Abstract
Mesenchymal stem cells (MSCs) have an inherent tropism for sites of inflammation, which are frequently present in sites of cancer, including prostatic lesions. MSCs have been defined as CD73/CD90/CD105 triple-positive cells in the absence of hematopoietic lineage markers with the ability to differentiate into multiple mesodermal lineages, including osteoblasts, adipocytes, and chondrocytes. Our group has previously demonstrated that MSCs represent between 0.01 and 1.1% of the total cells present in human prostatectomy tissue. In addition to their multi-lineage differentiation potential, MSCs are immunoprivileged in nature and have a range of immunomodulatory effects on both the innate and adaptive arms of the immune system. MSCs have been detected in an increasing array of tissues, and evidence suggests that they are likely present in perivascular niches throughout the body. These observations suggest that MSCs represent critical mediators of the overall immune response during physiological homeostasis and likely contribute to pathophysiological conditions as well. Chronic inflammation has been suggested as an initiating event and progression factor in prostate carcinogenesis, a process in which the immunosuppressive properties of MSCs may play a role. MSCs have also been shown to influence malignant progression through a variety of other mechanisms, including effects on tumor proliferation, angiogenesis, survival, and metastasis. Additionally, human bone marrow-derived MSCs have been shown to traffic to human prostate cancer xenografts in immunocompromised murine hosts. The trafficking properties and immunoprivileged status of MSCs suggest that they can be exploited as an allogeneic cell-based vector to deliver cytotoxic or diagnostic agents for therapy.
Keywords: mesenchymal stem cell, MSC, inflammation, prostate cancer, multipotent stromal cell
Introduction
The prostate is the most common organ in the human body to undergo neoplastic transformation when accounting for both benign and malignant lesions. Pathological benign prostatic hyperplasia (BPH) affects >50% of men over the age of 50 years with nonclinical incidence representing a far greater number (Berry et al. 1984). Similarly, autopsy studies have demonstrated histological prostate cancer in as many as 50% of men by the age of 50 years with a linear increase in incidence for each subsequent decade of life (Delongchamps et al. 2006). The etiology of BPH and prostate cancer is unclear; however, chronic inflammation has been suggested as a contributing factor in both (Nelson et al. 2003, De Marzo et al. 2007, Kramer et al. 2007, De Nunzio et al. 2011, Sfanos & De Marzo 2012). The prostate, by virtue of its anatomical nature, is among a subset of tissues with a direct route of access to the external environment. Due to this exposure, the prostate routinely comes into contact with potentially infectious agents and frequently contains focal sites of inflammation. Though inflammation is commonly present, external pathogens often cannot be identified within these lesions (De Nunzio et al. 2011), suggesting that the inflammatory response persists after the pathogen is cleared or non-pathogenic stimuli are responsible. Other causative agents of prostatic inflammation include dietary and hormonal factors, in addition to chemical and physical irritations resulting from urine reflux and corpora amylacea respectively (De Marzo et al. 2007, De Nunzio et al. 2011). Furthermore, damage to epithelial cells and glandular structure as a result of these factors can contribute to altered antigen processing and presentation, which can generate an autoimmune response if these peptides are not recognized as ‘self’ or tolerance is broken (De Nunzio et al. 2011, Jackson et al. 2012). Independent of the origin, a chronic inflammatory state can arise as made evident by an age-associated persistent presence of an infiltrating leukocyte population (Kramer et al. 2007, Nickel et al. 2008).
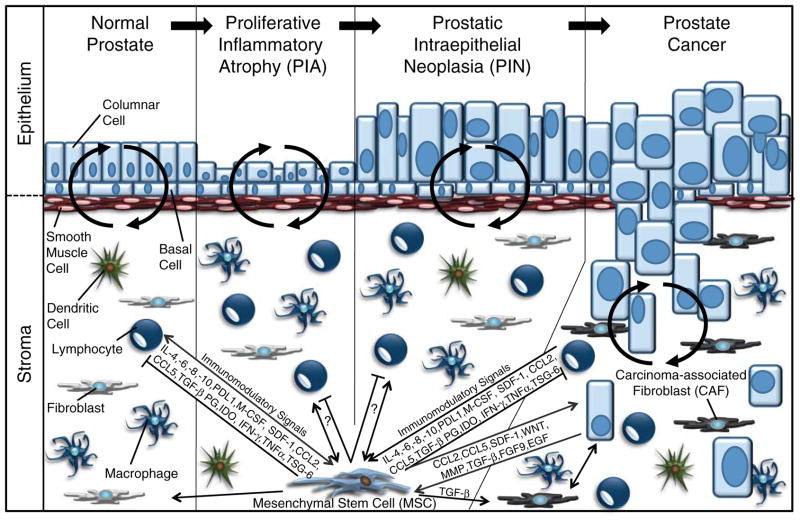
A heuristic model of prostate carcinogenesis suggests that prostate cancer progresses through proliferative inflammatory atrophy (PIA) and prostatic intraepithelial neoplasia (PIN) precursor lesions prior to malignant transformation (Fig. 1). PIA is defined as focal sites of hyperproliferative epithelial atrophy that are frequently associated with inflammation (De Marzo et al. 1999, Nelson et al. 2003). PIA is often adjacent to areas of PIN, which are characterized by intraductal cellular proliferation with no evidence of basement membrane and stromal invasion (Clouston & Bolton 2012). During both normal physiological processes and pathophysiological states, dynamic interactions initiated by paracrine mediators occur between the epithelium and cells normally restricted to the stroma, including smooth muscle cells, fibroblasts, bone marrow-derived mesenchymal stem cells (BM-MSCs), and various inflammatory cells. The latter of these have not only been associated with the initiation of prostate cancer but have also been suggested as potential drivers of its progression by virtue of DNA-damaging reactive oxygen species, a variety of immunosuppressive mechanisms, and the secretion of mitogenic and pro-angiogenic cytokines (Nelson et al. 2003, De Marzo et al. 2007, Sfanos & De Marzo 2012). Once the cancer cells have penetrated the basement membrane, they have direct contact with cells that were previously restricted to paracrine interactions, in addition to direct access to growth factors, survival signals, pro-invasion molecules, and extracellular matrix proteins. In total, these are collectively known as the tumor microenvironment and can have profound effects on cancer progression, malignancy, and therapeutic outcome (Cunha et al. 2003, Bissell & Hines 2011, Dayyani et al. 2011, Hanahan & Weinberg 2011, Brennen et al. 2012, Correia & Bissell 2012).
Figure 1.
MSCs in the normal and malignant prostate. A heuristic model of prostate carcinogenesis suggests that the normal gland progresses through proliferative inflammatory atrophy (PIA) and prostatic intraepithelial neoplasia (PIN) stages on its path to malignant transformation. MSCs likely have significant immunomodulatory roles not only in the normal prostate but throughout all stages of prostate cancer tumorigenesis and progression as well. These properties are mediated through the secretion of various chemokines (SDF1, CCL2, and CCL5), cytokines (IL4, IL6, IL8, IL10, M-CSF, IFN-γ, and TNFα) and other bioactive signaling molecules (TGF-β, PG, and IDO) that can indirectly affect carcinogenesis through leukocyte intermediates but also through direct effects on the cancer cells themselves. A full colour version of this figure is available via http://dx.doi.org/10.1530/ERC-13-0151.
Chemokines, such as CXCL12 (SDF-1), CCL5 (RANTES), and CCL2 (MCP-1) and the rest of the inflammation-associated secretory milieu, have been shown to recruit MSCs to these sites as a result of the high expression of chemokine and cytokine receptors on their surface (Spaeth et al. 2008). Recently, our group has demonstrated that MSCs are not only present at sites of human prostate cancer but also represent 0.01–1.1% of the total cells present in human prostatectomy tissue cores (Brennen et al. 2013). MSCs have been shown to be critical mediators of the overall immune response (Caplan 2009, Newman et al. 2009, English & Mahon 2011) and, therefore, may contribute to carcinogenesis through a variety of mechanisms, including stimulation of proliferation, angiogenesis, and metastasis, in addition to their immunosuppressive properties (Bergfeld & DeClerck 2010, Klopp et al. 2011). These latter properties may be particularly relevant in tumor progression as the cancer cells must escape immune surveillance and clearance to reach their full malignant potential (Fig. 1). Perhaps more importantly, the tumor trafficking properties of MSCs suggest that they could be used to deliver therapeutic or diagnostic agents to sites of prostate cancer, both primary and secondary lesions (Brennen et al. 2013).
MSCs: mesenchymal stem cells
Recently, there has been an increasing appreciation for the role of MSCs, also known as multipotent stromal cells, in modulating both innate and adaptive immune responses. These cells were initially characterized by Friedenstein et al. (1970) as clonogenic cells in culture that were multipotent stromal precursors. Throughout much of the early literature, these cells were referred to as colony-forming unit fibroblasts or CFU-Fs (Friedenstein et al. 1976), until Caplan proposed the term ‘Mesenchymal Stem Cells’ in 1991 (Caplan 1991). Over the ensuing years, there has been much debate regarding the appropriateness of this terminology (Horwitz et al. 2005, Bianco et al. 2008, Ho et al. 2008, Nombela-Arrieta et al. 2011); however, this continues to be the accepted consensus in the literature.
The International Society for Cell Therapy has minimally defined MSCs as plastic-adhering multipotent cells of fibroblastoid morphology with the ability to differentiate into cells of the osteogenic, adipogenic, and chondrogenic lineages (Pittenger et al. 1999, Dominici et al. 2006). MSCs have been further defined based upon the expression of CD90 (Thy-1), CD105 (endoglin), and CD73 (5′-nucleotidase) in the absence of hematopoietic lineage markers, including CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR expression (Dominici et al. 2006). Although some are more controversial than others, there is also evidence to suggest that MSCs can differentiate into myocytes (Wakitani et al. 1995, Crisan et al. 2008), fibroblasts (Lee et al. 2010), pericytes (Hirschi & D’Amore 1996, Crisan et al. 2008), and neurons (Woodbury et al. 2000, Hofstetter et al. 2002, Bertani et al. 2005, Krampera et al. 2007, Phinney & Prockop 2007), although neuronal differentiation appears to be correlated with the age of the donor (Hermann et al. 2010, Brohlin et al. 2012). The number of MSCs in an individual declines with age, as demonstrated by a 100-fold decrease in the ability of nucleated marrow cells to form colonies (CFU-F), from ~1 in 104 in newborns to ~1 in 106 in the elderly (Caplan 2009). Furthermore, while MSCs are generally thought to be derived from the mesoderm (Vodyanik et al. 2010), there is an initial wave of neuroectodermal MSCs during embryogenesis that arise from the neural crest (Takashima et al. 2007, Morikawa et al. 2009). Additional evidence suggests that the percentage of MSCs derived from the neural crest declines with age (Takashima et al. 2007), which may explain the loss of neuronal differentiation potential observed in MSCs derived from older donors. Additionally, the mesenchymoangioblast was also recently identified as a mesoderm-derived precursor able to generate both MSCs and endothelial cells (Vodyanik et al. 2010). MSCs have also been isolated from numerous peripheral tissues, including fat, skin, dental pulp, and pancreas (Zuk et al. 2002, da Silva Meirelles et al. 2006, Zhang et al. 2006, Davani et al. 2007, Crisan et al. 2008, Blasi et al. 2011) and are likely present in all tissues at low levels as part of a homeostatic surveillance mechanism.
