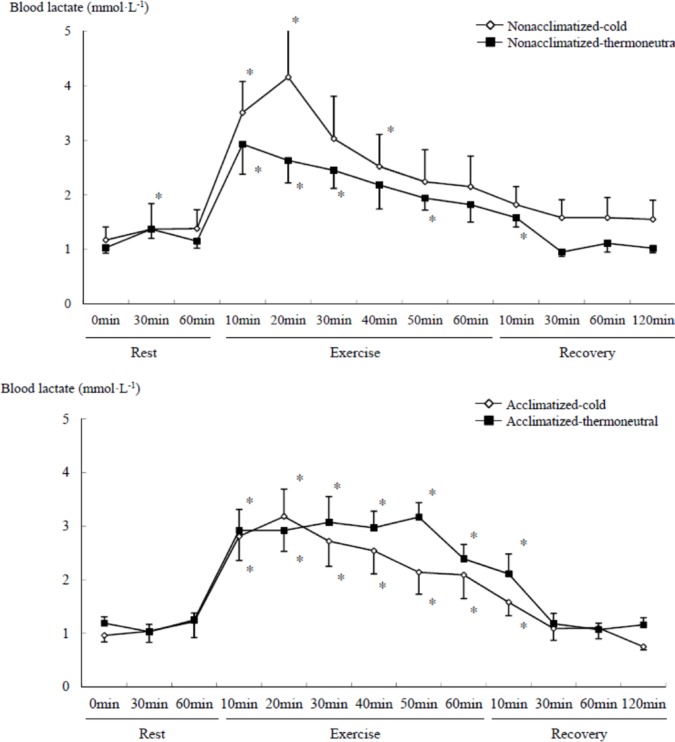
Blood lactate concentration significantly changed in both groups over time (time main effect, F=22.801, P<0.001) during exercise and recovery as compared to rest. Blood lactate concentration in the non-acclimatized group tended to be higher in the cold than the thermoneutral conditions. In the acclimatized group, lactate tended to be lower in the cold than the thermoneutral conditions, but these differences did not reach statistical significance (Figure 3). Blood lactate concentration had returned to resting values after 30 min of recovery in both groups.
FIG. 3.
COMPARISON OF BLOOD LACTATE CONCENTRATION DURING SUBMAXIMAL EXERCISE BETWEEN COLD AND THERMONEUTRAL CONDITIONS IN COLD-ACCLIMATIZED AND NON-ACCLIMATIZED GROUPS
Note: * P <0.05 compared to rest (0 min), Significant main effect by 3-way ANOVA: Time (P <0.001)
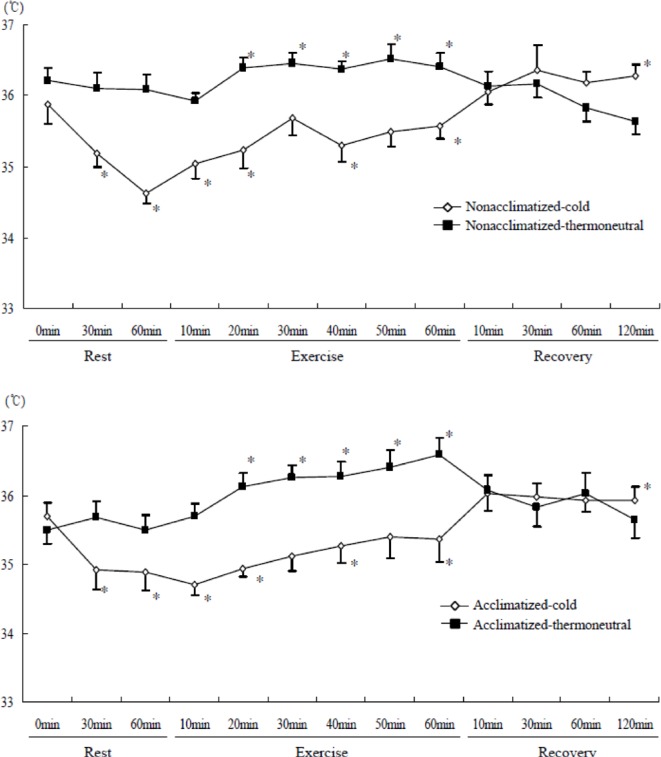
Tympanic temperature was significantly lower in the cold than the thermoneutral conditions (temperature main effect, F=20.710, P<0.001) in both groups. Tympanic temperature changed significantly in both groups over time (time main effect, F=12.312, P<0.001). There was a significant time × temperature effect (F=10.604, P<0.001) for tympanic temperature. Tympanic temperature was significantly lower compared to rest in both groups at the end of resting cold exposure, and also at the end of exercise in cold conditions. In contrast, tympanic temperature was significantly higher compared to rest after exercise in thermoneutral conditions (Figure 4).
FIG. 4.
COMPARISON OF TYMPANIC TEMPERATURE DURING SUBMAXIMAL EXERCISE BETWEEN COLD AND THERMONEUTRAL CONDITIONS IN COLD-ACCLIMATIZED AND NON-ACCLIMATIZED GROUPS
Note: * P <0.05 compared to rest (0 min), Significant main effect by 3-way ANOVA: Time (P <0.001), Temperature (P <0.001), Significant interaction time × temperature (P <0.001)
Based on the haematocrit values, plasma volume changed in both groups over time during the recovery phase after submaximal exercise. Haematocrit values significantly changed in both groups over time (time main effect, F=25.068, P<0.001). Although there were no statistically significant differences of main effects between temperature conditions or groups, haematocrit values of the non-acclimatized group had not returned to resting values after 120 min of recovery in post hoc tests (Table 2).
