Abstract
Purpose
Type I endoleak is known to be associated with sac enlargement and occasional rupture, therefore, the treatment of type I endoleak is recommended at the time of diagnosis. The aim of this study was to identify the significance of early type I endoleak found on completion angiography.
Methods
Between January 2000 and December 2012, a total of 86 patients underwent endovascular abdominal aortic aneurysm repair (EVAR) and 10 patients (11.6%) were diagnosed with type Ia endoleak on completion angiography. Clinical and radiologic data were reviewed retrospectively.
Results
Of the 10 patients, two underwent EVAR with custom-made stent-grafts in the initial stage and both of them needed immediate treatment: one case involved open repair while the other involved insertion of an additional stent-graft. In 8 patients, the amount of leakage decreased after repeated balloon molding. They were managed conservatively and followed up with computed tomography angiography within 2 weeks after EVAR. In 7 of the 8 cases, type Ia endoleaks disappeared. In one patient with a persistent endoleak and a folded posterior wall of the stent-graft, coil embolization was performed 1 week after EVAR. With a median follow-up of 12 months (range, 1-61 months), no patients showed recurrence of type I endoleak or sac expansion.
Conclusion
Type I endoleaks diagnosed on completion angiography sealed spontaneously in 7 of 10 patients (70.0%). In cases of decreased amounts of leakage after balloon molding, simple observation may be an alternative to repetitive procedures. The long-term follow-up of patients with self-sealed type I endoleaks is mandatory.
Keywords: Endovascular aneurysm repair, Abdominal aortic aneurysm, Endoleak
INTRODUCTION
Endovascular aortic aneurysm repair (EVAR) has been accepted as a standard procedure for an anatomically suitable infrarenal abdominal aortic aneurysm (AAA). Up to 70% of AAAs have been repaired with EVAR in the United States [1]. Although EVAR is less invasive and can be the treatment of choice for high-risk patients, it might lead to a great number of complications and re-interventions [2,3]. Several studies reported that about 15%-20% of patients underwent secondary intervention after EVAR [4-6]. Endoleaks were one of the main reasons for reintervention and Mehta et al. [5] reported that it accounted for 56.6% of all reinterventions cases.
Type I endoleaks occur because of inadequate sealing at the proximal and distal ends of the prosthesis. Type I endoleaks are known to be associated with high sac pressure, aneurysmal dilatation, and rupture of aneurysm, therefore treatment at the time of diagnosis is recommended [5,7]. However, 50%-60% of the leaks at completion of EVAR were reported to close spontaneously within the first postoperative month [8,9]. In a study of 28 patients with periprosthetic leaks, including 23 type I endoleaks, 50% of the type I endoleaks spontaneously sealed [10]. The prognosis of type I endoleaks depends on whether they are early or delayed and on whether they are accompanied by migration or malposition of the stent-graft.
The aims of this study were to identify the clinical significance of type I endoleak diagnosed on completion angiography after EVAR and to report the short-term outcomes of spontaneously sealed early type I endoleaks in a single institute.
METHODS
A total of 351 patients were diagnosed with infrarenal AAA between January 2000 and December 2012, and 86 patients underwent EVAR. A retrospective review of the medical records and radiologic studies of these patients was performed after the approval by the Institutional Review Board of Seoul National University Hospital (H-1303-031-471).
An endoleak is defined as persistent blood flow outside a graft and within an aneurysm sac. A type I endoleak is leakage from the proximal (Ia) or distal (Ib) attachment site. An early endoleak is defined as one that is found on completion angiography immediately after EVAR. It is distinguished from a delayed endoleak with or without graft migration that is not definite on completion angiography but occurs during follow-up. Of the 86 patients, 11 (12.8%) had an early type I endoleak diagnosed initially on completion angiography, including 10 type Ia endoleaks and 1 type Ib endoleak. Patients with type Ia endoleak were included in this study.
All procedures were performed with a fixed fluoroscopic unit. Open access through bilateral femoral arteriotomies were performed under general anesthesia in all patients. When the procedures were completed, the proximal, distal and overlapping sites of the grafts were routinely dilated with a molding balloon. After balloon molding, completion angiography was performed before the bilateral femoral sheaths were removed. If a type I endoleak was found on completion angiography, balloon molding was performed again. When the amount of leakage was reduced and the stent-graft was positioned as planned, all interventions were finished and follow-up computed tomography (CT) angiography was planned for the next 2 weeks. In patients without endoleaks or other complications on the final angiography, CT angiography was originally scheduled at 1, 6, and 12 months after the procedure and then annually thereafter.
