Abstract
States of under-nutrition are characterized by growth hormone resistance. Decreased total energy intake, as well as isolated protein-calorie malnutrition and isolated nutrient deficiencies result in elevated growth hormone levels and low levels of IGF-I. We review various states of malnutrition and a disease state characterized by chronic under-nutrition -- anorexia nervosa -- and discuss possible mechanisms contributing to the state of growth hormone resistance, including FGF-21 and SIRT1. We conclude by examining the hypothesis that growth hormone resistance is an adaptive response to states of under-nutrition, in order to maintain euglycemia and preserve energy.
Introduction
Insulin-like growth factor (IGF)-I is a hormone produced primarily by the liver and regulated by growth hormone (GH) secretion by the somatotroph cells of the anterior pituitary gland. Many, but not all, of the actions of GH are mediated by IGF-I. In addition to regulation by GH, IGF-I secretion is also responsive to nutritional cues. In obesity, a state of over-nutrition, GH secretion is decreased and may result in IGF-I levels which are lower than those in normal weight individuals (Maccario, et al. 1997), although bioactive IGF-I levels may be comparable to those of lean controls (Frystyk, et al. 2009). In states of under-nutrition, GH levels are normal or elevated in the setting of low IGF-I levels; therefore there is a state of GH resistance, with an inappropriate response to GH at the level of the liver. This state of acquired GH resistance is likely an adaptive response to decreased energy intake. A number of potential mechanisms of GH resistance in malnutrition have been elucidated, including possible hormonal determinants. This review will examine the current evidence of determinants of GH resistance in states of malnutrition (Figure 1).
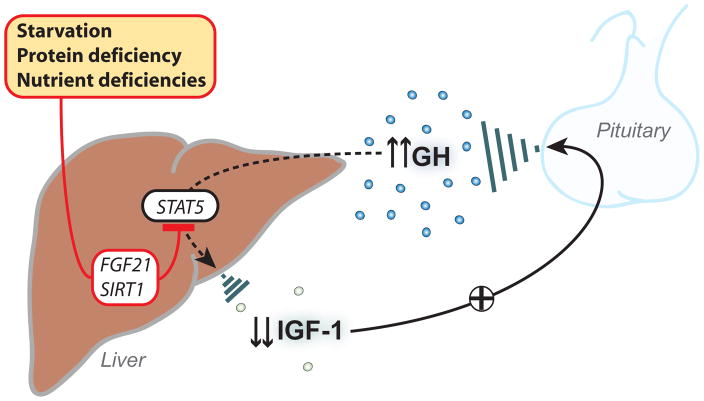
Figure 1. Potential causes and mediators of growth hormone (GH) resistance.
Starvation, protein-calorie malnutrition and isolated vitamin deficiencies can all lead to a state of GH resistance, with normal or elevated levels of GH coincident with low levels of insulin-like growth factor I (IGF-I). Recent evidence suggests that the state of GH resistance in starvation may result from a decrease in signal transducer and activator of transcription (STAT)5 phosphorylation, mediated by fibroblast growth factor-21 (FGF-21) and/or Sirtuin1 (SIRT1).
States of malnutrition
A number of models of malnutrition have been studied to better understand the state of GH resistance in under-nutrition. These models include total energy deficiency, isolated protein deficiency (kwashiorkor) -- a state in which individuals consume an appropriate number of calories but have profoundly low levels of protein intake -- and anorexia nervosa, a psychiatric disease which is characterized by low body weight and self-induced starvation. There are a number of other types of malnutrition as well, such as isolated nutrient deficiencies, which also may result in GH resistance. These various states of malnutrition will be discussed in detail below.
Starvation
Malnutrition may be due to isolated energy deficiencies -- such as protein-calorie deficiency -- or total energy deficiency, the extreme case of which is starvation. States of total energy deficiency result in a GH resistance state. IGF-I levels decrease by approximately 50% after only four days of fasting (Grinspoon, et al. 1995). Fasting individuals have elevated GH levels coupled with the low IGF-I levels (Fichter, et al. 1986; Ho, et al. 1988) and also may have hyper-responsive GH secretion after thyrotropin releasing hormone stimulation (Shimizu, et al. 1991). The fact that endogenous hyper-secretion of GH does not result in elevated IGF-I levels suggests that fasting either decreases GH receptors in the liver, or there is a post-receptor defect resulting in an inability of GH to stimulate IGF-I production. In animal models of starvation, low IGF-I levels are coupled with decreased GH receptor mRNA (Straus and Takemoto 1990) and decreased GH binding (Baxter, et al. 1981), suggesting that GH receptor down-regulation in the liver contributes to the state of GH resistance.
Exogenous GH is also unable to stimulate IGF-I production during fasting. The effects of exogenous GH in the fed versus the fasted state were investigated in subjects with isolated GH deficiency (Merimee, et al. 1982). Subjects were treated with 5mg of GH twice per day for five days, either while consuming an ad lib diet or during a five day fast (Merimee et al. 1982). In response to GH, IGF-I levels increased 10-fold in the fed state but only doubled in the fasted state, demonstrating resistance to exogenous GH during fasting (Merimee et al. 1982). Interestingly, in a study of acromegalics undergoing a 36hr fast, serum IGF-I levels did not decrease in the acromegalics whereas levels decreased in the fasted control patients (Grottoli, et al. 2008). Therefore, it is likely that adaptations to fasting are abnormal in individuals with baseline GH hyper-secretion, although the effects of a longer-term fast in individuals with acromegaly are unknown.