TABLE 2.
CHANGES OF HAEMATOCRIT VALUE, CORTISOL AND CALPROTECTIN CONCENTRATIONS AT REST, BEFORE AND AFTER SUBMAXIMAL EXERCISE
| Variables | Group | Condition | Rest | Before exercise | Recovery phase (min) | 3 way ANOVA | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 0 | 30 | 60 | 120 | ||||||
| Hematocrit % | Acclimatized | Cold | 42.22 | 43.60* | 43.89* | 42.06 | 41.90 | 42.42 | Ti : P<0.001 Te : NS Gr : NS Interaction(Ti×Te) : P<0.001 |
| 0.87 | 0.72 | 0.97 | 0.88 | 0.86 | 0.75 | ||||
|
| |||||||||
| Thermoneutral | 41.50 | 41.63 | 43.13* | 41.52 | 41.37 | 42.17 | |||
| 0.85 | 0.80 | 0.88 | 0.92 | 0.87 | 0.98 | ||||
|
| |||||||||
| Non-acclimatized | Cold | 43.75 | 44.34* | 44.88* | 43.45 | 43.77 | 44.59 | ||
| 0.90 | 0.67 | 0.90 | 0.90 | 0.92 | 1.12 | ||||
|
| |||||||||
| Thermoneutral | 43.10 | 43.03 | 45.15* | 43.52 | 43.59 | 44.55* | |||
| 0.92 | 0.93 | 1.02 | 1.09 | 0.94 | 0.99 | ||||
|
| |||||||||
| Cortisol (nmol · L-1) | Acclimatized | Cold | 228.25 | 153.15* | 262.71 | 247.45 | 143.03 | 128.79* | Ti : P<0.001 Te : NS Gr : NS Interaction(Te×Gr): P<0.05 |
| 27.59 | 21.93 | 47.18 | 51.04 | 28.72 | 23.56 | ||||
|
| |||||||||
| Thermoneutral | 245.96 | 196.39 | 339.83 | 320.51 | 220.09 | 167.28 | |||
| 29.14 | 22.87 | 44.17 | 43.95 | 24.94 | 34.18 | ||||
|
| |||||||||
| Non-acclimatized | Cold | 273.97 | 178.29* | 277.39 | 291.41 | 238.15 | 211.12 | ||
| 48.64 | 30.90 | 65.22 | 75.84 | 63.76 | 37.52 | ||||
|
| |||||||||
| Thermoneutral | 213.88 | 179.72 | 269.31 | 298.63 | 213.18 | 140.49* | |||
| 30.65 | 37.27 | 47.42 | 49.41 | 52.75 | 26.38 | ||||
|
| |||||||||
| Calprotectin (g · L-1) | Acclimatized | Cold | 4.04 | 4.60* | 9.26* | 10.37* | 9.76* | 9.95* | Ti : P<0.001 Te : P<0.05 Gr : NS Interaction : NS |
| 0.95 | 0.88 | 1.13 | 1.20 | 1.07 | 1.58 | ||||
|
| |||||||||
| Thermoneutral | 4.46 | 3.91* | 9.46* | 7.47* | 10.75* | 8.98* | |||
| 0.07 | 0.85 | 1.03 | 0.92 | 1.34 | 0.79 | ||||
|
| |||||||||
| Non-acclimatized | Cold | 5.00 | 5.10 | 11.86* | 9.74* | 11.67* | 11.25* | ||
| 0.67 | 0.70 | 1.54 | 1.19 | 1.27 | 1.54 | ||||
|
| |||||||||
| Thermoneutral | 4.07 | 3.39* | 9.75* | 8.19* | 9.16* | 10.07* | |||
| 0.50 | 0.44 | 1.40 | 1.08 | 1.01 | 1.00 | ||||
Note: Values are mean and SE, P<0.05 compared to rest, Ti: time, Te: temperature, Gr: group, NS: no significance
Plasma cortisol concentration changed in both groups over time during the recovery phase after submaximal exercise (time main effect, F=7.304, P<0.001). However, there were no significant differences between conditions or groups. Plasma cortisol concentration in the acclimatized group tended to be lower during the recovery phase after exercise in the cold than the thermoneutral conditions, while that of the non-acclimatized group did not decrease after exercise in cold exposure or during the recovery time (Table 2). Plasma cortisol concentration significantly decreased as compared to rest after 60 min exposure to cold or thermoneutral conditions before exercise in both groups and temperature conditions.