MSCs: tissue of origin
MSCs isolated from these peripheral tissues are frequently thought of as equivalent to those derived from bone marrow due to significantly overlapping properties; however, there is accumulating evidence to suggest that there are differences between these populations, including their expression profiles (Panepucci et al. 2004, Wagner et al. 2005, Park et al. 2007, Noel et al. 2008, Jansen et al. 2010, Strioga et al. 2012) and differentiation potential (Sakaguchi et al. 2005, Musina et al. 2006, Strioga et al. 2012). These differences may reflect a ‘memory’, epigenetic or otherwise, associated with distinct signaling events and cellular interactions that occur between MSCs and unique microenvironments. For example, multiple studies have shown that MSCs isolated from fat tissue, or adipose-derived stem cells (ADSCs), have an increased propensity to form adipocytes relative to those derived from bone marrow (Sakaguchi et al. 2005, Musina et al. 2006). Both synovium- and BM-MSCs seem to have a greater ability to generate chondrocytes than ADSCs (Sakaguchi et al. 2005, Afizah et al. 2007). Additionally, ADSCs generate osteoblasts with less efficiency relative to their bone marrow-derived counterparts (Sakaguchi et al. 2005). However, other studies have shown that both BM-MSCs and ADSCs have equal osteoblast and adipocyte differentiation potential (De Ugarte et al. 2003, Krampera et al. 2007, Noel et al. 2008, Pachon-Pena et al. 2011). Our own studies suggest that MSCs from the prostates of young, healthy men selectively lose their adipocyte differentiation ability (W N Brennen, S Chen and J T Isaacs 2013, unpublished observations), while those isolated from cancerous prostates in older men retain their tri-lineage differentiation potential (Brennen et al. 2013), which may reflect their more recent exodus from the bone marrow and represent a more naïve commitment status.
Importantly, inter-individual variation in the proliferative capacity and differentiation potential of donor-derived MSCs can make the interpretation of such comparisons difficult, which can be further compounded by differences in optimal culture conditions that are yet to be fully standardized for MSCs obtained from alternative tissue sources (Huang et al. 2005, Sakaguchi et al. 2005, Wagner et al. 2005, Ho et al. 2008, Pevsner-Fischer et al. 2011, Rada et al. 2011, Brennen et al. 2013). This variability can be alleviated, in part, by comparing a panel of tissue-specific MSCs isolated from a single individual. Indeed, such studies appear to confirm observations suggesting a restricted differentiation potential related to a tissue-of-origin ‘memory’ (Sakaguchi et al. 2005, Afizah et al. 2007). For instance, BM-MSCs have greater chondrogenic potential than ADSCs isolated from the same patient (Huang et al. 2005, Afizah et al. 2007). Sakaguchi et al. (2005) also demonstrated distinct differences in the differentiation efficiencies of patient-matched MSCs isolated from multiple tissues and expanded under similar conditions. Furthermore, gene expression and proteomic analyses of MSCs from different sources have also demonstrated distinct profiles despite significant similarities (Wagner et al. 2005, Noel et al. 2008, Jansen et al. 2010). For example, increased expression of osteogenesis-and angiogenesis-associated genes was measured in BM-MSCs and umbilical cord MSCs relative to each other respectively (Panepucci et al. 2004). Minimally, these observations highlight the heterogeneity of isolated MSC populations with regard to their differentiation potential, embryonic lineage, tissue source, and donor age.
MSCs in the clinic
Numerous clinical trials over the last decade were designed to exploit the multipotent differentiation potential of MSCs for a range of pathological conditions, including myocardial infarction (MI), spinal cord injury, and osteogenesis imperfecta. While promising results were obtained in early phase clinical trials, the high hopes for these MSC-based regenerative strategies were largely unrealized in the accompanying phase III trials. Follow-up on these studies suggested a lack of long-term tissue engraftment (<1%) with no evidence of differentiation into the anticipated cell types following systemic administration (Ankrum & Karp 2010). In contrast to the results from in vitro differentiation assays, these clinical observations questioned the assumption that MSC’s primary role in tissue repair is to reconstitute damaged cell types. However, despite the lack of differentiation, there were positive therapeutic effects observed in select patients from these trials. Concurrent laboratory investigations led to an emerging realization that MSCs function through trophic and immunomodulatory mechanisms based on the secretion of bioactive molecules (Krampera et al. 2003, Le Blanc et al. 2003b, Aggarwal & Pittenger 2005, Zimmet & Hare 2005, Iso et al. 2007, Prockop 2007, Caplan 2009). MSCs have been shown to secrete numerous growth factors, cytokines, and chemokines, in addition to pro-angiogenic, anti-apoptotic, and anti-inflammatory signals, including transforming growth factor β (TGF-β), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 6 (IL6), regulated on activation, normal T cell expressed and secreted (RANTES), CCL2, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), prostaglandins (PGs), and IL10, to name a few (Newman et al. 2009, Zhukareva et al. 2010, English & Mahon 2011). Although some groups only detect IL10 in MSC-leukocyte co-cultures (Tse et al. 2003, Beyth et al. 2005, Rasmusson et al. 2005), others have reported the constitutive expression of IL10 by MSCs in monoculture as well (Aggarwal & Pittenger 2005, Barry et al. 2005, Coffelt et al. 2009, Mougiakakos et al. 2011, Technau et al. 2011). Additionally, molecular profiling has revealed that MSCs express a large repertoire of cytokine and chemokine receptors that are believed to mediate their trafficking to inflammatory sites (Spaeth et al. 2008). The paracrine effects of these secreted molecules likely explain the observed clinical benefits seen thus far and have formed the underlying rationale for the majority of current MSC-based clinical trials designed to treat various inflammatory and autoimmune disorders.
To date, a number of clinical trials have been completed in which ex vivo expanded MSCs have been administered for applications as diverse as enhancing cardiac function post-MI, promoting hematopoietic stem cell engraftment, mitigating graft-vs-host-disease (GVHD), and treating a host of autoimmune disorders (Lazarus et al. 2005, Le Blanc et al. 2008, Hare et al. 2009, Garcia-Gomez et al. 2010). The most commonly studied application of systemic allogeneic MSCs has been as a means to decrease or prevent GVHD. Two phase III trials enrolling a total of 452 patients have evaluated the efficacy of allogeneic MSCs in patients with GVHD have been completed. While neither trial met its primary endpoint of complete response, there were some clinical benefits observed in those with steroid-refractory GVHD. These results ultimately lead to the approval in Canada of Prochymal, a pre-manufactured, universal donor MSC product, in acute pediatric GVHD (Prasad et al. 2011, Osiris Therapeutics 2012). More importantly, as was true with earlier phase studies, no adverse events were noted after infusion of MSCs in any patient being treated on either GVHD protocol. A variety of phase I/II studies testing the effect of MSCs in the setting of MI, chronic obstructive pulmonary disease, liver cirrhosis, lupus, and type II diabetes have also been reported (Hare et al. 2009, Jiang et al. 2011, Zhang et al. 2012, Li et al. 2013, Weiss et al. 2013). Thus far, no significant MSC-related adverse events have been reported across these various phase I/II studies (Lalu et al. 2012). Currently, a number of additional randomized trials are underway utilizing MSCs in a range of different diseases (Garcia-Gomez et al. 2010). In summary, the combined results from a large number of trials indicate that i.v. administration of unmodified human BM-MSCs, whether autologous or allogeneic, can be safely administered to patients without producing significant side effects.
MSCs: firemen of the immune system
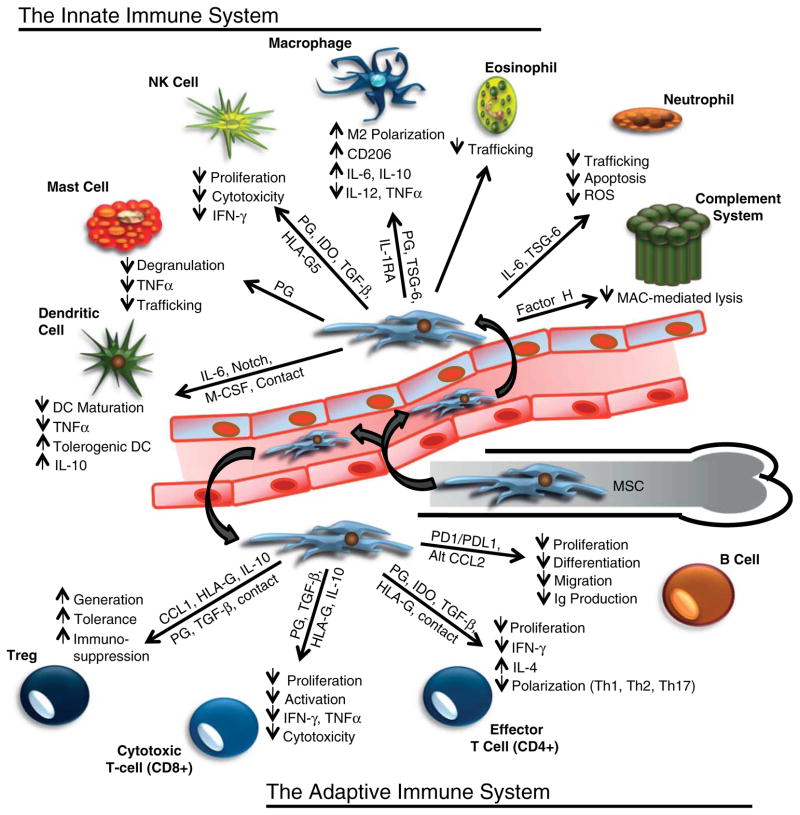
MSCs are generally thought to be non-immunogenic due to their lack of both MHC-II expression and the associated co-stimulatory molecules (Tse et al. 2003). Importantly, MSCs do express low levels of MHC-I, which prevents them from being recognized and lysed by NK cells (Newman et al. 2009). Furthermore, the constitutive expression of factor H makes MSCs resistant to complement-mediated lysis (Tu et al. 2010). Secretion of factor H extends this protection to other cells in the local microenvironment and represents one of many mechanisms through which MSCs suppress both the innate and adaptive immune responses (Fig. 2). MSCs inhibit the proliferation and activation of NK cells through expression of PGs and indoleamine dioxygenase (IDO; English & Mahon 2011). MSCs secreted PGs have also been shown to suppress mast cell degranulation, trafficking, and tumor necrosis factor α (TNFα) expression (Brown et al. 2011), in addition to promoting macrophage M2 polarization (Prockop 2013). Secretion of TSG-6 by MSCs has multiple anti-inflammatory properties, including inhibition of TLR2-induced NF-κB signaling in macrophages by blocking CD44 stimulation, abrogation of neutrophil migration, and suppression of pro-inflammatory protease activity (Lee et al. 2009, Prockop & Oh 2012, Prockop 2013). Additionally, MSCs block the secretion of pro-inflammatory cytokines from activated macrophages, prevent the oxidative burst associated with neutrophil function, and suppress eosinophil trafficking to inflammatory tissues (Newman et al. 2009, English & Mahon 2011). MSC-derived IL6 inhibits dendritic cell (DC) maturation from monocytes, in addition to suppressing the expression of MHC-II and the CD40 and CD86 co-stimulatory molecules required for T-cell activation (Djouad et al. 2007). By blocking DC maturation and antigen presentation, MSCs induce a tolerogenic phenotype in which DCs downregulate the expression of pro-inflammatory cytokines, such as TNFα, while upregulating the expression of IL10 and other anti-inflammatory cytokines (Aggarwal & Pittenger 2005).
Figure 2.
Immunosuppressive properties of MSCs on both the innate and adaptive arms of the immune system. MSC trafficking from the bone marrow in response to an inflammatory stimulus, in addition to MSCs, already present in the local microenvironment can profoundly affect the overall immune response. The immunosuppressive effects of MSCs are mediated through both direct cell contact in some cases, and the secretion of numerous paracrine signals that effect proliferation, survival, trafficking, maturation, polarization, activation, cytotoxicity, and the secretion of additional inflammatory mediators. These effects occur between MSCs and nearly all components of both the innate and adaptive immune system, which suggests that MSCs may represent a central hub in the regulatory networks of the immune system. A full colour version of this figure is available via http://dx.doi.org/10.1530/ERC-13-0151.