Protein deficiency
Isolated protein deficiency, with otherwise normal energy intake, also results in a state of GH resistance. Studies in children with protein-calorie malnutrition demonstrate elevated GH levels and low IGF-I levels with normalization after nutritional recovery (Hintz, et al. 1978; Olusi, et al. 1977; Robinson and Picou 1977; Soliman, et al. 1986). In adults who were fasted and then re-fed a protein-deficient diet, IGF-I levels increased significantly less than in adults fed an iso-caloric, protein-sufficient diet (Isley, et al. 1983).
Importantly, in animal models with low IGF-I levels due to protein-deficiency, pharmacological doses of GH do not increase IGF-I levels to the normal range or normalize growth (Thissen, et al. 1991a; Thissen, et al. 1990), but increasing dietary protein does increase IGF-I levels. In animal models, varying the protein content of food did not affect plasma GH levels, whereas IGF-I levels increased significantly with increasing protein content (Reeves, et al. 1979). Similarly, in a population of elderly individuals, six months of protein supplementation increased IGF-I levels by 85% (Schurch, et al. 1998) and in individuals with type 2 diabetes mellitus, IGF-I levels increased by 30% in those fed a diet consisting of 30% protein for five weeks as compared to those fed a diet consisting of 15% protein (Gannon and Nuttall 2011).
Unlike in starvation, in which a decrease in GH receptors is likely contributing to the GH resistant state, in protein deficiency, the GH resistance state is likely due to a post-receptor defect. In animal models of protein deficiency, GH binding is not reduced (Maes, et al. 1988; Thissen et al. 1990) and IGF-I mRNA production in response to exogenous GH is similar in hypophysectomized rats on a protein-deficient versus protein-sufficient diet (Thissen et al. 1991a). Protein deficiency not only results in GH resistance, but also likely results in a state of end-organ resistance to IGF-I. Rats with low IGF-I levels due to protein restriction and a control population of rats with low IGF-I levels due to hypophysectomy were treated with recombinant human IGF-I at a dose of 300 micrograms/day (Thissen, et al. 1991b). Serum IGF-I levels normalized in both groups but increases in body weight, tibial epiphyseal widening and tail growth only occurred in the hypophysectomized rats, not the protein-restricted rats (Thissen et al. 1991b). Therefore in addition to a state of GH resistance, there may also be a state of IGF-I resistance in protein-restriction.
Anorexia nervosa
Anorexia nervosa is a psychiatric disorder, predominantly affecting women, with a lifetime prevalence of 2.2% (Keski-Rahkonen, et al. 2007). The disease is characterized by self-imposed starvation and an inability to maintain a normal weight for height (Association 1994). Individuals with anorexia nervosa are in a state of chronic starvation. Like individuals with total energy deficiency and isolated protein deficiency, individuals with anorexia nervosa are GH resistant. In girls and women with anorexia nervosa, basal and pulsatile GH secretion is increased and IGF-I levels are low (Garfinkel, et al. 1975; Misra, et al. 2003; Scacchi, et al. 1997; Stoving, et al. 1999). Systemic IGF-I levels in anorexia nervosa are approximately 50% of those of normal weight women and levels of IGF binding protein-3, the predominant binding protein for IGF-I, are low (Counts, et al. 1992). Yet bioactive IGF-I levels are also low (Stoving, et al. 2007), suggesting that the low IGF-I levels in anorexia nervosa are not simply due to decreased levels of binding proteins but due to decreased bioactive IGF-I. The low IGF-I levels in women with anorexia nervosa are exquisitely sensitive to nutritional repletion--- after only three days of hyper-alimentation therapy, levels have been shown to increase by approximately 50% (Hotta, et al. 2000).
Individuals with anorexia nervosa also have an exaggerated response to GH releasing hormone (GHRH) (Rolla, et al. 1990) and abnormal GH suppressibility after a glucose load (Tamai, et al. 1991). In healthy individuals, pre-treatment with a cholinergic muscarinic antagonist blocks GH secretion in response to GH releasing factor (Massara, et al. 1984). The mechanism by which this occurs may be an increase in somatostatin release by the hypothalamus – a potent inhibitor of GH secretion. In contrast, in girls with anorexia nervosa, pretreatment with a cholinergic muscarinic antagonist does not block the exaggerated GH response to GHRH (Rolla et al. 1990; Tamai, et al. 1990), suggesting that there may be an abnormality in the release of or response to somatostatin.
We investigated whether supra-physiologic doses of GH can overcome the state of GH resistance in women with anorexia nervosa (Fazeli, et al. 2010a). Subjects (N=21) were randomized to either placebo or recombinant human GH treatment (mean maximum daily dose: 1.4 mg/day) for 12 weeks (Fazeli et al. 2010a). At the conclusion of the study, IGF-I levels did not differ between the groups, demonstrating that even very high levels of exogenous GH cannot overcome the insensitivity to GH, at the level of the liver, in anorexia nervosa (Fazeli et al. 2010a). One purported mechanism for the GH resistance state in anorexia nervosa is the down regulation of GH receptors in the liver (Counts et al. 1992). The failure of supra-physiologic doses of GH to increase IGF-I levels supports this hypothesis.
Isolated nutrient deficiencies
Isolated vitamin deficiencies may also cause a state of GH resistance. Vitamin A deficient rats have lower IGF-I levels as compared to pair-fed controls despite comparable GH levels (Mohan and Jaya Rao 1980). As compared to similar weight controls, children with vitamin A deficiency have normal GH levels coincident with lower IGF-I levels (Mohan and Jaya Rao 1979). Similarly, rats fed a diet deficient in vitamin B6 for four weeks were found to have low IGF-I levels and normal GH levels (Rao and Mohan 1982).