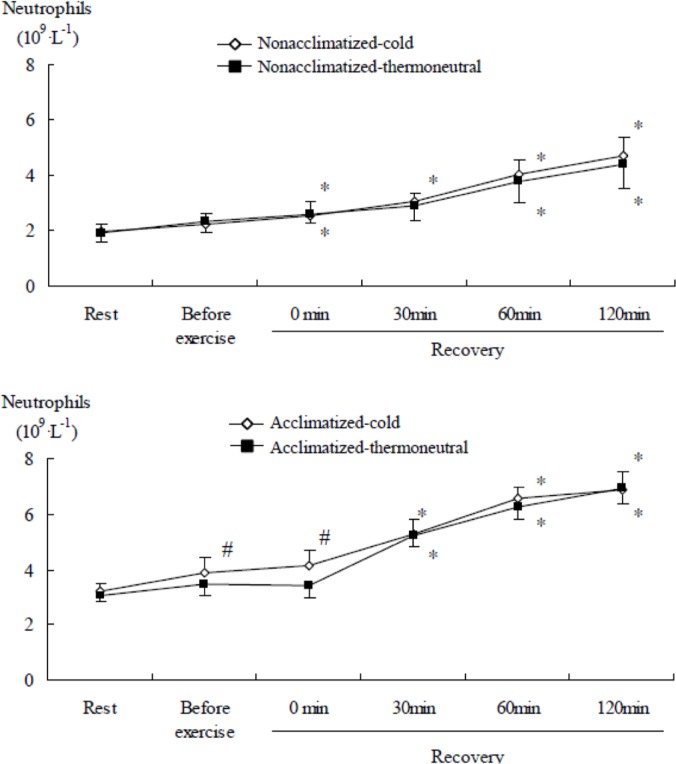
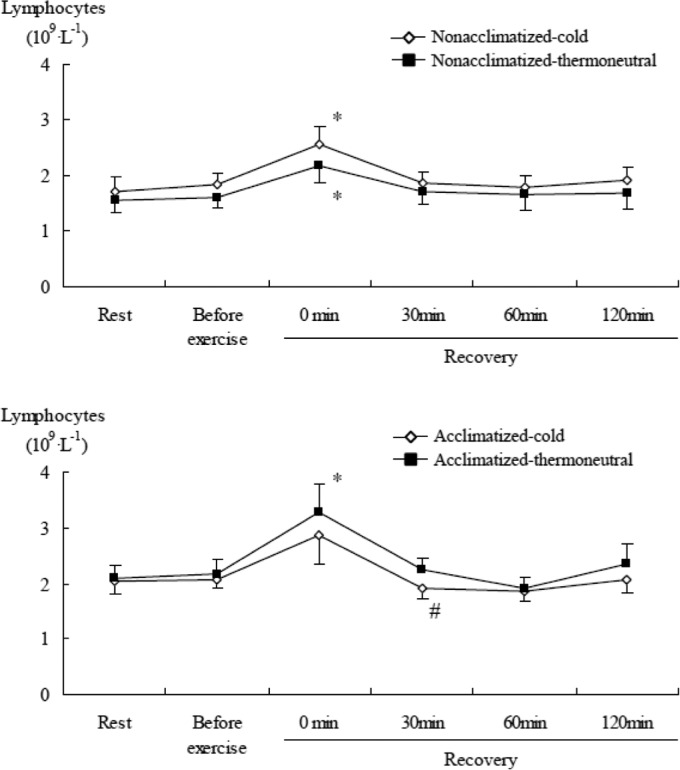
During the recovery phase after submaximal exercise, there was an increase in the numbers of total leukocytes (Table 3), neutrophils, lymphocytes, and monocytes (all time main effects, P<0.001). The number of neutrophils in the acclimatized group was significantly (P<0.05) higher before exercise and immediately after sub-maximal exercise in the cold than the thermoneutral conditions, while the non-acclimatized group showed no significant difference between cold and thermoneutral conditions (Figure 5). Lymphocyte counts in the non-acclimatized group were higher immediately after submaximal exercise in the cold than in thermoneutral conditions. Conversely, in the acclimatized group, they were lower after exercise in the cold than in thermoneutral conditions. The concentration of lymphocytes in the acclimatized group was significantly (P<0.05) lower at 30 min of recovery in the cold than the thermoneutral conditions, while in the non-acclimatized group lymphocytes tended to be higher in the cold than the thermoneutral conditions (Figure 6). Monocytes did not show a significant difference between conditions or groups.
TABLE 3.
CHANGES OF TOTAL LEUKOCYTES, CD3+ AND CD19+ CONCENTRATIONS AT REST, BEFORE AND AFTER SUBMAXIMAL EXERCISE
| Variables | Group | Condition | Rest | Before exercise | Recovery phase (min) | 3 way ANOVA | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 0 | 30 | 60 | 120 | ||||||
| Leukocytes (109 · L-1) | Acclimatized | Cold | 5.90 | 6.53 | 7.32* | 8.02* | 9.01* | 10.09* | Ti : P<0.001 Te : NS Gr : NS Interaction(Te×Gr): P<0.05 |
| 0.40 | 0.54 | 0.67 | 0.48 | 1.37 | 0.80 | ||||
|
| |||||||||
| Thermoneutral | 5.26 | 5.22 | 7.56* | 6.14* | 7.53* | 8.03* | |||
| 0.33 | 0.29 | 0.38 | 0.39 | 0.46 | 0.58 | ||||
|
| |||||||||
| Non-acclimatized | Cold | 4.09 | 4.53 | 5.64* | 5.26* | 6.36* | 7.01* | ||
| 0.33 | 0.37 | 0.36 | 0.50 | 0.83 | 0.82 | ||||
|
| |||||||||
| Thermoneutral | 5.03 | 4.94 | 7.25* | 6.70* | 7.52* | 8.19* | |||
| 0.69 | 0.72 | 1.17 | 1.46 | 1.34 | 1.32 | ||||
|
| |||||||||
| CD3+(106 · L-1) | Acclimatized | Cold | 1.29 | 1.01 | 0.83 | 0.90 | 0.82* | 0.62* | Ti : P<0.001 Te : NS Gr : NS Interaction : NS |
| 0.26 | 0.30 | 0.15 | 0.33 | 0.17 | 0.12 | ||||
|
| |||||||||
| Thermoneutral | 1.64 | 1.49 | 1.15 | 1.11* | 0.83* | 0.73* | |||
| 0.24 | 0.26 | 0.19 | 0.24 | 0.17 | 0.13 | ||||
|
| |||||||||
| Non-acclimatized | Cold | 1.38 | 1.27 | 1.30 | 1.11 | 0.97 | 0.82 | ||
| 0.34 | 0.25 | 0.34 | 0.23 | 0.22 | 0.20 | ||||
|
| |||||||||
| Thermoneutral | 2.22 | 1.96 | 1.86 | 2.02 | 1.72 | 1.47 | |||
| 0.55 | 0.48 | 0.44 | 0.64 | 0.55 | 0.46 | ||||
|
| |||||||||
| CD19+(106 · L-1) | Acclimatized | Cold | 0.27 | 0.30 | 0.29 | 0.28 | 0.20* | 0.19* | Ti : P <0.01 Te : NS Gr : NS Interaction : NS |
| 0.05 | 0.07 | 0.05 | 0.10 | 0.04 | 0.04 | ||||
|
| |||||||||
| Thermoneutral | 0.32 | 0.34 | 0.29 | 0.25 | 0.18* | 0.16* | |||
| 0.04 | 0.05 | 0.06 | 0.05 | 0.03 | 0.03 | ||||
|
| |||||||||
| Non-acclimatized | Cold | 0.35 | 0.32 | 0.46 | 0.29 | 0.30 | 0.19 | ||
| 0.07 | 0.07 | 0.10 | 0.04 | 0.08 | 0.03 | ||||
|
| |||||||||
| Thermoneutral | 0.29 | 0.34 | 0.31 | 0.38 | 0.33 | 0.26 | |||
| 0.08 | 0.14 | 0.08 | 0.14 | 0.14 | 0.13 | ||||
Note: Values are mean and SE, P <0.05 compared to rest, Ti : time, Te : temperature, Gr : group, NS : no significance
FIG. 5.