RESULTS
Of the 86 patients, early type Ia endoleaks occurred in 10 patients (11.6%). The median age of the patients was 75 years (range, 57-87 years). Eight male and 2 female patients were included. The median maximal diameter of the AAA was 57 mm (range, 49-87 mm). The median length and diameter of the aneurysmal neck were 30 mm (range, 17-45 mm) and 23 mm (range, 18-30 mm), respectively. The median angles between the suprarenal aorta and proximal neck, and the proximal neck and aneurysm were 72° (range, 20°-90°) and 55° (range 25°-90°), respectively. Stent-grafts used during the EVAR procedures were Zenith (n = 4), Excluder (n = 2), Endurant (n = 2), and custom-made (n = 2). The demographics and aneurysm-related characteristics of the patients are summarized in Table 1.
Table 1.
Baseline characteristics of patients with early type I endoleaks
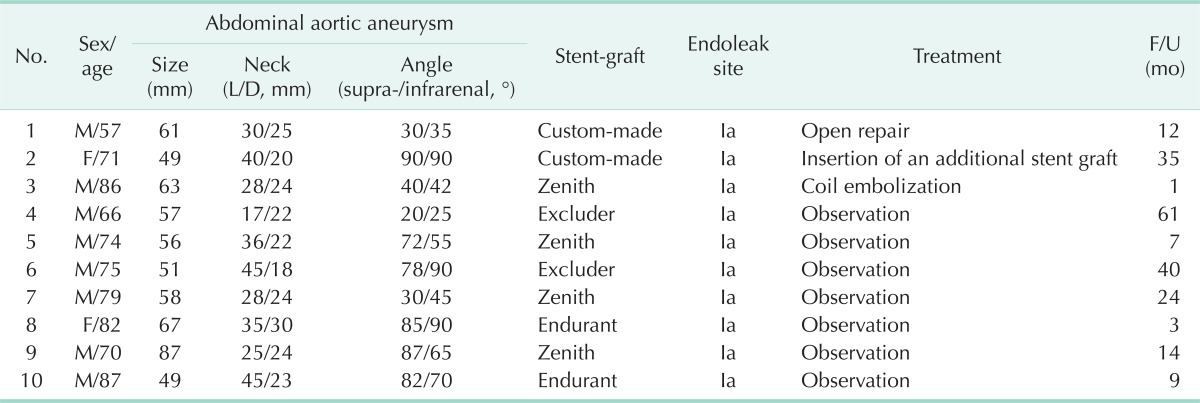
L/D, length/diameter; F/U, follow-up.
Of the 10 patients with early type Ia endoleaks, 2 received treatment immediately after diagnosis of type I endoleak. Both of them underwent EVAR using a custom-made stent-graft during our initial period. The first patient was a 57-year-old male. The aneurysm was 61 mm in diameter, and the angles between the suprarenal aorta and proximal neck, and the suprarenal aorta and aneurysm were 30° and 35°, respectively. The length and diameter of the neck were 30 mm and 25 mm, respectively. He underwent EVAR with a custom-made stent-graft, however, a large amount of leakage from the proximal attachment site was found immediately after the procedure with distal migration of the stent graft. He was treated with open repair. The other patient was a 71-year-old female with an AAA of 49 mm in diameter. The angles between the suprarenal aorta and proximal neck, and the suprarenal aorta and aneurysm were both 90°. The length and diameter of the neck were 40 mm and 20 mm, respectively. She also underwent EVAR using a custom-made stent-graft. There was a moderate amount of leakage on completion angiography because of inadequate coverage of the proximal neck. She was treated by insertion of an additional custom-made stent-graft 5 days later.