In a rodent model, both zinc deficiency and magnesium deficiency are associated with decreased IGF-I levels in the setting of normal GH levels (Dorup, et al. 1991). Young rats fed a magnesium-deficient diet were found to have significantly lower serum IGF-I levels as compared to pair-fed controls, whereas basal GH levels and GH levels after stimulation with GH releasing factor were similar in the magnesium-deficient group as compared to an ad lib fed group (Dorup et al. 1991). Similarly, young rats fed a zinc-deficient diet were also found to have significantly lower serum IGF-I levels as compared to pair-fed controls but levels of GH were similar in the zinc-deficient rats and the ad lib fed zinc-sufficient rats after treatment with GH releasing factor, suggesting a state of GH resistance (Dorup et al. 1991). In postmenopausal women, zinc intake is positively associated with IGF-I levels, even after adjustment for possible confounders, but because GH levels were not measured in this study, it is not known if GH resistance contributes to the lower IGF-I levels (Devine, et al. 1998).
Potassium-deficient diets have also been studied in an animal model (Flyvbjerg, et al. 1991). Rats consuming a potassium-deficient fodder had significantly lower serum IGF-I levels compared to pair-fed controls, but in this case the response to GH releasing factor stimulation was significantly less in the potassium-deficient group as compared to the controls (Flyvbjerg et al. 1991). Therefore, the low IGF-I levels in potassium-deficient rodents are not due to GH resistance but likely due to down-regulation of an appropriately functioning GH-IGF-I axis.
Effects of dietary composition
In in vitro studies, saturated fatty acids, but not unsaturated fatty acids, inhibit promoter activity at the GH receptor gene and thereby decrease GH receptor mRNA and protein levels (Thimmarayappa, et al. 2006), suggesting that saturated fatty acids may contribute to GH resistance. In a large cohort of healthy women (n=1000), a cross-sectional examination of plasma IGF-I levels and various dietary components demonstrated a positive association between IGF-I levels and total energy intake (Holmes, et al. 2002). There was also a positive association between IGF-I levels and increasing levels of protein intake (Holmes et al. 2002). Smaller human studies (n=115) have also investigated the cross-sectional relationship between IGF-I and dietary composition (Kaklamani, et al. 1999). Positive associations between IGF-I and fats, red meat and oil consumption have been reported as well as an inverse association between IGF-I levels and carbohydrate intake (Kaklamani et al. 1999). Importantly, GH levels were not measured in these studies and therefore we do not know if these differences in IGF-I levels are GH mediated or due to GH insensitivity.
Mechanisms of GH resistance
GH is secreted by somatotroph cells in the anterior pituitary gland and binds to GH receptors in many tissues, including hepatocytes. The binding of GH to its receptor activates Janus kinase (JAK)2 which leads to phosphorylation of signal transducer and activator of transcription (STAT)5. STAT5 is subsequently translocated into the nucleus where it is able to bind to regulatory elements of target genes including IGF-I. Recently, two proteins have been shown to be important regulators of GH resistance in states of nutritional deprivation: fibroblast growth factor (FGF)-21 and Sirtuin (SIRT)1. FGF-21 and SIRT1 both induce GH resistance via STAT5 inhibition.
FGF-21
FGF-21, a member of the fibroblast growth factor family of proteins, is a hormone produced in the liver (Nishimura, et al. 2000) and adipocytes (Zhang, et al. 2008). Serum FGF-21 levels correlate with body mass index and also with FGF-21 mRNA expression in subcutaneous fat (Zhang et al. 2008), suggesting that serum FGF-21 levels primarily originate in adipose tissue. FGF-21 also stimulates insulin-independent glucose uptake in adipocytes via the glucose transporter-1 (Kharitonenkov, et al. 2005) and is currently being investigated as a therapeutic agent in individuals with type 2 diabetes mellitus (Gaich, et al. 2013). In animal models, starvation induces FGF-21 production in the liver via a mechanism requiring peroxisome proliferator-activated receptor (PPAR)-α (Inagaki, et al. 2007; Lundasen, et al. 2007). FGF-21 subsequently induces peroxisome proliferator-activated receptor γ coactivator protein (PGC)-1α expression, leading to fatty acid oxidation and increased gluconeogenesis (Potthoff, et al. 2009). In humans, a very low calorie diet and prolonged fasting result in increased levels of FGF-21 (Galman, et al. 2008; Mraz, et al. 2009). Therefore, FGF-21 may act differently in states of nutritional sufficiency as compared to states of nutritional deprivation.
In a transgenic animal model, FGF-21 transgenic mice have high levels of GH and significantly lower levels of IGF-I compared to wild-type mice (Inagaki, et al. 2008), consistent with GH resistance. The mechanism of GH resistance in FGF-21 transgenic mice is a decrease in STAT5 phosphorylation (Inagaki et al. 2008). Similarly, we have shown that FGF-21 levels are significantly higher in adolescent girls with anorexia nervosa as compared to normal-weight girls after controlling for percent body fat and insulin resistance, suggesting that the increased levels of FGF-21 in anorexia nervosa are due to increased levels of liver-derived FGF-21 (Fazeli, et al. 2010b). We have also demonstrated a significant positive relationship between GH area under the curve and FGF-21 and an inverse relationship between IGF-I and elevated levels of FGF-21, after controlling for percent body fat and insulin resistance (Fazeli et al. 2010b). These data suggest that FGF-21 may be a mediator of GH resistance in the human model of chronic starvation -- anorexia nervosa.