COMPARISON OF NEUTROPHIL CONCENTRATION AFTER SUBMAXIMAL EXERCISE BETWEEN COLD AND THERMONEUTRAL CONDITIONS IN COLD-ACCLIMATIZED AND NON-ACCLIMATIZED GROUPS
Note: * P <0.05 compared to rest (0 min), Significant main effect by 3-way ANOVA: Time (P <0.001), Significant interaction time × group × temperature (P =0.004), # P <0.05 compared to thermoneutral conditions
FIG. 6.
COMPARISON OF LYMPHOCYTE CONCENTRATION AFTER SUBMAXIMAL EXERCISE BETWEEN COLD AND THERMONEUTRAL CONDITIONS IN COLD-ACCLIMATIZED AND NON-ACCLIMATIZED GROUPS
Note: Significant main effect by 3-way ANOVA: Time (P <0.001), * P <0.05 compared to rest (0 min), # P <0.05 compared to thermoneutral conditions
Plasma calprotectin concentration changed in both groups over time during the recovery phase after submaximal exercise (time main effect, F=50.597, P<0.001), and was significantly different between temperature conditions (F=4.827, P=0.048). After 60 min of resting exposure to cold or thermoneutral conditions before exercise, calprotectin levels increased in the cold, whereas levels decreased in thermoneutral conditions (Table 2). Calprotectin tended to be higher in the non-acclimatized group during the recovery phase after exercise in the cold than the thermoneutral conditions, but the acclimatized group showed no difference. During the recovery phase after submaximal exercise, there was an increase in the ratio of calprotectin to neutrophils in both groups, and the acclimatized group had a significantly higher ratio (P<0.05) immediately after submaximal exercise as compared to rest in thermoneutral conditions. The non-acclimatized group tended to have a greater increase than the acclimatized group with no statistical difference (Figure 7).
FIG. 7.
COMPARISON OF THE RATIO OF CALPROTECTIN TO NEUTROPHILS AFTER SUBMAXIMAL EXERCISE BETWEEN COLD AND THERMONEUTRAL CONDITIONS IN COLD-ACCLIMATIZED AND NON-ACCLIMATIZED GROUPS
Note: * P <0.05 compared to rest (0 min), Significant main effect by 3-way ANOVA: Time (P <0.001)
CD3+ cells significantly decreased in both groups over time (time main effect, F=11.748, P<0.001) during recovery after submaximal exercise as compared to rest (Table 3). The cell number of the acclimatized group was significantly lower after 60 min of recovery than rest in both conditions.
CD19+ cells significantly changed in both groups over time (time main effect, F=4.035, P<0.01) during recovery as compared to rest. CD19+ cells in the non-acclimatized group tended to be higher immediately after submaximal exercise in the cold as compared to rest or during resting cold exposure (before exercise) with no statistical difference (Table 3). CD19+ cells in the acclimatized group were significantly lower at 60 min of the recovery phase than rest in both conditions.