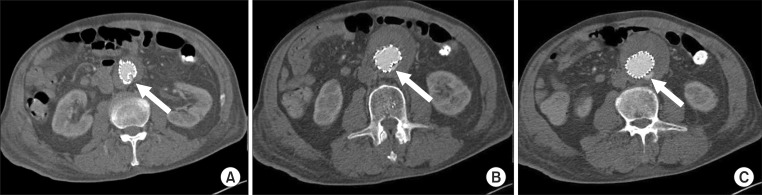
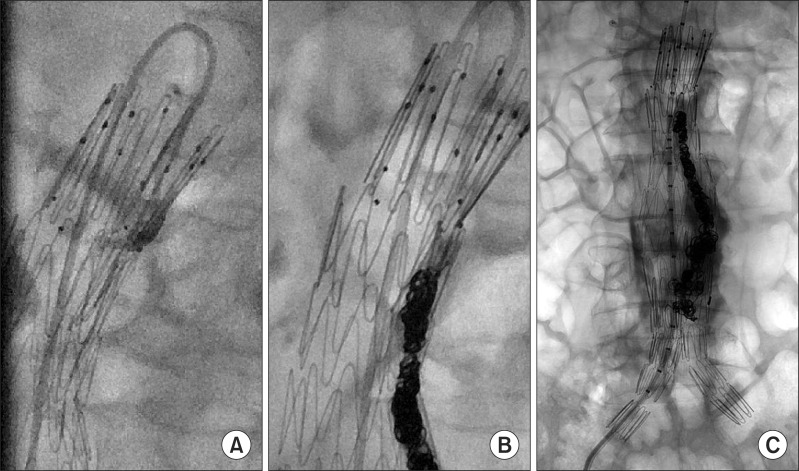
Eight of the 10 patients with early type Ia endoleaks showed reduced amounts of leakage after balloon molding and the stent-grafts were positioned as planned. They were managed conservatively and evaluated with CT angiography within 2 weeks after EVAR. All except one patient showed spontaneously sealed type I endoleaks on follow-up CT angiography. A persistent type Ia endoleak with the posterior wall of the stent-graft folded was found in one patient (Fig. 1). He was treated with coil embolization 1 week after the EVAR. A microcatheter was advanced into the space between the stent-graft and the aortic wall. Then, the endoleak was treated with embolization using MicroNester coils (Cook Medical, Bloomington, IN, USA) and glue (Fig. 2).
Fig. 1.
(A-C) Computed tomography angiography demonstrating type I endoleak with posterior wall of stent-graft folded (arrows).
Fig. 2.
(A-C) Fluoroscopic image demonstrating selective catheterization of the type Ia endoleak and deployment of coils into the space.
With a median follow-up of 12 months (range, 1-61 months), no further interventions for the type I endoleaks were required. The patients treated with an additional stent graft were followed-up for 35 months and there were no recurrences of endoleak on CT angiography. The patients treated with coil embolization 7 days after EVAR was lost to follow-up. In 7 patients who were managed conservatively, no recurrences of type I endoleak or sac expansion were observed on surveillance CT angiography (Table 2). The size of the aneurysm on CT angiography was reduced in 4 patients and remained unchanged in 3 patients.
Table 2.
The changes of size of aneurysmal sac in patients treated conservatively
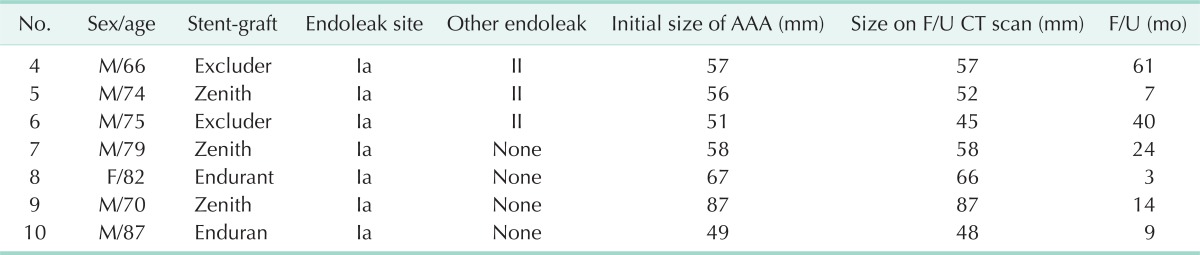
AAA, abdominal aortic aneurysm; F/U, follow-up; CT, computed tomography.
DISCUSSION
An endoleak is defined as a failure to totally exclude an AAA from continued perfusion and pressurization [11]. Type I endoleaks are caused by an inadequate seal at the proximal and distal end of a stent-graft. They could result in a rupture of the aneurysm because of persistent blood flow into the aneurysmal sac, therefore treatment at the time of diagnosis is recommended [12]. The EUROSTAR registry shows the significance of type I and III endoleaks as having a 2% risk of conversion after EVAR and a 1% annual risk of rupture [8]. Metha et al. [5], have reported that the most common cause of delayed AAA rupture after EVAR was a type I endoleak.