SIRT1
Sirtuin 1 (SIRT1), a class III histone deacetylase is regulated by nutrient availability and promotes gluconeogenesis and fatty acid oxidation during periods of fasting (Gillum, et al. 2010). Recently SIRT1 was shown to play a role in GH resistance in states of starvation (Yamamoto, et al. 2013). SIRT1 knock-down mice that underwent a 48 hour fast had higher serum levels of IGF-I and higher IGF-I mRNA levels in the liver when compared to wild-type fasted mice, suggesting that SIRT1 mediates GH resistance in states of under-nutrition (Yamamoto et al. 2013). Like FGF-21, the mechanism by which SIRT1 acts is a reduction in STAT5 phosphorylation; this decrease in STAT5 phosphorylation is a result of SIRT1 deacetylating lysine residues on STAT5 (Yamamoto et al. 2013).
Insulin
Type 1 diabetes mellitus is also a state of GH resistance (Horner, et al. 1981; Lundbaek, et al. 1970; Yde 1969). Therefore insulin has also been investigated as a possible determinant of GH resistance. GH receptors in the liver are reduced in diabetic female rats and this decrease correlates with the decreased serum insulin levels (Baxter and Turtle 1978). GH receptors also increase in response to treatment with insulin (Baxter and Turtle 1978) and n vitro models demonstrate up-regulation of GH receptors by insulin (Leung, et al. 2000). Therefore insulin levels, which are low in states of nutritional deprivation such as fasting, may in part mediate GH resistance by down-regulation of GH receptors in the liver. Although, this is unlikely to be the only mechanism by which GH resistance is induced in states of under-nutrition, as studies in diabetic rats have demonstrated independent effects of insulin and a low-protein diet (Maiter, et al. 1989).
IGF-I
The low IGF-I levels in the GH resistance state -- and the resultant decrease in negative feedback at the level of the pituitary -- contribute to the elevated GH levels characteristic of GH resistance. In a study of healthy, fasting men, a recombinant human IGF-I infusion resulted in a significant decrease in serum GH levels (Hartman, et al. 1993). Similarly, in a study of women with anorexia nervosa, administration of recombinant human IGF-I (20mcg/kg) significantly reduced mean basal GH concentrations, although levels remained higher than those in normal weight controls (Gianotti, et al. 2000).
Other hormonal factors
Triiodothyronine
Triiodothyronine (T3) may play a role in the GH resistance state induced by fasting (Ikeda, et al. 1990). Plasma IGF-I and T3 levels are both low in fasted rats (Ikeda et al. 1990). Subcutaneous injections of T3 (5mcg/kg) normalized T3 levels in fasted rats and plasma IGF-I levels also increased significantly, suggesting that T3 may play a role in mediating GH resistance in states of under-nutrition (Ikeda et al. 1990).
Leptin
Levels of leptin, an anorexigenic hormone secreted by adipocytes, are low in states of under-nutrition (Frederich, et al. 1995) and these low levels may mediate the GH resistance state. In sheep, an animal model which also demonstrates GH resistance in response to fasting, ovariectomized ewes which were fasted for 72 hours and infused with leptin centrally had levels of GH which were lower than those of the vehicle-treated, fasted, ovariectomized ewes (Henry, et al. 2004). GH levels in the leptin-treated ewes were comparable to levels in ad lib fed ewes (Henry et al. 2004). Therefore, central leptin may reduce GH secretion and the low levels of leptin during starvation may result in an increase in GH levels, contributing to the GH resistance state.
In humans, children with protein-energy malnutrition have significantly lower levels of leptin and IGF-I as compared to healthy controls and significantly higher levels of GH. Leptin levels are also positively associated with IGF-I and inversely associated with GH (Kilic, et al. 2004; Soliman, et al. 2000). When women with hypothalamic amenorrhea, a state of chronic nutritional deprivation, were treated with recombinant human methionyl leptin for three months -- resulting in a normalization of leptin levels -- IGF-I levels increased to levels comparable to euleptinemic controls, even after controlling for estradiol levels and changes in weight (Chan, et al. 2008). Therefore low leptin levels may be a mediator of GH resistance in states of nutritional deprivation.
Testosterone
Testosterone has also been shown to be a significant predictor of IGF-I levels in women with anorexia nervosa (Brick, et al. 2010). In a group of women with anorexia nervosa and normal weight controls, free testosterone was found to be a significant and independent predictor of IGF-I levels and was able to account for 36% of the variability in IGF-I levels after controlling for other possible hormonal predictors including estradiol (Brick et al. 2010). Therefore, the low testosterone levels in anorexia nervosa (Miller, et al. 2007) and other states of starvation (Klibanski, et al. 1981) may contribute to the state of GH resistance.
Growth hormone resistance is an adaptive response to under-nutrition
In states of under-nutrition, GH resistance may be an adaptive response. Elevated GH levels may be necessary to maintain euglycemia, whereas decreased levels of IGF-I help conserve energy during periods of nutritional deprivation.
Mobilization of fat stores
Elevated GH levels may be important for mobilization of fat stores in states of malnutrition. Mice with adult-onset, isolated GH-deficiency and their GH-replete littermates were calorie restricted for 11 days (Gahete, et al. 2013); GH levels increased in the GH-replete mice but not in the GH-deficient mice in response to the calorie deprivation (Gahete et al. 2013). Importantly, the loss of fat mass and subsequent increase in free fatty acid levels were significantly greater in the GH-replete mice as compared to the GH-deficient mice (Gahete et al. 2013), suggesting that mobilization of fat stores during starvation is a significant, and likely adaptive, consequence of the elevated GH levels. In humans, GH has also been shown to be a critical factor in increasing the rate of lipolysis during fasting (Sakharova, et al. 2008).