Inhibition of MHC-II-mediated antigen presentation by DCs prevents T-cell activation and proliferation. Furthermore, MSCs promote the generation of regulatory T cells; suppress Th1, Th2, and Th17 polarization; and inhibit the proliferation and activation of cytotoxic T cells (CTLs); thereby, shifting the T-cell response to an immunosuppressive state (Newman et al. 2009, English & Mahon 2011). Multiple studies have also demonstrated that MSCs inhibit B-cell activation, proliferation, migration, and immunoglobulin expression (Corcione et al. 2006, Newman et al. 2009). These effects are mediated by both soluble factors and direct cell–cell contact; the latter of which activates programmed death pathway-1 (PD-1) signaling and is at least partially responsible for the attenuation of B-cell proliferation and altered cytokine receptor expression in mice (Augello et al. 2005). These observations suggest that a primary physiological function of MSCs is to promote an immunosuppressive microenvironment (Fig. 2). This MSC-mediated immunosuppression likely represents a critical negative feedback mechanism to prevent unchecked chronic inflammation. Together with other regulatory mechanisms, this negative feedback helps to prevent an uncontrolled self-reinforcing ‘cytokine storm’, or hypercytokinemia, that can lead to increased vascular permeability, tissue edema, autoimmune disorders, fibrosis, acute respiratory distress syndrome, organ failure, cancer, or even death in extreme cases (Osterholm 2005, La Gruta et al. 2007).
MSCs: immunoprivileged or not?
While MSCs are traditionally thought to be non-immunogenic and immunosuppressive due to the properties described above, some recent studies have suggested that MSCs may be immunogenic under certain conditions (Eliopoulos et al. 2005, Nauta et al. 2006, Huang et al. 2010). The rejection of allogeneic MSCs in immunocompetent MHC mismatched mice was associated with an increase in infiltrating CTLs, natural killer T cells, and NK cells (Huang et al. 2010). Additionally, while syngeneic murine MSCs were associated with tolerance to both donor and recipient antigens in an allogeneic bone marrow transplant model, the same study also demonstrated that transplantation of MHC-matched MSCs and BM into an allogeneic recipient decreased engraftment efficiency (Nauta et al. 2006). In contrast, no effect on BM engraftment was observed when MSCs from a third-party donor were used. Furthermore, while allogeneic MSCs only triggered rejection when they were MHC-matched to the BM donor, both third-party and BM-matched MSCs were able to induce a memory T-cell response, which strongly suggests that allogeneic murine MSCs are immunogenic (Nauta et al. 2006). Unlike human MSCs, which only express MHC-II following stimulation with interferon (IFN)-γ, the murine MSCs used in these studies express low levels of MHC-II under non-stimulated conditions (Eliopoulos et al. 2005). Unsurprisingly, this basal expression of MHC-II renders murine MSCs immunogenic in an allogeneic setting and likely explains the results observed in these studies.
Like humans and other higher order mammals, rat MSCs, in contrast to their murine cousins, do not express MHC-II antigens under basal conditions (Newman et al. 2009); however, induction of MHC-II expression was detected following myogenic, endothelial, and smooth muscle differentiation (Huang et al. 2010). Furthermore, these differentiated MSCs were cleared from recipient tissues and donor-specific alloantibodies were detected in the serum of recipient rats at 5 weeks post-injection. Similarly, expression of HLA-DR, a MHC-II antigen, is induced following chondrogenic differentiation of adipose-derived human MSCs (ADSCs) in vitro (Technau et al. 2011). Interestingly, these chondrocyte-differentiated ADSCs continued to express the immunosuppressive HLA-G antigen and secrete IL10, suggesting that they may retain their immunosuppressive properties post-differentiation. Increased immunogenicity following differentiation would potentially explain the lack of data demonstrating long-term engraftment in patients following allogeneic MSC infusion. Indeed, Niemeyer et al. (2008) found no evidence of BM-MSCs that were osteogenically induced ex vivo prior to infusion in recipient animals; by contrast, undifferentiated BM-MSCs were detected in all recipients following xenotransplantation. Additionally, both allogeneic and autologous BM-MSCs are susceptible to complement-mediated lysis in the presence of serum following ex vivo culturing, despite the expression of factor H and other negative regulators (Li & Lin 2012).
Other studies have demonstrated that neither differentiated nor undifferentiated allogeneic MSCs induce a proliferative response in mixed lymphocyte cultures (Le Blanc et al. 2003a) or in a rabbit model of osteogenesis (Liu et al. 2006). Additionally, early studies failed to detect alloantibodies against MSCs in the serum of patients receiving therapeutic doses of allogeneic MSCs (Le Blanc et al. 2004, Sundin et al. 2007), suggesting that the immunoprivileged phenotype of MSCs remains dominant even if they do undergo differentiation in vivo. However, recent studies have reported the presence of anti-donor antibodies in the serum of a minority of patients. Weak alloimmune reactions were detected in 3.7% of patients in the POSEIDON randomized trial comparing allogeneic to autologous BM-MSC therapy for ischemic cardiomyopathy (Hare et al. 2012). In a press release reporting the results from Mesoblast’s phase 2 trial evaluating MSCs in patients with cardiovascular disease, anti-donor antibodies were detected in 13% of patients (PRNewswire 2011). Importantly, no adverse clinical effects were associated with the presence of alloantibodies in either of these studies.
Ex vivo culturing conditions, particularly with respect to FBS, have been shown to affect the immunogenicity of MSCs and may explain some of the mixed results observed between laboratories (Sundin et al. 2007, Newman et al. 2009). Multiple trials evaluating the use of autologous MSCs for a variety of conditions have recently been completed or are in progress, and reports on their engraftment efficiency compared with their allogeneic counterparts will address this possibility in a more definitive manner. Importantly, there have been no adverse clinical events, immunological or otherwise, associated with either systemic or local administration of MSCs in the thousands of patients that have been accrued in these trials (Ankrum & Karp 2010, Lalu et al. 2012), which emphasizes the overall safety of MSC-based therapeutic strategies. The spontaneous malignant transformation of human MSCs during prolonged expansion ex vivo has also been raised as a potential safety concern regarding their clinical use; however, reports on this phenomenon were later corrected or retracted by admissions of contamination in the MSC cultures with other cancer cell lines (Garcia et al. 2010, Torsvik et al. 2010, Vogel 2010, Klopp et al. 2011). Of note, patients enrolled in MSC-based clinical trials often receive multiple doses of >108 cells, and no transformation of MSCs in these patients have been reported to date. A recent autopsy study of 18 patients receiving infusions of HLA-mismatched MSCs found no evidence of ectopic tissue formation or malignant tumors derived from donor MSCs (von Bahr et al. 2012). Furthermore, in eight patients with tissue samples collected more than 50 days post-infusion, low levels of MSC donor DNA (<1/1000) were only detected in the lung and kidney of a single patient each. These data corroborate previous clinical observations, suggesting that MSCs have limited long-term engraftment capabilities, which serves to highlight the overall lack of tumorigenic potential for these cells in an allogeneic therapeutic setting.
MSCs: complexities and immunostimulatory properties
There is accumulating evidence to suggest that the interactions between MSCs and immunological effector cells are more complex than those previously appreciated (Fig. 3). For example, MSCs are known to inhibit the IL2-stimulated proliferation of resting NK cells; however, activated NK cells are not only more resistant to MSC-mediated proliferative suppression but have also been shown to lyse both autologous and allogeneic MSCs in the absence of IFN-γ (Spaggiari et al. 2006). This lysis occurs as a result of the expression of NK-activating ligands by MSCs (Spaggiari et al. 2006). Binding of these ligands to their cognate receptors on the surface of NK cells triggers their recognition and destruction by NK cells, despite the low levels of MHC-I expression on MSCs. Exposure to IFN-γ in an inflammatory microenvironment significantly upregulates MHC-I expression on MSCs and protects them from NK-mediated lysis (Eliopoulos et al. 2005, Spaggiari et al. 2006). Additionally, IFN-γ-stimulated MSCs also express MHC-II and can function as antigen-presenting cells (APCs; Chan et al. 2006, Stagg et al. 2006). Interestingly, these antigen-presenting properties are biphasic and only present during a narrow range of IFN-γ concentrations with high levels leading to a decrease in APC functions (Chan et al. 2006). MSCs also possess direct antimicrobial activity mediated through the secretion of cathelicidin hCAP-18/LL-37, a peptide with activity against both Gram-positive and -negative bacteria (Krasnodembskaya et al. 2010).
Figure 3.
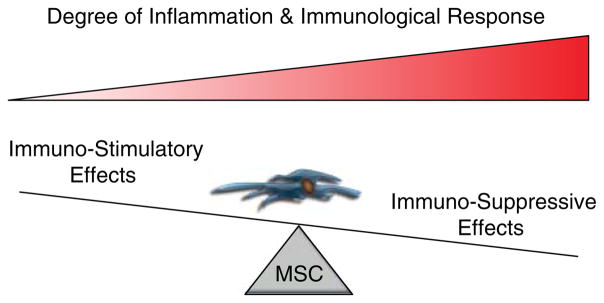
The dichotomous role of MSCs in modulating the immune response depends on the degree of the immunological assault. Evidence suggests that during the initial stages of an inflammatory response, MSCs can behave as antigen-presenting cells and have immunostimulatory effects that activate an adaptive immune response following PAMP recognition and IFN-γ stimulation. As concentrations of IFN-γ, TNFα, and other inflammatory cytokines rise during prolonged inflammation and the lymphocyte-to-MSC ratio increases, the immunosuppressive properties gain dominance and serve as a negative feedback mechanism to prevent unchecked chronic inflammation that can contribute to pathogenesis. A full colour version of this figure is available via http://dx.doi.org/10.1530/ERC-13-0151.
The differential activation of TLR signaling in MSCs has also been shown to be a critical mediator of their immunomodulatory properties (Pevsner-Fischer et al. 2007, Liotta et al. 2008). TLR-2 stimulation suppresses MSC differentiation, while promoting their proliferation and immunosuppressive phenotype (Pevsner-Fischer et al. 2007). By contrast, TLR-3 and -4 signaling inhibits this immunosuppressive activity without affecting their differentiation potential (Liotta et al. 2008). Activation of different TLR signaling pathways in response to various pathogen-associated molecular patterns (PAMPs) has also been proposed to explain the ability of MSCs to promote tissue repair and control the inflammatory reaction without negatively impacting the ability of the immune system to fight off invading pathogens (English & Mahon 2011). These observations suggest a model where MSCs would function in a dichotomous manner depending on the nature of the infectious insult and the extent of the immunological response. MSCs would initially behave as APCs to activate an adaptive response following PAMP recognition and IFN-γ stimulation, and the immunosuppressive effects would take dominance during prolonged inflammation with increasing IFN-γ levels (Fig. 3). In further support of this model, the effect of MSCs on lymphocyte proliferation seems to be dependent on the MSC-to-lymphocyte ratio present. Low ratios of MSCs to lymphocytes, as would be seen in the initial phases of inflammation, stimulated lymphocyte proliferation through soluble paracrine mediators, whereas, higher ratios, which may occur after a prolonged inflammatory response, resulted in inhibition of lymphocyte proliferation (Bocelli-Tyndall et al. 2009). Evolutionarily, this negative feedback mechanism would serve to limit the immune response and prevent an unbridled leukocytic infiltrate from initiating a self-reinforcing loop of chronic inflammation, which could potentially lead to associated pathological conditions. This parallels a recent model proposed by English & Mahon (2011) in which they describe MSCs as a sort of inflammatory rheostat or ‘licensing switch’ to modulate the immune response. Additional support for the role of IFN-γ in regulating the immunomodulatory properties of MSCs comes from observations demonstrating that MSC-mediated suppression of T-cell proliferation is enhanced by IFN-γ secreted by activated NK and T cells (Krampera et al. 2006, English et al. 2007). Furthermore, in contrast to wild-type MSCs, IFN-γ receptor 1-null MSCs were unable to prevent GVHD in mice, suggesting that inflammation and IFN-γ signaling in particular are required for the immunosuppressive effects of MSCs (Ren et al. 2008b). Importantly, these immunomodulatory properties are probably not dictated by IFN-γ alone but are the result of a complex interplay between IFN-γ, TNFα, and the entire panoply of inflammatory cytokines, chemokines, and signaling molecules present within the local microenvironment.