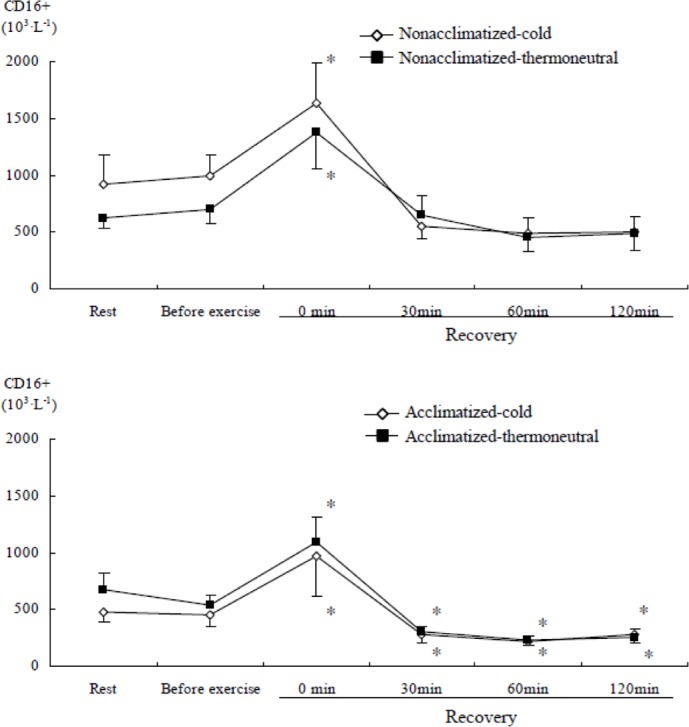
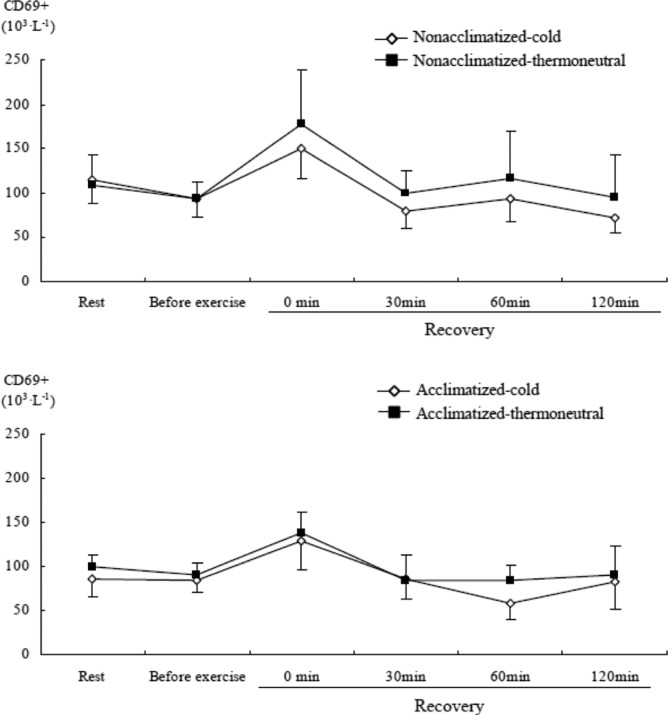
CD16+ and CD69+ cells significantly changed in both groups over time (time main effect, F=19.859, P<0.001 & F=2.901, P<0.05) during recovery after submaximal exercise as compared to rest. Immediately after submaximal exercise in both groups, there was an increase in CD16+ and CD69+ cells as compared to rest and before exercise in cold or thermoneutral conditions (Figure 8 & Figure 9). CD16+ cells in the acclimatized group were significantly lower after 30 min of the recovery phase than rest in both conditions.
FIG. 8.
COMPARISON OF CD16+ CELL CONCENTRATION AFTER SUBMAXIMAL EXERCISE BETWEEN COLD AND THERMONEUTRAL CONDITIONS IN COLD-ACCLIMATIZED AND NON-ACCLIMATIZED GROUPS
Note: * P <0.05 compared to rest (0 min), Significant main effect by 3-way ANOVA: Time (P <0.001)
FIG. 9.
COMPARISON OF CD69+ CELL CONCENTRATION AFTER SUBMAXIMAL EXERCISE BETWEEN COLD AND THERMONEUTRAL CONDITIONS IN COLD-ACCLIMATIZED AND NON-ACCLIMATIZED GROUPS
Note: Significant main effect by 3-way ANOVA: Time (P =0.019)
DISCUSSION
Cold exposure has an additive effect on physiological responses to exercise. In the present study, heart rate was lower in the cold than in thermoneutral conditions during submaximal exercise for both groups. This response was likely due to the parasympathetic activation of the baroreflex receptor, and a blunted epinephrine response which serves to prevent an increase in core temperature during exercise in cold conditions [27]. Defending core temperature against cold exposure to limit heat loss is mediated by peripheral vasoconstriction. Peripheral vasoconstriction redistributes blood to the core, causing increased stroke volumes and a slightly decreased heart rate [25]. However, in the present study heart rate during exercise was similar between the two groups, which suggests that acclimatization did not influence the catecholamine response to exercise.
Peripheral vasoconstriction decreases peripheral blood flow and reduces convective heat transfer from the body's core to skin during cold exposure. Consequently, heat loss is reduced and core temperature is maintained, but skin temperature decreases, thereby reducing the thermal gradient between the skin and the environment. This may explain why tympanic temperature was significantly lower in the cold than the thermoneutral conditions in this study.
Interestingly, although the differences in blood lactate concentration did not reach statistical significance, lactate tended to be higher in the non-acclimatized group in cold versus thermoneutral conditions. Conversely, in the acclimatized group, lactate tended to be lower in cold versus thermoneutral conditions. The higher blood lactate concentration during exercise in the cold is a result of lower aerobic metabolism and reduced clearance of lactate as compared to thermoneutral conditions [34]. The lower blood lactate concentration in the acclimatized group in the cold versus thermoneutral conditions may be due to adaptive responses to cold conditions. Such responses may involve activation of the sympatho-adrenal system and greater lipid metabolism during exercise in cold as compared to thermoneutral conditions [38].
Increases in concentrations of total leukocytes, neutrophils, lymphocytes and monocytes during the recovery phase after submaximal exercise represent a mild and transient immune perturbation, which is considered to be beneficial to immunosurveillance [9, 24]. The sustained elevation of total leukocytes and neutrophils after 120 min of recovery may be related to elevated cortisol levels in cold and thermoneutral conditions. Cold is known to affect leukocyte mobilization [16, 4] and can suppress lymphocyte functional activities [1, 10]. However, contrary to our expectations, there were no significant differences in total leukocytes and subsets between temperature conditions or groups. Others have reported that the concentration of leukocytes and monocytes at rest was significantly higher in cold-acclimatized swimmers versus non-acclimatized swimmers [12]. These findings suggest that acute cold exposure does not necessarily suppress immune function, and chronic adaptation to cold conditions may not modify changes in immune function following exercise in the cold.