Maintenance of euglycemia
Elevated GH levels may also be important for maintaining euglycemia in states of starvation. Ghrelin is an orexigenic hormone produced primarily in the fundus of the stomach. Importantly, there are ghrelin receptors -- called GH secretagogue receptors -- in the anterior pituitary gland and octanoylated ghrelin acts as a potent stimulator of GH secretion (Kojima and Kangawa 2005). In an animal model, elimination of ghrelin O-acyltransferase (GOAT) -- which attaches octanoate to proghrelin -- results in mice which lack octanoylated ghrelin or acylated ghrelin (Zhao, et al. 2010). GOAT knock-out mice on normal or high-fat diets grow and gain weight normally but when calorie-restricted, the GOAT knock-out mice become profoundly hypoglycemic in contrast to the wild-type, calorie-restricted mice (Zhao et al. 2010). GH levels were also found to be two-fold higher in the wild-type mice as compared to the GOAT knock-out mice and infusion of GH was able to rescue the GOAT knock-out mice from hypoglycemia and normalize blood sugars, suggesting that an important role of elevated GH levels in states of malnutrition is to maintain euglycemia (Zhao et al. 2010). Although there is recent controversy regarding the role of ghrelin and GH in protecting against hypoglycemia during periods of calorie restriction (Gahete et al. 2013; Yi, et al. 2012), it is clear that the increasing levels of GH serve to mobilize energy during periods of malnutrition and are an adaptive response to under-nutrition.
Decreased energy expenditure
Elevated GH levels are potentially an important means of mobilizing fat stores and maintaining euglycemia in states of under-nutrition. Yet if the GH-IGF-I axis remained intact during states of nutritional deprivation, the elevated levels of GH would result in elevated IGF-I levels, leading to increased energy expenditure on growth and therefore incur a survival disadvantage in states of malnutrition. Therefore GH resistance, with an inability of GH to appropriately stimulate IGF-I production, is likely an adaptive mechanism to preserve energy during periods of under-nutrition.
Conclusions
Our evolutionary past was marked by periods of under-nutrition. These periods of under-nutrition were characterized by isolated protein-calorie deficiency, isolated vitamin deficiencies and even starvation and survival depended on the body’s ability to mobilize energy stores and prevent hypoglycemia. GH plays a key role in mobilizing energy and therefore elevated GH levels confer a survival advantage during periods of under-nutrition. Yet increasing energy expenditure for growth during these periods -- which would result if the GH-IGF-I axis remained intact and IGF-I levels increased -- would be disadvantageous in states of under-nutrition. Therefore, the elevated GH levels and low IGF-I levels characteristic of GH resistance are an important adaptive response to calorie and nutrient deprivation and an important mechanism for survival.
Acknowledgments
Sources of support: K23 DK094820 (Fazeli), R01 DK052625 (Klibanski)
Footnotes
Disclosures: AK receives support for an investigator initiated study from Rhythm Pharmaceuticals.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV) 4. Washington, DC: 1994. [Google Scholar]
- Baxter RC, Bryson JM, Turtle JR. The effect of fasting on liver receptors for prolactin and growth hormone. Metabolism. 1981;30:1086–1090. doi: 10.1016/0026-0495(81)90052-4. [DOI] [PubMed] [Google Scholar]
- Baxter RC, Turtle JR. Regulation of hepatic growth hormone receptors by insulin. Biochem Biophys Res Commun. 1978;84:350–357. doi: 10.1016/0006-291x(78)90177-8. [DOI] [PubMed] [Google Scholar]
- Brick DJ, Gerweck AV, Meenaghan E, Lawson EA, Misra M, Fazeli P, Johnson W, Klibanski A, Miller KK. Determinants of IGF1 and GH across the weight spectrum: from anorexia nervosa to obesity. Eur J Endocrinol. 2010;163:185–191. doi: 10.1530/EJE-10-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JL, Williams CJ, Raciti P, Blakeman J, Kelesidis T, Kelesidis I, Johnson ML, Thorner MO, Mantzoros CS. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases IGF-I in leptin deficiency states. J Clin Endocrinol Metab. 2008;93:2819–2827. doi: 10.1210/jc.2008-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB., Jr The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75:762–767. doi: 10.1210/jcem.75.3.1381372. [DOI] [PubMed] [Google Scholar]
- Devine A, Rosen C, Mohan S, Baylink D, Prince RL. Effects of zinc and other nutritional factors on insulin-like growth factor I and insulin-like growth factor binding proteins in postmenopausal women. Am J Clin Nutr. 1998;68:200–206. doi: 10.1093/ajcn/68.1.200. [DOI] [PubMed] [Google Scholar]
- Dorup I, Flyvbjerg A, Everts ME, Clausen T. Role of insulin-like growth factor-1 and growth hormone in growth inhibition induced by magnesium and zinc deficiencies. Br J Nutr. 1991;66:505–521. doi: 10.1079/bjn19910051. [DOI] [PubMed] [Google Scholar]