MSCs and the inflammatory prostate
A model in which MSCs play a primary role in modulating the immune response implies that these cells are present in, or continuously fluxing through, all tissues. Accumulating evidence supports this model. In addition to the bone marrow, MSCs have been isolated from a growing list of tissues, including adipose tissue, skin, muscle, dental pulp, pancreas, intestine, lung, and peripheral blood (Zuk et al. 2002, Kassis et al. 2006, Zhang et al. 2006, Davani et al. 2007, Lama et al. 2007, Crisan et al. 2008, Lanzoni et al. 2009, Blasi et al. 2011), with all available evidence suggesting that they reside in perivascular niches within all tissues (da Silva Meirelles et al. 2006, Crisan et al. 2008). While this is a rare, but detectable, population of cells within these tissues under homeostatic conditions, there is a dramatic influx from the bone marrow in response to an inflammatory insult (Spaeth et al. 2008, Newman et al. 2009). It is well known that the prostate is bombarded with inflammatory and infectious agents throughout an individual’s lifetime, with as many as 80% of men showing evidence of a leukocytic infiltrate in their prostate when biopsied (De Marzo et al. 2007, Nickel et al. 2008, Sfanos & De Marzo 2012). Additionally, the formation of corpora amylacea, which are aggregates of inflammatory proteins, is thought to begin early in life and increase with age, becoming highly prevalent within the prostates of older men (Sfanos & De Marzo 2012). The presence of prostatic inflammation, or prostatitis, likely extends to all men at some point in their lives as many inflammatory stimuli will be resolved without generating overt clinical symptoms.
Therefore, it is unsurprising that MSCs can also be isolated from the prostates of both young and old men (Brennen et al. 2013, W N Brennen and J T Isaacs 2013, unpublished observations). Lin et al. (2007) isolated cells consistent with an MSC phenotype from BPH tissue, a disease characterized by a hyper-proliferative stroma. Despite their ability to differentiate into the myogenic, adipogenic, and osteogenic lineages, the authors concluded that these cells did not represent MSCs due to their inability to generate neural cells, a property later shown to decrease with age (Hermann et al. 2010, Brohlin et al. 2012), thereby explaining the lack of this particular differentiation potential in BPH cells isolated from older men. Additionally, MSCs incorporate into the re-growing prostates of castrated mice following testosterone supplementation (Placencio et al. 2010). In addition to older men with prostate cancer, our own laboratory has cultivated MSCs (CD90+/FAP+/CD105+/CD73+/HLA-DR−) from the prostate of a 20-year-old healthy organ donor (S Chen, W N Brennen and J T Isaacs 2013, unpublished observations), suggesting that MSCs are present in the prostate throughout an individual’s lifetime to varying degrees.
Chronic inflammation is thought to be an initiating event for prostatic carcinogenesis (Nelson et al. 2003, De Marzo et al. 2007). Numerous factors have been implicated in the initiation of an inflammatory microenvironment within the prostate, including diet, infectious agents, physical trauma induced by corpora amylacea, hormonal changes, and urine reflux (Sfanos & De Marzo 2012). Independent of the cause, the resulting inflammatory signals act as a chemoattractant for circulating BM-MSCs (Fig. 1) due to the extensive array of chemokine and cytokine receptors expressed on their cell surface (Spaeth et al. 2008). CXCL12 (SDF-1), CCL5 (RANTES), and CCL2 (MCP-1), in particular, have been shown to be highly overexpressed in prostate cancer (Sun et al. 2003, Vaday et al. 2006, Fujita et al. 2010), all of which have also been implicated in MSC trafficking to inflammatory sites (Spaeth et al. 2008). Multipotent MSCs of mouse origin have been isolated from prostate cancer xenografts using a side population assay (Santamaria-Martinez et al. 2009). Additional evidence consistent with the presence of MSCs in human prostate cancer includes the characteristic overexpression of CD90 (True et al. 2010), a marker of not only MSCs but also endothelial cells, hematopoietic precursors, neurons, thymocytes, and NK cells. Interestingly, in a series of prostate cancer tissue samples with high CD90 expression, these same authors showed a non-comparable increase in CD45-positive cells, suggesting that the increased CD90 expression was not merely due to excessive leukocyte infiltration (Liu et al. 2004). Importantly, not all these extra CD90-positive cells are likely to represent bona fide MSCs as this population also includes MSCs at various stages of differentiation, endothelial cells, hematopoietic progenitors, and carcinoma-associated fibroblasts (CAF). Furthermore, while CD90 expression is significantly elevated in malignant prostatic lesions, rare CD90+ cells can also be detected in normal prostate tissue (Zhao & Peehl 2009, True et al. 2010), which is consistent with the presence of a small population of MSCs in all tissues. CD90-positive cells have also been identified in cultures isolated from primary human prostatic stromal cells (Zhao & Peehl 2009). While the authors of this study concluded that these cells did not represent MSCs, it should be noted that CD90hi cells were only compared with CD90lo, rather than CD90-negative cells. Additionally, the differentiation potential of these two CD90-positive populations was not investigated. We would suggest that both these populations likely represent MSCs, albeit potentially ones at different stages of differentiation or lineage commitment. Our own studies clearly indicate the presence of MSCs in multiple primary prostate cancer specimens obtained directly from the operating room prior to expansion in tissue culture (Brennen et al. 2013). While CD90 expression has been proposed as a potential cancer biomarker (True et al. 2010), the relationship between CD90 expression and PIA or PIN, which are believed to be prostate cancer precursor lesions, has not been studied. Coupled with other characteristic MSC markers, this would help to determine whether MSCs traffic to these inflammatory precursor lesions as an early event in prostate carcinogenesis.
MSCs: effects on tumor progression and metastasis
The role of MSCs in the pathogenesis of cancer is complex and likely related to the balance of competing pro- and anti-tumorigenic forces. Numerous mechanisms have been proposed to play a role in the ability of MSCs to promote tumor growth, including stimulation of proliferation, angiogenesis, and metastasis, in addition to the immunosuppressive properties described earlier. Co-inoculation of MSCs with tumor cells has been shown to increase xenograft growth in models of melanoma and lymphoma, in addition to colon, breast, and lung cancer (Bergfeld & DeClerck 2010, Klopp et al. 2011). Tumor growth can be further fueled by promoting an increased tumor vasculature through the secretion of pro-angiogenic factors by MSCs, including VEGF, TGF-β, platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF) (Bergfeld & DeClerck 2010, Bianchi et al. 2011). MSCs are also frequently found in perivascular niches and can promote vessel stabilization through pericyte-like functions (da Silva Meirelles et al. 2006, Crisan et al. 2008, Bianchi et al. 2011). Additionally, MSCs have been shown to enhance the metastatic potential of breast and colon cancer cells in xenograft models (Karnoub et al. 2007, Klopp et al. 2011), in addition to promoting a pro-tumorigenic environment in the bone marrow (Bergfeld & DeClerck 2010). The immunosuppressive properties of MSCs have also been proposed as a mechanism to enable the tumor to escape host immune surveillance (Bergfeld & DeClerck 2010). Extensive reviews on the relationship between MSCs and cancer have previously been published elsewhere (Bergfeld & DeClerck 2010, Bianchi et al. 2011, Klopp et al. 2011).
While the pro-tumorigenic role of MSCs is more easily understood, there are also a large number of studies demonstrating anti-tumorigenic effects of MSCs for reasons that are less clear (Klopp et al. 2011) but include pro-inflammatory effects and the downregulation of survival signals mediated through the Akt and Wnt pathways (Ohlsson et al. 2003, Khakoo et al. 2006, Qiao et al. 2008). An attempt to reconcile these conflicting observations has recently been discussed by Marini and colleagues, who conclude that there is currently no clear explanation for these divergent findings (Klopp et al. 2011). The dichotomous role of MSCs in the immune system likely plays a role in this tumorigenic response; however, both pro- and anti-tumorigenic effects have been observed in both immunocompromised and immunocompetent animals, suggesting that this relationship is more complex than merely a function of their immunomodulatory properties.
MSCs: role in prostate carcinogenesis
Specifically, with regard to MSCs and prostate cancer, several in vitro investigations have attempted to understand how the interactions between these two cell types may contribute to carcinogenesis in both the primary and the metastatic tumor microenvironments (Fig. 1). FGF-9 and paracrine factors secreted by bone metastatic PC3 cells stimulate osteoblastic differentiation of human BM-MSCs, whereas conditioned medium from non-metastatic CWR22Rv1 cells did not (Fritz et al. 2011). This is particularly interesting in light of the well-known observation that prostate cancer frequently generates osteoblastic lesions when it metastasizes to the bone. Later studies demonstrated that the pro-osteoblastic effect of PC3-conditioned media (PC3-CM) was due to the presence of epidermal growth factor receptor (EGFR) ligands, which also stimulated the proliferation of human BM-MSCs, but suppressed adipocyte and osteoclast differentiation (Borghese et al. 2012). Interestingly, significant levels of new bone formation in vivo were only observed when MSCs were injected intra-tibially in the presence of PC3 cancer cells but not in their absence (Chanda et al. 2009). PC3-CM also stimulated IL6 and CCL5 secretion by MSCs, the latter of which led to increased cell migration; reciprocally, MSCs not only promoted PC3 proliferation and colony formation but also protected them from docetaxel-induced toxicity through paracrine mediators (Borghese et al. 2012). Of note, in response to radiation and cytotoxic chemotherapies, stromal cells in the prostate tumor microenvironment were recently shown to secrete paracrine factors, including WNT16B, that promote the survival of adjacent cancer cells and lead to enhanced therapeutic resistance (Sun et al. 2012). Ye et al. (2012) have shown that media conditioned by human BM-MSCs not only upregulates MMP-2/-9 expression in PC3 cells but also promotes their migration and invasion via TGF-β signaling pathways. Additionally, oncostatin M was recently shown to induce both TGF-β1 and periostin expression in human ADSCs and promote PC3 adhesion (Lee et al. 2013).
To date, there have only been a limited number of studies investigating the effect of MSCs on prostate tumor growth in vivo, and the majority of those have demonstrated little to no effect (Table 1). It should be mentioned, however, that most of these studies have utilized the PC3 cell line, and therefore, these analyses should be extended into other models before making generalized conclusions. Khakoo et al. (2006) demonstrated that human BM-MSCs suppress tumor growth in a model of Kaposi’s sarcoma by inhibiting Akt activation in a cell contact-dependent manner; however, when co-cultured with PC3 cells, these same MSCs had no effect on phospho-Akt levels, nor did they alter xenograft growth in immunocompromised animals. No effect on tumor weight or animal survival was observed in mice bearing PC3 tumors who received three weekly i.v. injections of 2×106 human BM-MSCs (Wang et al. 2012). Rat BM-MSCs transduced with the herpes simplex virus thymidine kinase gene (HSV-TK) had no effect on PC3 xenograft growth in the absence of ganciclovir treatment (Song et al. 2011). Additionally, human BM-MSCs had no effect on tumor take or growth rates when co-injected with DU145 cells (Pessina et al. 2011). A study by Zhang et al. (2011) demonstrated that rat BM-MSCs injected into already established tumors had no effect on PC3 xenograft growth. Additionally, C3H10T1/2 embryonic murine MSCs co-injected with PC3 cells also had no effect on intratibial tumor growth (Fritz et al. 2008). By contrast, Chanda et al. (2009) showed that adult murine MSCs injected into already established intratibial PC3 tumors suppressed their growth and promoted bone regeneration, although the effect was less pronounced than when the MSCs were co-injected with the tumor cells simultaneously. Using the TRAMP-C2 model, Ren et al. (2008a) demonstrated that murine BM-MSCs had no effect on lung metastasis when injected 10 days post-tumor cell inoculation. Zolochevska et al. (2012) showed that human ADSCs had no effect on PC3 xenograft growth in immunocompromised animals nor did murine ADSC significantly stimulate xenograft growth in the immunocompetent TRAMP-C2-Ras model. Lee et al. (2013) also demonstrated that co-inoculation of human ADSC had no effect on PC3-M xenograft growth in the absence of oncostatin M.
Table 1.