Castellani et al. [5] reviewed the literature and concluded that cold-air exposure increased leukocyte and granulocyte counts regardless of whether the subjects exercised or had an elevated core temperature before cold exposure, while changes in lymphocyte, monocyte and NK cell count changes were more variable. In the present study, submaximal exercise stimulated changes in total leukocytes and lymphocyte subsets during the recovery phase. The increase in total leukocytes during recovery was primarily due to an increase in neutrophils. A more pronounced increase in neutrophils, but not in lymphocytes, was found after exercise in this study. These results showed a similar tendency to previous studies [19, 22]. The other previous works explained that increased neutrophils after endurance exercise are due to the disruption of skeletal muscle [37]. Alternatively, the attenuated response of neutrophils to repeated sessions of endurance exercise can be an adaptive mechanism for preventing pathophysiological reactions [36]. However, the higher neutrophil count in the acclimatized group in cold conditions may indicate the effect of cold exposure itself, and it is possible that neutrophils are mobilized in response to exercise as well as cold stress.
Calprotectin is an inflammatory mediator released by neutrophils [41]. Plasma calprotectin concentration and the ratio of calprotectin to neutrophils increased after submaximal exercise, indicating increased neutrophil activity after exercise. As calprotectin increased after 60 min exposure to cold conditions before exercise, this finding suggests that calprotectin is also released in response to cold stress itself. Calprotectin increased to a greater extent in the non-acclimatized group compared to the acclimatized group. Therefore, unaccustomed exercise in cold conditions may stimulate an acute inflammatory response. However, this concept is not supported, as there was a lack of any significant difference between the two groups in the ratio of calprotectin to neutrophils immediately after submaximal exercise (Figure 7).
Monocytes are deployed rapidly from the marginal pool into the circulation during the first minutes of strenuous exercise, but their numbers quickly decrease upon cessation of activity [3]. Additionally, prolonged cold exposure substantially magnified the extent of monocytosis, and such cold-enhanced recruitment of monocytes is presumably mediated by pronounced sympathetic nervous system (SNS) activation accompanying prolonged cold stress [29]. However, monocyte concentration remained elevated at 120 min of the recovery phase without a difference in these responses between different conditions or groups in our results.
The concentration of lymphocytes in the acclimatized group tended to be lower immediately after submaximal exercise in the cold than the thermoneutral conditions. In contrast, although the difference did not reach statistical significance, lymphocyte counts in the non-acclimatized group tended to be higher in the cold than the thermoneutral conditions. The greater lymphocyte response in the non-acclimatized group after exercise in cold conditions may be associated with acute phase responses of the immune system due to lack of adaptation to cold conditions. Exercising in cold water attenuated the leukocytosis observed after exercise in hot water [8], and the reduced cortisol response following exercise in cold water might be favourable for immune function and host defence [14].
Cold exposure causes plasma norepinephrine concentrations (a marker of SNS activity) to increase [6, 7]. Also, the SNS mediates and modulates immune function [31] and thus cold exposure can indirectly affect immune system responses through activation of the SNS. The rise in circulating leukocytes during cold exposure may be attributed to a norepinephrine-mediated demargination of white blood cells [31]. Although the effects of cold exposure on plasma cortisol concentrations are equivocal [13, 40], plasma cortisol can modulate the immune system [31], and the cortisol response pattern to exercise has been considered to be predictive of an individual's adaptation to specific forms of stress [18]. In this study, plasma cortisol concentration decreased after 60 min of rest, but increased following exercise, and then fell after 1 hour into the recovery phase. The results of the present study indicate that lymphocytes and plasma cortisol concentration increase transiently after exercise independent of cold acclimatization. Interestingly, in contrast with the acclimatized group, lymphocyte counts were higher in the non-acclimatized group after exercise in the cold than in thermoneutral conditions, and did not decrease after exercise in cold exposure even during recovery. Therefore, we suggest that acclimatization to exercise in the cold may influence exercise-induced changes of lymphocytes and cortisol concentration.
The increase in total lymphocytes immediately after exercise was primarily due to elevated NK (CD16+) cells, together with an increase in activated T lymphocytes (CD69+). The mild and transient increase of NK cells and activated T lymphocytes after submaximal exercise could be described as a beneficial response to immune perturbation [18]. Shepherd and Shek [33] suggested that severe chilling acutely suppresses several cellular and humoral components of the immune response, including a reduction in NK cell count. However, we did not find evidence for the impairment of immunosurveillance in response to cold stress during exercise. Although we found a decrease in CD16+ cells during recovery, it is difficult to conclude that immunosurveillance was in fact impaired. Brenner et al. [4] suggested that heart rate, rectal temperature, and plasma stress hormones may mediate the changes in total leukocytes, granulocytes, monocytes, and several lymphocyte subsets. In addition, they suggested that the modest rise in circulating leukocyte and neutrophil numbers during cold exposure may be attributed to a norepinephrine-mediated mobilization of demarginated cells, and reported increased NK cell activity during cold exposure after exercise. Furthermore, NK cell activity showed the greatest change after exercise in 18°C water. Contrary to our expectations, we did not find prominent differences in responses of lymphocyte subsets between conditions or groups after exercise. The results of the present study also indicate that lymphocyte subsets increase transiently after exercise independent of cold acclimatization. McFarlin and Mitchell [23] reported similar changes of NK cell activity and leukocytosis through a similar design to our study. Increased mobilization of total lymphocytes, NK cells, and activated T lymphocytes into the circulation immediately after exercise is associated with an increased plasma cortisol response. This relationship demonstrates that there is a carryover effect on these immune cells with stress hormones after submaximal exercise [30].