- Fazeli PK, Lawson EA, Prabhakaran R, Miller KK, Donoho DA, Clemmons DR, Herzog DB, Misra M, Klibanski A. Effects of recombinant human growth hormone in anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2010a;95:4889–4897. doi: 10.1210/jc.2010-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PK, Misra M, Goldstein M, Miller KK, Klibanski A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J Clin Endocrinol Metab. 2010b;95:369–374. doi: 10.1210/jc.2009-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichter MM, Pirke KM, Holsboer F. Weight loss causes neuroendocrine disturbances: experimental study in healthy starving subjects. Psychiatry Res. 1986;17:61–72. doi: 10.1016/0165-1781(86)90042-9. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A, Dorup I, Everts ME, Orskov H. Evidence that potassium deficiency induces growth retardation through reduced circulating levels of growth hormone and insulin-like growth factor I. Metabolism. 1991;40:769–775. doi: 10.1016/0026-0495(91)90001-d. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96:1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frystyk J, Brick DJ, Gerweck AV, Utz AL, Miller KK. Bioactive insulin-like growth factor-I in obesity. J Clin Endocrinol Metab. 2009;94:3093–3097. doi: 10.1210/jc.2009-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahete MD, Cordoba-Chacon J, Luque RM, Kineman RD. The rise in growth hormone during starvation does not serve to maintain glucose levels or lean mass but is required for appropriate adipose tissue response in female mice. Endocrinology. 2013;154:263–269. doi: 10.1210/en.2012-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The Effects of LY2405319, an FGF21 Analog, in Obese Human Subjects with Type 2 Diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Gannon MC, Nuttall FQ. Effect of a high-protein diet on ghrelin, growth hormone, and insulin-like growth factor-I and binding proteins 1 and 3 in subjects with type 2 diabetes mellitus. Metabolism. 2011;60:1300–1311. doi: 10.1016/j.metabol.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Garfinkel PE, Brown GM, Stancer HC, Moldofsky H. Hypothalamic-pituitary function in anorexia nervosa. Arch Gen Psychiatry. 1975;32:739–744. doi: 10.1001/archpsyc.1975.01760240067005. [DOI] [PubMed] [Google Scholar]
- Gianotti L, Pincelli AI, Scacchi M, Rolla M, Bellitti D, Arvat E, Lanfranco F, Torsello A, Ghigo E, Cavagnini F, et al. Effects of recombinant human insulin-like growth factor I administration on spontaneous and growth hormone (GH)-releasing hormone-stimulated GH secretion in anorexia nervosa. J Clin Endocrinol Metab. 2000;85:2805–2809. doi: 10.1210/jcem.85.8.6743. [DOI] [PubMed] [Google Scholar]
- Gillum MP, Erion DM, Shulman GI. Sirtuin-1 regulation of mammalian metabolism. Trends Mol Med. 2010 doi: 10.1016/j.molmed.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon SK, Baum HB, Peterson S, Klibanski A. Effects of rhIGF-I administration on bone turnover during short-term fasting. J Clin Invest. 1995;96:900–906. doi: 10.1172/JCI118137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottoli S, Gasco V, Mainolfi A, Beccuti G, Corneli G, Aimaretti G, Dieguez C, Casanueva F, Ghigo E. Growth hormone/insulin-like growth factor I axis, glucose metabolism, and lypolisis but not leptin show some degree of refractoriness to short-term fasting in acromegaly. J Endocrinol Invest. 2008;31:1103–1109. doi: 10.1007/BF03345660. [DOI] [PubMed] [Google Scholar]
- Hartman ML, Clayton PE, Johnson ML, Celniker A, Perlman AJ, Alberti KG, Thorner MO. A low dose euglycemic infusion of recombinant human insulin-like growth factor I rapidly suppresses fasting-enhanced pulsatile growth hormone secretion in humans. J Clin Invest. 1993;91:2453–2462. doi: 10.1172/JCI116480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BA, Goding JW, Tilbrook AJ, Dunshea FR, Blache D, Clarke IJ. Leptin-mediated effects of undernutrition or fasting on luteinizing hormone and growth hormone secretion in ovariectomized ewes depend on the duration of metabolic perturbation. J Neuroendocrinol. 2004;16:244–255. doi: 10.1111/j.0953-8194.2004.01157.x. [DOI] [PubMed] [Google Scholar]
- Hintz RL, Suskind R, Amatayakul K, Thanangkul O, Olson R. Plasma somatomedin and growth hormone values in children with protein-calorie malnutrition. J Pediatr. 1978;92:153–156. doi: 10.1016/s0022-3476(78)80099-7. [DOI] [PubMed] [Google Scholar]
- Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, Thorner MO. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest. 1988;81:968–975. doi: 10.1172/JCI113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:852–861. [PubMed] [Google Scholar]
- Horner JM, Kemp SF, Hintz RL. Growth hormone and somatomedin in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1981;53:1148–1153. doi: 10.1210/jcem-53-6-1148. [DOI] [PubMed] [Google Scholar]