Effects of MSCs on prostate cancer growth in preclinical animal models
| Effect on tumor growth
|
Species (MSC)
|
Tissue source
|
Mouse strain
|
Tumor model
|
Immunogenicity
|
MSC:cancer cell ratio (no. of MSCs)
|
Delivery route
|
Timing (post-inoculation)
|
MSC injections (#)
|
Results
|
Proposed mechanism
|
Reference
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| – | Human | Bone marrow | SCID | PC3 | Allogeneic | 1:6.7 (3×105) | MSC: i.v.; tumor: s.c. | d14, 21, 28+ (35 and 42) | Growth: 3; survival: 5 | No effect on tumor growth or survival | Wang et al. (2012) | |
| – | Human | Bone marrow | Nude | PC3 | Allogeneic | 1:1.25 (4×106) | MSC: i.v.; tumor: s.c. | d0, 3, and 10 | 3 | No effect on tumor growth | Khakoo et al. (2006) | |
| – | Human | Bone marrow | NOD/SCID | DU145 | Allogeneic | 1:5 (0.4×106) | MSC: s.c.; tumor: s.c. | d0 (co-inoculation) | 1 | No effect on tumor take or growth | Pessina et al. (2011) | |
| – | Human | Adipose | Nude | PC3 | Allogeneic | 1:5 (2×105) | MSC: s.c.; tumor: s.c. | d0 (co-inoculation) | 1 | No effect on tumor growth | Zolochevska et al. (2012) | |
| – | Human | Adipose | Nude | PC3-M | Allogeneic | 1:1 (1×106) | MSC: s.c.; tumor: s.c. | d0 (co-inoculation) | 1 | No effect on tumor growth | Lee et al. (2013) | |
| ↓/↑ | Human | Adipose | Nude | PC3 | Allogeneic | s.c.: 2:5 (1.2– 1.6×106) i.v. 1:1.5 or 1:1 (2–3×106) | MSC: s.c. or i.v. tumor: s.c. | s.c.: d0 (co-inoculation) i.v.: d7 and d18 | s.c.: 1 i.v.: 1 or 2 | s.c.: increased tumor growth and mortality by a couple of days i.v.: suppressed tumor growth | None proposed | Cavarretta et al. (2010) |
| ↑ | Human | Adipose | Nude | PC3 | Allogeneic | 1:2 (1×106) | MSC: s.c. (left flank); tumor: s.c. (right flank) | d7 | 1 | Increased tumor growth | CXCL12/CXCR4 axis; increased capillary density; FGF2 expression | Lin et al. (2010) |
| ↑ | Human | Adipose | Nude | MDA-PCa- 118b | Allogeneic | 1:10 (1×105) | MSC: s.c.; tumor: s.c. | d0 (co-inoculation) | 1 | Increased tumor growth | None proposed | Prantl et al. (2010) |
| ↑ | Human | Bone marrow | Nude | PC3 | Allogeneic | 1:5 (1×106) | MSC: s.c.; tumor: s.c. | d0 (co-inoculation) | 1 | Increased tumor growth | TGF-β signaling | Ye et al. (2012) |
| ↑ | Human | Bone marrow | SCID | PC3 | Allogeneic | 1:100 (2×103) | MSC: s.c.; tumor: s.c. | d0 (co-inoculation) | 1 | Increased tumor growth | CXCL16/CXCR6 axis | Jung et al. (2013) |
| ↓ | Mouse | Bone marrow | SCID | PC3 | Allogeneic | 5:1 (5×105) | MSC: I.T.; tumor: I.T. | d1 or d14 | 1 | Suppressed tumor growth effect less pronounced in established tumors; promoted bone regeneration in the presence of cancer cells | New bone formation restricted growth of cancer cells | Chanda et al. (2009) |
| – | Mouse | Bone marrow | SCID | PC3 | Allogeneic | 1:1 and 3:1 (0.5 and 1.5×106) | MSC: I.T.; tumor: I.T. | d0 (co-inoculation) | 1 | No effect on tumor growth at either ratio | Fritz et al. (2008) | |
| – | Mouse | Bone marrow | C57BL/6 | TRAMP-C2 | Syngeneic | 1:2.5 (2×105) | MSC: i.v.; tumor: i.v. | d10 | 1 | No effect on lung metastasis | Ren et al. (2008a, b) | |
| – | Mouse | Adipose | C57BL/6 | TRAMP-C2-Ras | Syngeneic | 1:5 (4×105) | MSC: s.c.; tumor: s.c. | d0 (co-inoculation) | 1 | No effect on tumor growth | Zolochevska et al. (2012) | |
| – | Rat | Bone marrow | Nude | PC3 | Allogeneic | 1:20 (1×106) | MSC: i.v.; tumor: s.c. | d3 and 10 | 2 | No effect on tumor growth | Song et al. (2011) | |
| – | Rat | Bone marrow | Nude | PC3 | Allogeneic | 1:1 (2×106) | MSC: I.T.; tumor: s.c. | d14, 21, and 28 | 3 | No effect on tumor growth | Zhang et al. |
By contrast, Lin et al. (2010) showed that implanted ADSCs were recruited to PC3 xenografts on the opposite flank via the CXCL12/CXCR4 axis where they stimulated tumor growth, at least partially through enhanced angiogenesis and FGF2 expression. A study by Cavarretta et al. (2010) also suggested that unmodified human ADSCs co-injected subcutaneously with PC3 cells accelerated tumor growth and mortality by a few days; however, systemically administered ADSCs expressing cytosine deaminase (CD) significantly suppressed PC3 tumor growth even in the absence of 5-fluorocytosine (5-FC) treatment. Additionally, Prantl et al. (2010) reported increased tumor growth when MDA-PCA-118b cells were co-inoculated with human ADSCs. In contrast to previous reports, Ye et al. (2012) observed a significant increase in tumor volumes when PC3 cells were co-injected with human BM-MSCs. Taichman and colleagues recently reported that human BM-MSC stimulated PC3 xenograft growth when co-inoculated at ratios of 1:100 through a CXCR6/CXCL16-dependent mechanism (Jung et al. 2013). They further demonstrated that the recruitment of murine MSC to murine RM1 prostate cancer tumors in vivo was CXCL16 dependent and the number of MSCs present in the tumor correlated with tumor growth. Furthermore, CXCR6 signaling in BM-MSC induced their conversion to a CXCL12-expressing CAF phenotype, which has been implicated in prostate cancer metastasis (Jung et al. 2013).
These conflicting results regarding the influence of MSCs on prostate cancer growth may be due to differences in the ratio of MSCs to tumor cells, the absolute number of MSCs injected, or the timing of their administration relative to tumor inoculation. Furthermore, there does not seem to be a clear relationship between the immunogenicity of the MSCs and tumor cells used nor the immunological status of the xenograft hosts (Table 1). Of note, a recent study by Marini and colleagues suggested that local ADSCs were more likely to be integrated into the fibrovascular network of the early tumor, whereas their bone marrow-derived counterparts were more likely to be localized to the tumor periphery where they may play a role in tissue remodeling and metastasis (Kidd et al. 2012). Interestingly, there does seem to be a higher incidence of pro-tumorigenic effects observed in experiments using ADSCs compared with BM-MSCs in the prostate-specific studies described earlier and in those reviewed by Klopp et al. (2011), but this is not exclusively true.
Both ADSCs and BM-MSCs have also been shown to give rise to CAF (Fig. 1; Mishra et al. 2008, 2009, Paunescu et al. 2011, Kidd et al. 2012, Jung et al. 2013), which have been implicated in nearly all stages of prostate cancer carcinogenesis, including initiation, progression, invasion, and metastasis (Chung 1991, Olumi et al. 1999, Bhowmick et al. 2004, Franco et al. 2010, Giannoni et al. 2010, Brennen et al. 2012, Li et al. 2012). Human prostate-derived CAF co-implanted with initiated but non-tumorigenic human prostate epithelium into immunocompromised murine hosts significantly enhances tumor growth (Olumi et al. 1999). Loss of TGF-β responsiveness in fibroblasts through genetic manipulation results in murine PIN-like lesions (Bhowmick et al. 2004) and promotes mixed bone lesions in intratibial models of metastasis (Li et al. 2012). Conditioned media from activated fibroblasts promotes epithelial-to-mesenchymal transition in PC3 cells in vitro, in addition to stimulating invasiveness and prostasphere formation (Giannoni et al. 2010). These same authors went on to demonstrate that prostate-derived CAF enhanced PC3 aggressiveness in vivo by promoting tumor formation and facilitating lung micrometastases (Giannoni et al. 2010). The role of CAF in the progression of tumors from multiple tissues, including breast, colon, and pancreas in addition to the prostate, are well described and have been extensively reviewed elsewhere (Kalluri & Zeisberg 2006, Orimo & Weinberg 2006, Franco et al. 2010, Shimoda et al. 2010). Our own studies suggest that CAF derived from human prostates are enriched in MSCs (Brennen et al. 2013). These seemingly contradictory observations regarding the well-known tumor-promoting properties of CAF and the lack of any effect in the majority of the studies described earlier using MSCs serves to further reinforce the idea that MSCs isolated from different compartments have divergent phenotypes. Perhaps unsurprisingly, this implies that prostate-derived CAF are different than the BM-MSCs from which at least a subset of them is derived. These differences likely arise as a result of their developmental origin and distinct signaling events received through interactions with the tissue and tumor microenvironments in which they are found. Additionally, these CAF may pass through an ADSC intermediate stage depending on their mode of recruitment, which may further add to the complexity and heterogeneity observed in the phenotypic and functional differences observed in these cells (Kidd et al. 2012).
MSCs: tumor-targeting vectors
Available evidence strongly suggests that the inherent tropism of MSCs for tumor tissue can be exploited to deliver therapeutic and diagnostic agents. Indeed, much preclinical work has already been performed in this area using MSCs derived from a variety of species and tissue sources (Ciavarella et al. 2011, Shah 2012). In addition to the tumor-targeting properties of MSCs, their immunoprivileged nature suggests that large quantities of these cells can be harvested from a healthy donor, expanded, and manipulated ex vivo prior to infusion into multiple allogeneic patients as an ’off-the-shelf’ therapy. This latter point not only makes this therapeutic strategy more practical with regard to time and cost but also alleviates ethical considerations related to re-infusing the cancer patient’s own (autologous) cells with regard to their potential to influence tumor malignancy.
A common theme of these strategies is to utilize genetic engineering techniques to generate MSCs that express various molecules with anticancer properties, which are then delivered to the tumor by the MSCs via systemic circulation. Generally, these MSC-delivered anticancer agents fall into one of several categories: immunostimulatory agents, oncolytic viruses, growth factor antagonists, pro-apoptotic factors, anti-angiogenic compounds, or prodrug-converting enzymes. Marini et al. pioneered the use of adenoviral transduced MSCs to deliver IFN-β to sites of cancer and have demonstrated efficacy in preclinical models of melanoma, breast, and pancreatic cancer (Studeny et al. 2002, 2004, Kidd et al. 2010). Delivery of IFN-β by genetically engineered MSCs has also shown efficacy in models of prostate bone and lung metastasis (Ren et al. 2008a, Chanda et al. 2009). Additional immunostimulatory agents, including IL2, IL7, IL12, IL18, IL23, and CX3CL1, have also been engineered into the MSC genome and used to treat a variety of preclinical cancer models, such as glioma, melanoma, Ewing’s sarcoma, and renal cell carcinoma (Nakamura et al. 2004, Elzaouk et al. 2006, Duan et al. 2009, Gao et al. 2010, Gunnarsson et al. 2010).
Multiple groups have also begun developing MSCs as delivery vectors for oncolytic viruses (Nakashima et al. 2010). Cell-based delivery of oncolytic viruses cannot only enhance the tumor-targeting potential of these viruses but can also reduce their neutralization by shielding them from pre-exisntig antiviral antibodies (Mader et al. 2009, Huang et al. 2013). Dembinski et al. (2010) demonstrated that delivery of a conditionally replicating fiber-modified adenoviral vector using MSCs reduced off-target infection and systemic toxicity following i.p. injection in a model of disseminated ovarian cancer. The Pereboeva and Curiel groups have also shown increased efficacy and survival following therapy with conditionally replicating adenovirus-transduced MSCs in ovarian xenograft and breast cancer lung metastasis models (Komarova et al. 2006, Stoff-Khalili et al. 2007).