CONCLUSIONS
In summary, both acclimatized and non-acclimatized groups exhibited lower heart rates in the cold than the thermoneutral conditions during submaximal exercise. An increase in the numbers of total leukocytes, neutrophils, lymphocytes and monocytes during recovery after sub-maximal exercise is a mild and transient immune perturbation. The increase in total leukocytes during recovery was primarily due to an increase in neutrophils. Lymphocytes, lymphocyte subsets, and plasma cortisol concentration increase transiently after exercise independent of cold acclimatization. However, the acclimatized group activated neutrophils after exercise in cold exposure, whereas the non-acclimatized group activated lymphocytes and cortisol after exercise in cold exposure. Acclimatization to exercise in the cold does not appear to influence exercise-induced immune changes in cold conditions, with the possible exception of neutrophils, lymphocytes and cortisol concentration.
Conflict of interest
The authors declared no conflict of interest
REFERENCES
- 1.Beillin B, Shavit Y, Razumovsky J, Wolloch Y, Zeidel A, Bessler H. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology. 1998;89:1133–1140. doi: 10.1097/00000542-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Bittel J.H.M, Nonotte-Varly C, Livecchi-Gonnot G.H, Savourey G.L.M, Hanniquet A.M. Physical fitness and thermoregulatory reactions in a cold environment in men. J. Appl. Physiol. 1988;65:1984–1989. doi: 10.1152/jappl.1988.65.5.1984. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Chanez P, Mercier J, Prefaut C. Monocytes, exercise and the inflammatory response. Exer. Immunol. Rev. 1996;2:35–44. [Google Scholar]
- 4.Brenner I.K.M, Castellani J.W, Gabaree C, Young A.J, Zamecnik J, Shepherd R.J, Shek P.N. Immune changes in humans during cold exposure: effects of prior heating and exercise. J. Appl. Physiol. 1999;87:699–710. doi: 10.1152/jappl.1999.87.2.699. [DOI] [PubMed] [Google Scholar]
- 5.Castellani J.W, Brener I.K.M, Rhind S.G. Cold exposure: human immune responses and intracellular cytokine expression. Med. Sci. Sports Exerc. 2002;34:2013–2020. doi: 10.1097/00005768-200212000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Castellani J.W, Young A.J, Degroot D.W, Stulz D.A, Cadarette B.S, Rhind S.G, Zamecnik J, Shek P.N, Sawka M.N. Thermoregulation during cold exposure after several days of exhaustive exercise. J. Appl. Physiol. 2001;90:939–946. doi: 10.1152/jappl.2001.90.3.939. [DOI] [PubMed] [Google Scholar]
- 7.Castellani J.W, Young A.J, Kain J.E, Rouse A, Sawka M.N. Thermoregulation during cold exposure: effects of prior exercise. J. Appl. Physiol. 1999;87:247–252. doi: 10.1152/jappl.1999.87.1.247. [DOI] [PubMed] [Google Scholar]
- 8.Cross M.C, Radomski M.W, VanHelder W.P, Rhind S.G, Shephard R.J. Endurance exercise with and without a thermal clamp: effects on leukocytes and leukocyte subsets. J. Appl. Physiol. 1996;81:822–829. doi: 10.1152/jappl.1996.81.2.822. [DOI] [PubMed] [Google Scholar]
- 9.Davis J.M, Murphy E.A, Brown A.S, Carmichael M.D, Ghaffar A, Mayer E.P. Effects of moderate exercise and oat beta-glucan on innate immune function and susceptibility to respiratory infection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R366–R372. doi: 10.1152/ajpregu.00304.2003. [DOI] [PubMed] [Google Scholar]
- 10.Davis S.L. Environmental modulation of the immune system via the endocrine system. Domest. Anim. Endocrinol. 1998;15:283–289. doi: 10.1016/s0739-7240(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 11.Dill D.B, Costill D.L. Calculation of percentage changes in volume of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 12.Dugue B, Leppanen E. Adaptation related to cytokines in man: effects of regular swimming in ice-cold water. Clin. Physiol. 2000;20:114–121. doi: 10.1046/j.1365-2281.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 13.Frank S.M, Higgins M.S, Fleisher L.A, Sitzmann J.V, Raff H, Breslow M.J. Adrenergic, respiratory, and cardiovascular effects of core cooling in humans. Am. J. Physiol. 1997;272:R557–R562. doi: 10.1152/ajpregu.1997.272.2.R557. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman-Goetz L, Pedersen B.K. Exercise and the immune system: a model of the stress response? Immunol. Today. 1994;15:382–387. doi: 10.1016/0167-5699(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs I, Romet T, Frim J, Hynes A. Effects of endurance fitness on responses to cold water immersion. Aviat. Space Environ. Med. 1984;55:715–720. [PubMed] [Google Scholar]
- 16.Jansky L, Pospisilva D, Honzova S, Ulicny B, Sramek P, Zeman V, Kaminkova J. Immune system of cold-exposed and cold-adapted humans. Eur. J. Appl. Physiol. 1996;72:445–450. doi: 10.1007/BF00242274. [DOI] [PubMed] [Google Scholar]
- 17.Jett D.M, Adams K.J, Stamford B.A. Cold exposure and exercise metabolism. Sports Med. 2006;36:643–656. doi: 10.2165/00007256-200636080-00002. [DOI] [PubMed] [Google Scholar]