- Hotta M, Fukuda I, Sato K, Hizuka N, Shibasaki T, Takano K. The relationship between bone turnover and body weight, serum insulin-like growth factor (IGF) I, and serum IGF-binding protein levels in patients with anorexia nervosa. J Clin Endocrinol Metab. 2000;85:200–206. doi: 10.1210/jcem.85.1.6321. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Fujiyama K, Hoshino T, Takeuchi T, Mashiba H, Tominaga M. Possible role of thyroid hormone in decreased somatomedin-C levels in diabetic and starved rats. Ann Nutr Metab. 1990;34:8–12. doi: 10.1159/000177564. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isley WL, Underwood LE, Clemmons DR. Dietary components that regulate serum somatomedin-C concentrations in humans. J Clin Invest. 1983;71:175–182. doi: 10.1172/JCI110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaklamani VG, Linos A, Kaklamani E, Markaki I, Koumantaki Y, Mantzoros CS. Dietary fat and carbohydrates are independently associated with circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 concentrations in healthy adults. J Clin Oncol. 1999;17:3291–3298. doi: 10.1200/JCO.1999.17.10.3291. [DOI] [PubMed] [Google Scholar]
- Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, Bulik CM, Kaprio J, Rissanen A. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry. 2007;164:1259–1265. doi: 10.1176/appi.ajp.2007.06081388. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic M, Taskin E, Ustundag B, Aygun AD. The evaluation of serum leptin level and other hormonal parameters in children with severe malnutrition. Clin Biochem. 2004;37:382–387. doi: 10.1016/j.clinbiochem.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Klibanski A, Beitins IZ, Badger T, Little R, McArthur JW. Reproductive function during fasting in men. J Clin Endocrinol Metab. 1981;53:258–263. doi: 10.1210/jcem-53-2-258. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000;85:4712–4720. doi: 10.1210/jcem.85.12.7017. [DOI] [PubMed] [Google Scholar]
- Lundasen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- Lundbaek K, Christensen NJ, Jensen VA, Johansen K, Olsen TS, Hansen AP, Orskov H, Osterby R. Diabetes, diabetic angiopathy, and growth hormone. Lancet. 1970;2:131–133. doi: 10.1016/s0140-6736(70)92706-6. [DOI] [PubMed] [Google Scholar]
- Maccario M, Valetto MR, Savio P, Aimaretti G, Baffoni C, Procopio M, Grottoli S, Oleandri SE, Arvat E, Ghigo E. Maximal secretory capacity of somatotrope cells in obesity: comparison with GH deficiency. Int J Obes Relat Metab Disord. 1997;21:27–32. doi: 10.1038/sj.ijo.0800356. [DOI] [PubMed] [Google Scholar]
- Maes M, Amand Y, Underwood LE, Maiter D, Ketelslegers JM. Decreased serum insulin-like growth factor I response to growth hormone in hypophysectomized rats fed a low protein diet: evidence for a postreceptor defect. Acta Endocrinol (Copenh) 1988;117:320–326. doi: 10.1530/acta.0.1170320. [DOI] [PubMed] [Google Scholar]
- Maiter D, Fliesen T, Underwood LE, Maes M, Gerard G, Davenport ML, Ketelslegers JM. Dietary protein restriction decreases insulin-like growth factor I independent of insulin and liver growth hormone binding. Endocrinology. 1989;124:2604–2611. doi: 10.1210/endo-124-5-2604. [DOI] [PubMed] [Google Scholar]
- Massara F, Ghigo E, Goffi S, Molinatti GM, Muller EE, Camanni F. Blockade of hp-GRF-40-induced GH release in normal men by a cholinergic muscarinic antagonist. J Clin Endocrinol Metab. 1984;59:1025–1026. doi: 10.1210/jcem-59-5-1025. [DOI] [PubMed] [Google Scholar]
- Merimee TJ, Zapf J, Froesch ER. Insulin-like growth factors in the fed and fasted states. J Clin Endocrinol Metab. 1982;55:999–1002. doi: 10.1210/jcem-55-5-999. [DOI] [PubMed] [Google Scholar]
- Miller KK, Lawson EA, Mathur V, Wexler TL, Meenaghan E, Misra M, Herzog DB, Klibanski A. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 2007;92:1334–1339. doi: 10.1210/jc.2006-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, Ott M, Herzog DB, Johnson ML, Klibanski A. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88:5615–5623. doi: 10.1210/jc.2003-030532. [DOI] [PubMed] [Google Scholar]
- Mohan PS, Jaya Rao KS. Plasma somatomedin activity in vitamin A deficient children. Clin Chim Acta. 1979;96:241–246. doi: 10.1016/0009-8981(79)90434-0. [DOI] [PubMed] [Google Scholar]
- Mohan PS, Jaya Rao KS. Sulfate metabolism in vitamin A-deficient rats. J Nutr. 1980;110:868–875. doi: 10.1093/jn/110.5.868. [DOI] [PubMed] [Google Scholar]
- Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, Matoulek M, Dostalova I, Humenanska V, Haluzik M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 2009;71:369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492:203–206. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- Olusi SO, Orrell DH, Morris PM, McFarlane H. A study of enocrine function in protein-energy malnutrition. Clin Chim Acta. 1977;74:261–269. doi: 10.1016/0009-8981(77)90293-5. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KS, Mohan PS. Plasma somatomedin activity, growth-hormone and insulin levels in vitamin B6 deficient rats. Horm Metab Res. 1982;14:580–582. doi: 10.1055/s-2007-1019086. [DOI] [PubMed] [Google Scholar]
- Reeves RD, Dickinson L, Lee J, Kilgore B, Branham B, Elders MJ. Effects of dietary composition on somatomedin activity in growing rats. J Nutr. 1979;109:613–620. doi: 10.1093/jn/109.4.613. [DOI] [PubMed] [Google Scholar]
- Robinson H, Picou D. A comparison of fasting plasma insulin and growth hormone concentrations in marasmic, kwashiorkor, marasmic-kwashiorkor and underweight children. Pediatr Res. 1977;11:637–640. doi: 10.1203/00006450-197705000-00003. [DOI] [PubMed] [Google Scholar]