The delivery of various prodrug-converting enzymes, including carboxylesterases, CD, and HSV-TK, have also generated provocative results in various preclinical models. Co-inoculation of MSCs expressing HSV-TK with PC3 prostate cancer cells inhibited xenograft growth when treated with ganciclovir, but not in its absence (Song et al. 2011). Furthermore, systemically delivered MSCs expressing HSV-TK showed efficacy against orthotopic pancreatic and hepatic xenograft growth and reduced the incidence of pancreatic metastasis (Zischek et al. 2009, Niess et al. 2011). Altaner and colleagues demonstrated that both co-inoculated and systemically administered ADSCs engineered to express CD significantly reduced tumor burden in animals bearing PC3 prostate cancer xenografts following daily doses of 5-FC (Cavarretta et al. 2010). This same group has also shown efficacy against HT-29 colon cancer and A375 melanoma xenograft growth in vivo using CD-transduced ADSCs (Kucerova et al. 2007, 2008). Additionally, MSCs engineered to express carboxylesterase, which metabolizes CPT-11 into an active topo-isomerase I inhibitor (SN-38), have shown efficacy against mouse models of glioma (Yin et al. 2011, Choi et al. 2012).
Additional strategies seeking to utilize the tumor-targeting properties of MSCs include the delivery of pro-apoptotic factors, such as TRAIL (Grisendi et al. 2010, Shah 2012); anti-angiogenic agents, such as thrombospondin-1 (van Eekelen et al. 2010) and endostatin (Yin et al. 2011); and growth factor antagonists, such as NK4 (Kanehira et al. 2007). An interesting approach recently described by Spitzweg et al. permits both imaging and therapy to be performed using MSCs transfected with the sodium iodide symporter (NIS), which is normally responsible for concentrating iodide in the thyroid (Knoop et al. 2011). NIS expression not only resulted in selective accumulation of iodine in hepatocellular tumors in mice, which made both 123I scintigraphy and 124I PET imaging possible but also abrogated xenograft growth following systemic administration of the radionuclide 131I.
While these strategies have shown great promise in numerous preclinical models, none have entered into clinical trials yet, although the relatively short time frame since their inception precludes any judgment on their eventual clinical potential. In fact, the world’s first unmodified MSC therapy only received approval as recently as 2012 in Canada for the treatment of GVHD (Osiris Therapeutics 2012). However, one attribute of these approaches that may ultimately harm their clinical translation is the failure to take into consideration the trafficking of MSCs to multiple sites throughout the body in addition to the tumor following systemic infusion after the initial entrapment in the lung, including the spleen, kidneys, liver, bone marrow, and other sites of inflammation and remodeling (Gao et al. 2001, Devine et al. 2003, Allers et al. 2004, Detante et al. 2009, Choi et al. 2011, von Bahr et al. 2012). This may increase the off-target/non-tumor effects and systemic toxicity associated with these therapies following infusion. One strategy to circumvent these potential off-target effects is the use of MSCs to deliver prodrugs that are activated in a tumor- or tissue-specific manner. As one example, ongoing studies in our own laboratory in collaboration with multiple other groups are seeking to develop MSCs as vectors to deliver prostate-specific antigen (PSA)-activated prodrugs (Denmeade et al. 2003) and protoxins (Williams et al. 2007) to sites of metastatic prostate cancer using multiple therapeutic platforms, including nanoparticle-loading strategies and genetic manipulation (Brennen et al. 2013). In this therapeutic scenario, prodrugs delivered by MSCs to nontarget tissues will not be activated due to the lack of enzymatically active PSA, which is only present in the prostate and at sites of prostate cancer metastases, thereby reducing systemic toxicity. Additionally, Karp and colleagues have demonstrated that MSC homing and engraftment in inflamed tissue can be increased by decorating their surface with proteins involved in leukocyte extravasation (Sarkar et al. 2011). Cell engineering strategies such as this and continued optimization of viral transduction methods for MSCs (Lin et al. 2012) will help translate these strategies into the clinic more efficiently.
Summary
In summary, MSCs have emerged as critical regulators of the immune response. The role of these cells in both the innate and adaptive immunity is complex and has yet to be fully elucidated. MSCs have a multitude of immunosuppressive properties through effects on nearly every component of the immune system. Additionally, MSCs also have immunostimulatory effects on many of these same components under specific conditions, particularly during the initial phases of an immunological assault. The balance between these competing forces, which is dictated by IFN-γ and the rest of the inflammatory cytokine milieu, plays a role in numerous pathological maladies, including cancer. MSCs and their progeny have a complex role in tumor biology with both pro- and anti-tumorigenic effects being described. While the immunomodulatory properties of these cells certainly play an important role in this relationship, available evidence suggests that the whole story is far more complex and dependent on numerous interactions with other cells present in the tumor microenvironment, both static residents and dynamic infiltrators. However, despite the incomplete understanding of MSC physiology, current data strongly suggest that these cells have an inherent tropism for tumor tissue based on the inflammatory microenvironment frequently present. These tumor trafficking properties, immunoprivileged nature, and expansion capabilities have the potential for exploitation as a cell-based delivery vector for therapeutic and diagnostic purposes. Cell-based treatment modalities attempting to harness the bodies’ own physiology for therapeutic benefit have gained traction over the last few years in a variety of diseases and are sure to represent a growing trend in promising anticancer strategies of the future.
Acknowledgments
The authors would like to acknowledge the Department of Defense (W N Brennen, Post-Doctoral Fellowship, W81XWH-12-1-0049), the Patrick C Walsh Fund (J T Isaacs), the Maryland Stem Cell Research Fund (J T Isaacs, RFA-MD-07-01), the Prostate Cancer Foundation/Movember Challenge Award (J T Isaacs), and National Cancer Institute Comprehensive Center (P50CA058236) and SPORE (P30CA006973) grants for their financial support.
Funding
S R Denmeade and J T Isaacs are consultants for GenSpera, Inc. and have received equity and financial compensation.
Footnotes
Declaration of interest
S R Denmeade and J T Isaacs have licensed PRX302 to and receive royalties from Sophiris Bio, Corp. Both these relationships for S R Denmeade and J T Isaacs have been disclosed and are under the management of the Johns Hopkins University School of Medicine Conflict of Interest Committee.
References
- Afizah H, Yang Z, Hui JH, Ouyang HW, Lee EH. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Engineering. 2007;13:659–666. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Allers C, Sierralta WD, Neubauer S, Rivera F, Minguell JJ, Conget PA. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation. 2004;78:503–508. doi: 10.1097/01.TP.0000128334.93343.B3. [DOI] [PubMed] [Google Scholar]
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends in Molecular Medicine. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. European Journal of Immunology. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- Barry FP, Murphy JM, English K, Mahon BP. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells and Development. 2005;14:252–265. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Reviews. 2010;29:249–261. doi: 10.1007/s10555-010-9222-7. [DOI] [PubMed] [Google Scholar]
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. Journal of Urology. 1984;132:474–479. doi: 10.1242/jcs.02511. [DOI] [PubMed] [Google Scholar]
- Bertani N, Malatesta P, Volpi G, Sonego P, Perris R. Neurogenic potential of human mesenchymal stem cells revisited: analysis by immunostaining, time-lapse video and microarray. Journal of Cell Science. 2005;118:3925–3936. doi: 10.1242/jcs.02511. [DOI] [PubMed] [Google Scholar]
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Borgonovo G, Pistoia V, Raffaghello L. Immunosuppres-sive cells and tumour microenvironment: focus on mesenchymal stem cells and myeloid derived suppressor cells. Histology and Histopathology. 2011;26:941–951. doi: 10.14670/HH-26.941. [DOI] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nature Medicine. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi A, Martino C, Balducci L, Saldarelli M, Soleti A, Navone SE, Canzi L, Cristini S, Invernici G, Parati EA, et al. Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angio-genic potential. Vascular Cell. 2011;3:5. doi: 10.1186/2045-824X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocelli-Tyndall C, Bracci L, Schaeren S, Feder-Mengus C, Barbero A, Tyndall A, Spagnoli GC. Human bone marrow mesenchymal stem cells and chondrocytes promote and/or suppress the in vitro proliferation of lymphocytes stimulated by interleukins 2, 7 and 15. Annals of the Rheumatic Diseases. 2009;68:1352–1359. doi: 10.1136/ard.2008.094003. [DOI] [PubMed] [Google Scholar]
- Borghese C, Cattaruzza L, Pivetta E, Normanno N, De Luca A, Mazzucato M, Celegato M, Colombatti A, Aldinucci D. Gefitinib inhibits the cross-talk between mesenchymal stem cells and prostate cancer cells leading to tumor cell proliferation and inhibition of docetaxel activity. Journal of Cellular Biochemistry. 2012;114:1135–1144. doi: 10.1002/jcb.24456. [DOI] [PubMed] [Google Scholar]
- Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibro-blasts as a novel chemotherapeutic strategy. Molecular Cancer Therapeutics. 2012;11:257–266. doi: 10.1158/1535-7163.MCT-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennen WN, Chen S, Denmeade SR, Isaacs JT. Quantification of mesenchymal stem cells (MSCs) at sites of human prostate cancer. Oncotarget. 2013;4:106–117. doi: 10.18632/oncotarget.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohlin M, Kingham PJ, Novikova LN, Novikov LN, Wiberg M. Aging effect on neurotrophic activity of human mesenchymal stem cells. PLoS ONE. 2012;7:e45052. doi: 10.1371/journal.pone.0045052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Nemeth K, Kushnir-Sukhov NM, Metcalfe DD, Mezey E. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clinical and Experimental Allergy. 2011;41:526–534. doi: 10.1111/j.1365-2222.2010.03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. Journal of Orthopaedic Research. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Why are MSCs therapeutic? New data: new insight. Journal of Pathology. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavarretta IT, Altanerova V, Matuskova M, Kucerova L, Culig Z, Altaner C. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Molecular Therapy. 2010;18:223–231. doi: 10.1038/mt.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-γ. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda D, Isayeva T, Kumar S, Hensel JA, Sawant A, Ramaswamy G, Siegal GP, Beatty MS, Ponnazhagan S. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in prostate cancer bone metastasis. Clinical Cancer Research. 2009;15:7175–7185. doi: 10.1158/1078-0432.CCR-09-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Ahn SY, Kim TS, Kim J, Kim BG, Han KH, Ban SJ, Kim HS, Choi Y, Lim CJ. Characterization and biodistribution of human mesenchymal stem cells transduced with lentiviral-mediated BMP2. Archives of Pharmacal Research. 2011;34:599–606. doi: 10.1007/s12272-011-0410-y. [DOI] [PubMed] [Google Scholar]
- Choi SA, Lee JY, Wang KC, Phi JH, Song SH, Song J, Kim SK. Human adipose tissue-derived mesenchymal stem cells: characteristics and therapeutic potential as cellular vehicles for prodrug gene therapy against brainstem gliomas. European Journal of Cancer. 2012;48:129–137. doi: 10.1016/j.ejca.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Chung LW. Fibroblasts are critical determinants in prostatic cancer growth and dissemination. Cancer Metastasis Reviews. 1991;10:263–274. doi: 10.1007/BF00050797. [DOI] [PubMed] [Google Scholar]
- Ciavarella S, Dominici M, Dammacco F, Silvestris F. Mesenchymal stem cells: a new promise in anticancer therapy. Stem Cells and Development. 2011;20:1–10. doi: 10.1089/scd.2010.0223. [DOI] [PubMed] [Google Scholar]
- Clouston D, Bolton D. In situ and intraductal epithelial proliferations of prostate: definitions and treatment implications. Part 1: prostatic intraepithelial neoplasia. BJU International. 2012;109(Suppl 3):22–26. doi: 10.1111/j.1464-410X.2012.11040.x. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, Tomchuck SL, Honer zu Bentrup K, Danka ES, Henkle SL, et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. PNAS. 2009;106:3806–3811. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resistance Updates. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. International Journal of Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- Davani B, Ikonomou L, Raaka BM, Geras-Raaka E, Morton RA, Marcus-Samuels B, Gershengorn MC. Human islet-derived precursor cells are mesenchymal stromal cells that differentiate and mature to hormone-expressing cells in vivo. Stem Cells. 2007;25:3215–3222. doi: 10.1634/stemcells.2007-0323. [DOI] [PubMed] [Google Scholar]