- 18.Kanaley J.A, Hartman M.L. Cortisol and growth hormone responses to exercise. Endocrinologist. 2002;12:421–432. [Google Scholar]
- 19.Keast D, Cameron K, Morton A.R. Exercise and the immune response. Sports Med. 1988;5:248–267. doi: 10.2165/00007256-198805040-00004. [DOI] [PubMed] [Google Scholar]
- 20.Kollias J, Boileau R, Buskirk E.R. Effects of physical conditioning in man on thermal responses to cold air. Int. J. Biometerol. 1972;16:389–402. doi: 10.1007/BF01553623. [DOI] [PubMed] [Google Scholar]
- 21.Kozyreva T.V, Eliseeva L.S. The immune system response to antigen in cold- and warm-adapted rats. J. Thermal. Biol. 2004;29:865–869. [Google Scholar]
- 22.McCarthy D.A, Dale M.M. The leukocytosis of exercise: a review and model. Sports Med. 1988;6:333–363. doi: 10.2165/00007256-198806060-00002. [DOI] [PubMed] [Google Scholar]
- 23.McFarlin B.K, Mitchell J.B. Exercise in hot and cold environments: differential effects on leukocyte number and NK cell activity. Aviat. Space Environ. Med. 2003;74:1231–1236. [PubMed] [Google Scholar]
- 24.Mooren F.C, Bloming D, Lechtermann A, Lerch M.M, Volker K. Lymphocyte apoptosis after exhaustive and moderate exercise. J. Appl. Physiol. 2002;93:147–153. doi: 10.1152/japplphysiol.01262.2001. [DOI] [PubMed] [Google Scholar]
- 25.Muza S.R, Young A.J, Bogart J.E, Pandolf K.B. Respiratory and cardiovascular responses to cold stress following repeated cold water immersion. Undersea Biomed. Res. 1988;15:165–178. [PubMed] [Google Scholar]
- 26.Nieman D.C, Henson D.A, Austin M.D, Brown V.A. The immune response to a 30-minute walk. Med. Sci. Sports Exerc. 2005;37:57–62. doi: 10.1249/01.mss.0000149808.38194.21. [DOI] [PubMed] [Google Scholar]
- 27.Parkin J.M, Carey M.F, Zhao S, Febbraio M.A. Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J. Appl. Physiol. 1999;86:902–908. doi: 10.1152/jappl.1999.86.3.902. [DOI] [PubMed] [Google Scholar]
- 28.Petersen A.M, Pedersen B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 29.Rhind S.G, Castellani J.W, Brenner I.K.M, Shepherd R.J, Zamecnik J, Montain S.J, Young A.J, Shek P.N. Intracellular monocyte and serum cytokine expression is modulated by exhausting exercise and cold exposure. Am. J. Physiol. 2001;281:R66–R75. doi: 10.1152/ajpregu.2001.281.1.R66. [DOI] [PubMed] [Google Scholar]
- 30.Ronsen O, Pedersen B.K, Oritsland T.R, Bahr R, Kjeldsen-Kragh J. Leukocyte counts and lymphocyte responsiveness associated with repeated bouts of strenuous endurance exercise. J. Appl. Physiol. 2001;91:425–434. doi: 10.1152/jappl.2001.91.1.425. [DOI] [PubMed] [Google Scholar]
- 31.Shephard R.J. Physical activity, training and the immune response; Carmel: In Cooper Publishing Group; 1997. pp. 8–9. [Google Scholar]
- 32.Shepherd R.J. Immune changes induced by exercise in an adverse environment. Can. J. Physiol. Pharmacol. 1998;76:539–546. doi: 10.1139/cjpp-76-5-539. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd R.J, Shek P.N. Cold exposure and Immune function. Can. J. Physiol. Pharmacol. 1998;76:828–836. doi: 10.1139/cjpp-76-9-828. [DOI] [PubMed] [Google Scholar]
- 34.Stainsby W.N, Brooks G.A. Control of lactic acid metabolism in contracting muscles during exercise. Exer. Spt. Sci. Rev. 1990;18:29–64. [PubMed] [Google Scholar]
- 35.Stocks J.M, Taylor N.A.S, Tipton M.J, Greenleaf J.E. Human physiological responses to cold exposure. Aviat. Space Environ. Med. 2004;75:444–457. [PubMed] [Google Scholar]
- 36.Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q, Sugawara K, Yamaya K, Sato K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999;87:1360–1367. doi: 10.1152/jappl.1999.87.4.1360. [DOI] [PubMed] [Google Scholar]
- 37.Thuma J.R, Gilders R, Verdun M, Loucks A.B. Circadian rhythm of cortisol confounds cortisol response to exercise: implications for future research. J. Appl. Physiol. 1995;78:1657–1664. doi: 10.1152/jappl.1995.78.5.1657. [DOI] [PubMed] [Google Scholar]
- 38.Tipton M.J, Franks E.M, Mereilly G.S, Mekjavic L.B. Substrate utilization during exercise and shivering. Eur. J. Appl. Physiol. 1997;76:103–108. doi: 10.1007/s004210050220. [DOI] [PubMed] [Google Scholar]
- 39.Wilkerson J.E, Raven P.B, Bolduan N.W, Horvath S.M. Adaptations in man's adrenal function in response to acute cold stress. J. Appl. Physiol. 1974;36:183–189. doi: 10.1152/jappl.1974.36.2.183. [DOI] [PubMed] [Google Scholar]
- 40.Wittert G.A, Or H.K, Livesey J.H, Richards A.M, Donald R.A, Espiner E.A. Vasopressin, corticotrophin-releasing factor, and pituitary adrenal responses to acute cold stress in normal humans. J. Clin. Endocrinol. Metab. 1992;75:750–755. doi: 10.1210/jcem.75.3.1517364. [DOI] [PubMed] [Google Scholar]
- 41.Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol. Pharm. Bull. 2003;26:753–760. doi: 10.1248/bpb.26.753. [DOI] [PubMed] [Google Scholar]