- Rolla M, Andreoni A, Belliti D, Ferdeghini M, Cristofani R, Muller EE. Effects of cholinergic muscarinic antagonist pirenzepine on GH response to GHRH 1-40 in patients with anorexia nervosa. Endocrinol Exp. 1990;24:195–204. [PubMed] [Google Scholar]
- Sakharova AA, Horowitz JF, Surya S, Goldenberg N, Harber MP, Symons K, Barkan A. Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J Clin Endocrinol Metab. 2008;93:2755–2759. doi: 10.1210/jc.2008-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scacchi M, Pincelli AI, Caumo A, Tomasi P, Delitala G, Baldi G, Cavagnini F. Spontaneous nocturnal growth hormone secretion in anorexia nervosa. J Clin Endocrinol Metab. 1997;82:3225–3229. doi: 10.1210/jcem.82.10.4275. [DOI] [PubMed] [Google Scholar]
- Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:801–809. doi: 10.7326/0003-4819-128-10-199805150-00002. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Miyazaki M, Shimomura Y, Kobayashi I. Altered hormonal status in a female deprived of food for 18 days. J Med. 1991;22:201–210. [PubMed] [Google Scholar]
- Soliman AT, ElZalabany MM, Salama M, Ansari BM. Serum leptin concentrations during severe protein-energy malnutrition: correlation with growth parameters and endocrine function. Metabolism. 2000;49:819–825. doi: 10.1053/meta.2000.6745. [DOI] [PubMed] [Google Scholar]
- Soliman AT, Hassan AE, Aref MK, Hintz RL, Rosenfeld RG, Rogol AD. Serum insulin-like growth factors I and II concentrations and growth hormone and insulin responses to arginine infusion in children with protein-energy malnutrition before and after nutritional rehabilitation. Pediatr Res. 1986;20:1122–1130. doi: 10.1203/00006450-198611000-00012. [DOI] [PubMed] [Google Scholar]
- Stoving RK, Chen JW, Glintborg D, Brixen K, Flyvbjerg A, Horder K, Frystyk J. Bioactive insulin-like growth factor (IGF) I and IGF-binding protein-1 in anorexia nervosa. J Clin Endocrinol Metab. 2007;92:2323–2329. doi: 10.1210/jc.2006-1926. [DOI] [PubMed] [Google Scholar]
- Stoving RK, Veldhuis JD, Flyvbjerg A, Vinten J, Hangaard J, Koldkjaer OG, Kristiansen J, Hagen C. Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. J Clin Endocrinol Metab. 1999;84:2056–2063. doi: 10.1210/jcem.84.6.5734. [DOI] [PubMed] [Google Scholar]
- Straus DS, Takemoto CD. Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Mol Endocrinol. 1990;4:91–100. doi: 10.1210/mend-4-1-91. [DOI] [PubMed] [Google Scholar]
- Tamai H, Kiyohara K, Mukuta T, Kobayashi N, Komaki G, Nakagawa T, Kumagai LF, Aoki TT. Responses of growth hormone and cortisol to intravenous glucose loading test in patients with anorexia nervosa. Metabolism. 1991;40:31–34. doi: 10.1016/0026-0495(91)90188-3. [DOI] [PubMed] [Google Scholar]
- Tamai H, Komaki G, Matsubayashi S, Kobayashi N, Mori K, Nakagawa T, Truong MP, Walter RM, Jr, Kumagai LF. Effect of cholinergic muscarinic receptor blockade on human growth hormone (GH)-releasing hormone-(1-44)-induced GH secretion in anorexia nervosa. J Clin Endocrinol Metab. 1990;70:738–741. doi: 10.1210/jcem-70-3-738. [DOI] [PubMed] [Google Scholar]
- Thimmarayappa J, Sun J, Schultz LE, Dejkhamron P, Lu C, Giallongo A, Merchant JL, Menon RK. Inhibition of growth hormone receptor gene expression by saturated fatty acids: role of Kruppel-like zinc finger factor, ZBP-89. Mol Endocrinol. 2006;20:2747–2760. doi: 10.1210/me.2006-0128. [DOI] [PubMed] [Google Scholar]
- Thissen JP, Triest S, Moats-Staats BM, Underwood LE, Mauerhoff T, Maiter D, Ketelslegers JM. Evidence that pretranslational and translational defects decrease serum insulin-like growth factor-I concentrations during dietary protein restriction. Endocrinology. 1991a;129:429–435. doi: 10.1210/endo-129-1-429. [DOI] [PubMed] [Google Scholar]
- Thissen JP, Triest S, Underwood LE, Maes M, Ketelslegers JM. Divergent responses of serum insulin-like growth factor-I and liver growth hormone (GH) receptors to exogenous GH in protein-restricted rats. Endocrinology. 1990;126:908–913. doi: 10.1210/endo-126-2-908. [DOI] [PubMed] [Google Scholar]
- Thissen JP, Underwood LE, Maiter D, Maes M, Clemmons DR, Ketelslegers JM. Failure of insulin-like growth factor-I (IGF-I) infusion to promote growth in protein-restricted rats despite normalization of serum IGF-I concentrations. Endocrinology. 1991b;128:885–890. doi: 10.1210/endo-128-2-885. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Iguchi G, Fukuoka H, Suda K, Bando H, Takahashi M, Nishizawa H, Seino S, Takahashi Y. SIRT1 regulates adaptive response of the growth hormone--insulin-like growth factor-I axis under fasting conditions in liver. Proc Natl Acad Sci U S A. 2013;110:14948–14953. doi: 10.1073/pnas.1220606110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yde H. The growth hormone dependent sulfation factor in serum from patients with various types of diabetes. Acta Med Scand. 1969;186:293–297. doi: 10.1111/j.0954-6820.1969.tb01479.x. [DOI] [PubMed] [Google Scholar]
- Yi CX, Heppner KM, Kirchner H, Tong J, Bielohuby M, Gaylinn BD, Muller TD, Bartley E, Davis HW, Zhao Y, et al. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS One. 2012;7:e32100. doi: 10.1371/journal.pone.0032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A. 2010;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]