- Dayyani F, Gallick GE, Logothetis CJ, Corn PG. Novel therapies for metastatic castrate-resistant prostate cancer. Journal of the National Cancer Institute. 2011;103:1665–1675. doi: 10.1093/jnci/djr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delongchamps NB, Singh A, Haas GP. The role of prevalence in the diagnosis of prostate cancer. Cancer Control. 2006;13:158–168. doi: 10.1177/107327480601300302. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. American Journal of Pathology. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nature Reviews. Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembinski JL, Spaeth EL, Fueyo J, Gomez-Manzano C, Studeny M, Andreeff M, Marini FC. Reduction of nontarget infection and systemic toxicity by targeted delivery of conditionally replicating viruses transported in mesenchymal stem cells. Cancer Gene Therapy. 2010;17:289–297. doi: 10.1038/cgt.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmeade SR, Jakobsen CM, Janssen S, Khan SR, Garrett ES, Lilja H, Christensen SB, Isaacs JT. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. Journal of the National Cancer Institute. 2003;95:990–1000. doi: 10.1093/jnci/95.13.990. [DOI] [PubMed] [Google Scholar]
- De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schroder F, Sciarra A, Tubaro A. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. European Urology. 2011;60:106–117. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells, Tissues, Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- Detante O, Moisan A, Dimastromatteo J, Richard MJ, Riou L, Grillon E, Barbier E, Desruet MD, De Fraipont F, Segebarth C, et al. Intravenous administration of 99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: in vivo imaging and biodistribu-tion. Cell Transplantation. 2009;18:1369–1379. doi: 10.3727/096368909X474230. [DOI] [PubMed] [Google Scholar]
- Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noel D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Duan X, Guan H, Cao Y, Kleinerman ES. Murine bone marrow-derived mesenchymal stem cells as vehicles for interleukin-12 gene delivery into Ewing sarcoma tumors. Cancer. 2009;115:13–22. doi: 10.1002/cncr.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen M, Sasportas LS, Kasmieh R, Yip S, Figueiredo JL, Louis DN, Weissleder R, Shah K. Human stem cells expressing novel TSP-1 variant have anti-angiogenic effect on brain tumors. Oncogene. 2010;29:3185–3195. doi: 10.1038/onc.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- Elzaouk L, Moelling K, Pavlovic J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Experimental Dermatology. 2006;15:865–874. doi: 10.1111/j.1600-0625.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- English K, Mahon BP. Allogeneic mesenchymal stem cells: agents of immune modulation. Journal of Cellular Biochemistry. 2011;112:1963–1968. doi: 10.1002/jcb.23119. [DOI] [PubMed] [Google Scholar]
- English K, Barry FP, Field-Corbett CP, Mahon BP. IFN-γ and TNF-α differentially regulate immunomodulation by murine mesenchymal stem cells. Immunology Letters. 2007;110:91–100. doi: 10.1016/j.imlet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Seminars in Cell & Developmental Biology. 2010;21:33–39. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and Tissue Kinetics. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Experimental Hematology. 1976;4:267–274. [PubMed] [Google Scholar]
- Fritz V, Noel D, Bouquet C, Opolon P, Voide R, Apparailly F, Louis-Plence P, Bouffi C, Drissi H, Xie C, et al. Antitumoral activity and osteogenic potential of mesenchymal stem cells expressing the urokinase-type plasminogen antagonist amino-terminal fragment in a murine model of osteolytic tumor. Stem Cells. 2008;26:2981–2990. doi: 10.1634/stemcells.2008-0139. [DOI] [PubMed] [Google Scholar]
- Fritz V, Brondello JM, Gordeladze JO, Reseland JE, Bony C, Yssel H, Noel D, Jorgensen C. Bone-metastatic prostate carcinoma favors mesenchymal stem cell differentiation toward osteoblasts and reduces their osteoclastogenic potential. Journal of Cellular Biochemistry. 2011;112:3234–3245. doi: 10.1002/jcb.23258. [DOI] [PubMed] [Google Scholar]
- Fujita K, Ewing CM, Getzenberg RH, Parsons JK, Isaacs WB, Pavlovich CP. Monocyte chemotactic protein-1 (MCP-1/CCL2) is associated with prostatic growth dysregulation and benign prostatic hyperplasia. Prostate. 2010;70:473–481. doi: 10.1002/pros.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells, Tissues, Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- Gao P, Ding Q, Wu Z, Jiang H, Fang Z. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Letters. 2010;290:157–166. doi: 10.1016/j.canlet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Garcia S, Bernad A, Martin MC, Cigudosa JC, Garcia-Castro J, de la Fuente R. Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Experimental Cell Research. 2010;316:1648–1650. doi: 10.1016/j.yexcr.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Garcia-Gomez I, Elvira G, Zapata AG, Lamana ML, Ramirez M, Castro JG, Arranz MG, Vicente A, Bueren J, Garcia-Olmo D. Mesenchymal stem cells: biological properties and clinical applications. Expert Opinion on Biological Therapy. 2010;10:1453–1468. doi: 10.1517/14712598.2010.519333. [DOI] [PubMed] [Google Scholar]
- Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial–mesenchymal transition and cancer stemness. Cancer Research. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- Grisendi G, Bussolari R, Cafarelli L, Petak I, Rasini V, Veronesi E, De Santis G, Spano C, Tagliazzucchi M, Barti-Juhasz H, et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Research. 2010;70:3718–3729. doi: 10.1158/0008-5472.CAN-09-1865. [DOI] [PubMed] [Google Scholar]
- Gunnarsson S, Bexell D, Svensson A, Siesjo P, Darabi A, Bengzon J. Intratumoral IL-7 delivery by mesenchymal stromal cells potentiates IFNgamma-transduced tumor cell immunotherapy of experimental glioma. Journal of Neuroimmunology. 2010;218:140–144. doi: 10.1016/j.jneuroim.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. Journal of the American Medical Association. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, List C, Habisch HJ, Vukicevic V, Ehrhart-Bornstein M, Brenner R, Bernstein P, Fickert S, Storch A. Age-dependent neuroectodermal differentiation capacity of human mesenchymal stromal cells: limitations for autologous cell replacement strategies. Cytotherapy. 2010;12:17–30. doi: 10.3109/14653240903313941. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovascular Research. 1996;32:687–698. doi: 10.1016/S0008-6363(96)00063-6. [DOI] [PubMed] [Google Scholar]
- Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320–330. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. PNAS. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. Journal of Orthopaedic Research. 2005;23:1383–1389. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCU-LATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- Huang YF, Chen MJ, Wu MH, Hung SC. The use of hypoxic cultured mesenchymal stem cell for oncolytic virus therapy. Cancer Gene Therapy. 2013;20:308–316. doi: 10.1038/cgt.2013.22. [DOI] [PubMed] [Google Scholar]
- Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochemical and Biophysical Research Communications. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CM, Flies DB, Mosse CA, Parwani A, Hipkiss EL, Drake CG. Strain-specific induction of experimental autoimmune prostatitis (EAP) in mice. Prostate. 2012;73:651–656. doi: 10.1002/pros.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen BJ, Gilissen C, Roelofs H, Schaap-Oziemlak A, Veltman JA, Raymakers RA, Jansen JH, Kogler G, Figdor CG, Torensma R, et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells and Development. 2010;19:481–490. doi: 10.1089/scd.2009.0288. [DOI] [PubMed] [Google Scholar]
- Jiang R, Han Z, Zhuo G, Qu X, Li X, Wang X, Shao Y, Yang S, Han ZC. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Frontiers of Medicine. 2011;5:94–100. doi: 10.1007/s11684-011-0116-z. [DOI] [PubMed] [Google Scholar]
- Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nature Communications. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature Reviews. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kanehira M, Xin H, Hoshino K, Maemondo M, Mizuguchi H, Hayakawa T, Matsumoto K, Nakamura T, Nukiwa T, Saijo Y. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Therapy. 2007;14:894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplantation. 2006;37:967–976. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. Journal of Experimental Medicine. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, Shi Y, Abbruzzese J, Konopleva M, Andreeff M, et al. Mesenchymal stromal cells alone or expressing interferon-β suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12:615–625. doi: 10.3109/14653241003631815. [DOI] [PubMed] [Google Scholar]
- Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, Andreeff M, Marini FC. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS ONE. 2012;7:e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., III Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop K, Kolokythas M, Klutz K, Willhauck MJ, Wunderlich N, Draganovici D, Zach C, Gildehaus FJ, Boning G, Goke B, et al. Image-guided, tumor stroma-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated NIS gene delivery. Molecular Therapy. 2011;19:1704–1713. doi: 10.1038/mt.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Molecular Cancer Therapeutics. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? European Urology. 2007;51:1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- Krampera M, Marconi S, Pasini A, Galie M, Rigotti G, Mosna F, Tinelli M, Lovato L, Anghileri E, Andreini A, et al. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone. 2007;40:382–390. doi: 10.1016/j.bone.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Research. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- Kucerova L, Matuskova M, Pastorakova A, Tyciakova S, Jakubikova J, Bohovic R, Altanerova V, Altaner C. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. Journal of Gene Medicine. 2008;10:1071–1082. doi: 10.1002/jgm.1239. [DOI] [PubMed] [Google Scholar]
- La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunology and Cell Biology. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. Journal of Clinical Investigation. 2007;117:989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzoni G, Alviano F, Marchionni C, Bonsi L, Costa R, Foroni L, Roda G, Belluzzi A, Caponi A, Ricci F, et al. Isolation of stem cell populations with trophic and immunoregulatory functions from human intestinal tissues: potential for cell therapy in inflammatory bowel disease. Cytotherapy. 2009;11:1020–1031. doi: 10.3109/14653240903253840. [DOI] [PubMed] [Google Scholar]
- Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, et al. Cotrans-plantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biology of Blood and Marrow Transplantation. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Experimental Hematology. 2003a;31:890–896. doi: 10.1016/S0301-472X(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scandinavian Journal of Immunology. 2003b;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. Journal of Clinical Investigation. 2010;120:3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Heo SC, Shin SH, Kwon YW, Do EK, Suh DS, Yoon MS, Kim JH. Oncostatin M promotes mesenchymal stem cell-stimulated tumor growth through a paracrine mechanism involving periostin and Betaig-h3. International Journal of Biochemistry & Cell Biology. 2013;45:1869–1877. doi: 10.1016/j.biocel.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 2012;120:3436–3443. doi: 10.1182/blood-2012-03-420612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sterling JA, Fan KH, Vessella RL, Shyr Y, Hayward SW, Matrisian LM, Bhowmick NA. Loss of TGF-β responsiveness in prostate stromal cells alters chemokine levels and facilitates the development of mixed osteoblastic/osteolytic bone lesions. Molecular Cancer Research. 2012;10:494–503. doi: 10.1158/1541-7786.MCR-11-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang D, Liang J, Zhang H, Sun L. Mesenchymal SCT ameliorates refractory cytopenia in patients with systemic lupus erythematosus. Bone Marrow Transplantation. 2013;48:544–550. doi: 10.1038/bmt.2012.184. [DOI] [PubMed] [Google Scholar]
- Lin VK, Wang SY, Vazquez DV, Xu C, Zhang S, Tang L. Prostatic stromal cells derived from benign prostatic hyperplasia specimens possess stem cell like property. Prostate. 2007;67:1265–1276. doi: 10.1002/pros.20599. [DOI] [PubMed] [Google Scholar]
- Lin G, Yang R, Banie L, Wang G, Ning H, Li LC, Lue TF, Lin CS. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010;70:1066–1073. doi: 10.1002/pros.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Lin Y, Lennon DP, Correa D, Schluchter M, Caplan AI. Efficient lentiviral transduction of human mesenchymal stem cells that preserves proliferation and differentiation capabilities. Stem Cells Translational Medicine. 2012;1:886–897. doi: 10.5966/sctm.2012-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26:279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- Liu AY, Roudier MP, True LD. Heterogeneity in primary and metastatic prostate cancer as defined by cell surface CD profile. American Journal of Pathology. 2004;165:1543–1556. doi: 10.1016/S0002-9440(10)63412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. Journal of Immunology. 2006;176:2864–2871. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- Mader EK, Maeyama Y, Lin Y, Butler GW, Russell HM, Galanis E, Russell SJ, Dietz AB, Peng KW. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an ortho-topic ovarian cancer therapy model. Clinical Cancer Research. 2009;15:7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Research. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PJ, Glod JW, Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Research. 2009;69:1255–1258. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Mabuchi Y, Niibe K, Suzuki S, Nagoshi N, Sunabori T, Shimmura S, Nagai Y, Nakagawa T, Okano H, et al. Development of mesenchymal stem cells partially originate from the neural crest. Biochemical and Biophysical Research Communications. 2009;379:1114–1119. doi: 10.1016/j.bbrc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826–4835. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- Musina RA, Bekchanova ES, Belyavskii AV, Sukhikh GT. Differentiation potential of mesenchymal stem cells of different origin. Bulletin of Experimental Biology and Medicine. 2006;141:147–151. doi: 10.1007/s10517-006-0115-2. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Therapy. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Kaur B, Chiocca EA. Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine & Growth Factor Reviews. 2010;21:119–126. doi: 10.1016/j.cytogfr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immuno-genic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. New England Journal of Medicine. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflammation & Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. European Urology. 2008;54:1379–1384. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer P, Vohrer J, Schmal H, Kasten P, Fellenberg J, Suedkamp NP, Mehlhorn AT. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008;10:784–795. doi: 10.1080/14653240802419302. [DOI] [PubMed] [Google Scholar]
- Niess H, Bao Q, Conrad C, Zischek C, Notohamiprodjo M, Schwab F, Schwarz B, Huss R, Jauch KW, Nelson PJ, et al. Selective targeting of genetically engineered mesenchymal stem cells to tumor stroma microenvironments using tissue-specific suicide gene expression suppresses growth of hepatocellular carcinoma. Annals of Surgery. 2011;254:767–774. doi: 10.1097/SLA.0b013e3182368c4f. discussion 774–765. [DOI] [PubMed] [Google Scholar]
- Noel D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, Jorgensen C, Cousin B. Cell specific differences between human adipose-derived and mesenchymal–stromal cells despite similar differentiation potentials. Experimental Cell Research. 2008;314:1575–1584. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nature Reviews. Molecular Cell Biology. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson LB, Varas L, Kjellman C, Edvardsen K, Lindvall M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Experimental and Molecular Pathology. 2003;75:248–255. doi: 10.1016/j.yexmp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Research. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- Osiris Therapeutics I. World’s First Approved Stem Cell Drug. In: Elchin E, editor. Osiris Receives Marketing Clearance from Health Canada for Prochymal. Columbia, MD: 2012. (available at: http://investor.osiris.com/releasedetail.cfm?ReleaseID=674658) [Google Scholar]
- Osterholm MT. Preparing for the next pandemic. New England Journal of Medicine. 2005;352:1839–1842. doi: 10.1056/NEJMp058068. [DOI] [PubMed] [Google Scholar]
- Pachon-Pena G, Yu G, Tucker A, Wu X, Vendrell J, Bunnell BA, Gimble JM. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. Journal of Cellular Physiology. 2011;226:843–851. doi: 10.1002/jcp.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepucci RA, Siufi JL, Silva WA, Jr, Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT, Zago MA. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22:1263–1278. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- Park HW, Shin JS, Kim CW. Proteome of mesenchymal stem cells. Proteomics. 2007;7:2881–2894. doi: 10.1002/pmic.200700089. [DOI] [PubMed] [Google Scholar]
- Paunescu V, Bojin FM, Tatu CA, Gavriliuc OI, Rosca A, Gruia AT, Tanasie G, Bunu C, Crisnic D, Gherghiceanu M, et al. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. Journal of Cellular and Molecular Medicine. 2011;15:635–646. doi: 10.1111/j.1582-4934.2010.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessina A, Bonomi A, Cocce V, Invernici G, Navone S, Cavicchini L, Sisto F, Ferrari M, Vigano L, Locatelli A, et al. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PLoS ONE. 2011;6:e28321. doi: 10.1371/journal.pone.0028321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- Pevsner-Fischer M, Levin S, Zipori D. The origins of mesenchymal stromal cell heterogeneity. Stem Cell Reviews. 2011;7:560–568. doi: 10.1007/s12015-011-9229-7. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Placencio VR, Li X, Sherrill TP, Fritz G, Bhowmick NA. Bone marrow derived mesenchymal stem cells incorporate into the prostate during regrowth. PLoS ONE. 2010;5:e12920. doi: 10.1371/journal.pone.0012920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prantl L, Muehlberg F, Navone NM, Song YH, Vykoukal J, Logothetis CJ, Alt EU. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate. 2010;70:1709–1715. doi: 10.1002/pros.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, Monroy R, Kurtzberg J. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biology of Blood and Marrow Transplantation. 2011;17:534–541. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- PRNewswire. Positive Results from Phase 2 Trial of Mesoblast’s Adult Stem Cell Therapy. Presented at the American Heart Association Annual Meeting; Orlando, FL and Melbourne, Australia. 2011. (available at: www.prnewswire.com/news-releases/positive-results-from-phase-2-trial-of-mesoblasts-adult-stem-cell-therapy-presented-at-the-american-heart-association-annual-meeting-133835958.html) [Google Scholar]
- Prockop DJ. “Stemness”, does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clinical Pharmacology and Therapeutics. 2007;82:241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Two negative feedback loops place mesenchymal stem/stromal cells (MSCs) at the center of early regulators of inflammation. Stem Cells. 2013 doi: 10.1002/stem.1400. [in press] [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Molecular Therapy. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L, Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Research. 2008;18:500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- Rada T, Reis RL, Gomes ME. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Reviews. 2011;7:64–76. doi: 10.1007/s12015-010-9147-0. [DOI] [PubMed] [Google Scholar]
- Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Experimental Cell Research. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Ren C, Kumar S, Chanda D, Kallman L, Chen J, Mountz JD, Ponnazhagan S. Cancer gene therapy using mesenchymal stem cells expressing interferon-β in a mouse prostate cancer lung metastasis model. Gene Therapy. 2008a;15:1446–1453. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008b;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis and Rheumatism. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- Santamaria-Martinez A, Barquinero J, Barbosa-Desongles A, Hurtado A, Pinos T, Seoane J, Poupon MF, Morote J, Reventos J, Munell F. Identification of multipotent mesenchymal stromal cells in the reactive stroma of a prostate cancer xenograft by side population analysis. Experimental Cell Research. 2009;315:3004–3013. doi: 10.1016/j.yexcr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Spencer JA, Phillips JA, Zhao W, Schafer S, Spelke DP, Mortensen LJ, Ruiz JP, Vemula PK, Sridharan R, et al. Engineered cell homing. Blood. 2011;118:e184–e191. doi: 10.1182/blood-2010-10-311464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60:199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. Mesenchymal stem cells engineered for cancer therapy. Advanced Drug Delivery Reviews. 2012;64:739–748. doi: 10.1016/j.addr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Seminars in Cell & Developmental Biology. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Song C, Xiang J, Tang J, Hirst DG, Zhou J, Chan KM, Li G. Thymidine kinase gene modified bone marrow mesenchymal stem cells as vehicles for antitumor therapy. Human Gene Therapy. 2011;22:439–449. doi: 10.1089/hum.2010.116. [DOI] [PubMed] [Google Scholar]
- Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Therapy. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell–natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon-γ-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- Stoff-Khalili MA, Rivera AA, Mathis JM, Banerjee NS, Moon AS, Hess A, Rocconi RP, Numnum TM, Everts M, Chow LT, et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Research and Treatment. 2007;105:157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells and Development. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Research. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. Journal of the National Cancer Institute. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. Journal of Cellular Biochemistry. 2003;89:462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS. Treatment-induced damage to the tumor microenvir-onment promotes prostate cancer therapy resistance through WNT16B. Nature Medicine. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin M, Ringden O, Sundberg B, Nava S, Gotherstrom C, Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92:1208–1215. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Technau A, Froelich K, Hagen R, Kleinsasser N. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy. 2011;13:310–317. doi: 10.3109/14653249.2010.504769. [DOI] [PubMed] [Google Scholar]
- Torsvik A, Rosland GV, Svendsen A, Molven A, Immervoll H, McCormack E, Lonning PE, Primon M, Sobala E, Tonn JC, et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track – letter. Cancer Research. 2010;70:6393–6396. doi: 10.1158/0008-5472.CAN-10-1305. [DOI] [PubMed] [Google Scholar]
- True LD, Zhang H, Ye M, Huang CY, Nelson PS, von Haller PD, Tjoelker LW, Kim JS, Qian WJ, Smith RD, et al. CD90/THY1 is overexpressed in prostate cancer-associated fibroblasts and could serve as a cancer biomarker. Modern Pathology. 2010;23:1346–1356. doi: 10.1038/modpathol.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- Tu Z, Li Q, Bu H, Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells and Development. 2010;19:1803–1809. doi: 10.1089/scd.2009.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66:124–134. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G. Cell biology. To scientists’ dismay, mixed-up cell lines strike again. Science. 2010;329:1004. doi: 10.1126/science.329.5995.1004. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental Hematology. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Wang GX, Zhan YA, Hu HL, Wang Y, Fu B. Mesenchymal stem cells modified to express interferon-β inhibit the growth of prostate cancer in a mouse model. Journal of International Medical Research. 2012;40:317–327. doi: 10.1177/147323001204000132. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Casaburi R, Flannery R, Leroux-Williams M, Tashkin DP. A placebo-controlled randomized trial of mesenchymal stem cells in chronic obstructive pulmonary disease. Chest. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Merchant RF, Garrett-Mayer E, Isaacs JT, Buckley JT, Denmeade SR. A prostate-specific antigen-activated channel-forming toxin as therapy for prostatic disease. Journal of the National Cancer Institute. 2007;99:376–385. doi: 10.1093/jnci/djk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. Journal of Neuroscience Research. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ye H, Cheng J, Tang Y, Liu Z, Xu C, Liu Y, Sun Y. Human bone marrow-derived mesenchymal stem cells produced TGFβ contributes to progression and metastasis of prostate cancer. Cancer Investigation. 2012;30:513–518. doi: 10.3109/07357907.2012.692171. [DOI] [PubMed] [Google Scholar]
- Yin J, Kim JK, Moon JH, Beck S, Piao D, Jin X, Kim SH, Lim YC, Nam DH, You S, et al. hMSC-mediated concurrent delivery of endostatin and carboxylesterase to mouse xenografts suppresses glioma initiation and recurrence. Molecular Therapy. 2011;19:1161–1169. doi: 10.1038/mt.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Engineering. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu W, Qian H, Zhu W, Zhang R. Mesenchymal stem cells modified to express lentivirus TNF-α Tumstatin(45–132) inhibit the growth of prostate cancer. Journal of Cellular and Molecular Medicine. 2011;15:433–444. doi: 10.1111/j.1582-4934.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. Journal of Gastroenterology and Hepatology. 2012;27(Suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Peehl DM. Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate. 2009;69:991–1000. doi: 10.1002/pros.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukareva V, Obrocka M, Houle JD, Fischer I, Neuhuber B. Secretion profile of human bone marrow stromal cells: donor variability and response to inflammatory stimuli. Cytokine. 2010;50:317–321. doi: 10.1016/j.cyto.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Zimmet JM, Hare JM. Emerging role for bone marrow derived mesenchymal stem cells in myocardial regenerative therapy. Basic Research Cardiology. 2005;100:471–481. doi: 10.1007/s00395-005-0553-4. [DOI] [PubMed] [Google Scholar]
- Zischek C, Niess H, Ischenko I, Conrad C, Huss R, Jauch KW, Nelson PJ, Bruns C. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Annals of Surgery. 2009;250:747–753. doi: 10.1097/SLA.0b013e3181bd62d0. [DOI] [PubMed] [Google Scholar]
- Zolochevska O, Yu G, Gimble JM, Figueiredo ML. Pigment epithelial-derived factor and melanoma differentiation associated gene-7 cytokine gene therapies delivered by adipose-derived stromal/mesenchymal stem cells are effective in reducing prostate cancer cell growth. Stem Cells and Development. 2012;21:1112–1123. doi: 10.1089/scd.2011.0247. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]