There are several treatment methods for type I endoleaks, and results with endovascular treatment have been encouraging [9]. Proximal stent graft extension, insertion of a Palmaz stent, and coil embolization can be attempted. When adequate proximal sealing, while maintaining the renal artery flow, is difficult, open conversion-stent graft explants and aortoiliac reconstruction-is required.
Even though the risk of type I endoleaks is generally accepted, type I endoleaks during the early postoperative period seal spontaneously in half of the cases. Fifty to 60% of leaks at the completion of EVAR close within the first postoperative month, but rarely after the first month of EVAR [10,11,13]. In the report of the consensus meeting about endoleaks, the answers of experts showed a bimodal distribution and half of them thought that 10%-50% of type I endoleaks could seal, but rarely after 2 weeks [7].
In our case experiences, 7 of 10 patients (70.0%) with type Ia endoleaks on completion angiography closed spontaneously. Of the 3 patients who did not show spontaneous sealing, 2 underwent EVAR in the early period and showed the migration or mis-sizing of stent-grafts. With second generation endografts, only one patient underwent an additional treatment for an early type I endoleak. He showed a folded posterior wall of the stent-graft on CT angiography 1 week after EVAR. Since this folded wall was difficult to close spontaneously due to structural problems in the stent graft, immediate coil embolization of the perigraft space was done. When migration, malposition, or other device-related problems occur, spontaneous closure of the leakage will not be possible and prompt intervention should be considered. In cases of decreased amounts of leakage after balloon molding without migration or device-related problems, simple observation with surveillance CT angiography for 2 to 4 weeks may be alternative to aggressive repetitive procedures. We performed the completion angiography with the sheaths in both femoral arteries and a stiff guidewire that was inserted into the aorta. This may have distorted the anatomy of the aorta, especially the aneurysm neck, and added pressure to the placed stent graft. Type I endoleaks on completion angiography can be influenced and exaggerated by these factors.
During the study period, we experienced 2 patients with delayed type I endoleaks with stent graft migration found on CT angiography 1 week after EVAR. Both of them were treated with open conversion and insertion of an additional stent graft. When a type I endoleak is not definite on completion angiography but occurs on surveillance imaging studies, there is a strong possibility of stent graft migration. This migration can also be caused by neck dilatation, but such cases are rare [12]. In delayed type I endoleaks, the leakage is less likely to be sealed and dilatation of aneurysm would occur. Unlike early type I endoleak, prompt treatment should be considered in delayed type I endoleaks.
A study with the EUROSTAR registry showed that a large diameter of the aneurismal neck, the degree of angulation of the aneurismal neck, and team experience with less than 30 procedures were associated with a higher incidence of type I or III endoleaks [11]. Several morphologic risk factors have been reported to be risk factors for type I endoleaks, including a large aneurysm, and a heavily calcified or wide neck [14,15]. Intraoperative factors, such as the type of stent graft, stent graft over-sizing and residual uncovered landing zones, were reported to be the causes of graft migration and type I endoleaks [16,17]. Preoperative planning and patient selection are important to prevent type I endoleaks. In addition, to avoid unnecessary procedures for early type I endoleak, further studies to identify predictive factors of spontaneous sealing type I endoleaks are warranted. Our ability to determine risk factors was limited due to the small sample size of this study.
There have been few reports on the long-term results of spontaneously sealed type I endoleaks. In this study, of the 8 patients managed conservatively, none showed sac enlargement or recurrent endoleaks. However, the median follow-up period was 14 months (mean, 20.9 months; range, 3-61 months) and 3 were lost to follow-up within a year. Long-term surveillance with CT angiography is mandatory in patients with self-sealed type I endoleaks.
In conclusion, type I endoleaks diagnosed on completion angiography sealed spontaneously in 7 of 10 patients (70.0%). In cases of decreased amounts of leakage after balloon molding without migration of the stent graft, simple observation with surveillance CT angiography may be an alternative to repetitive procedures. The long-term follow-up of patients with self-sealed type I endoleaks is mandatory.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Patel VI, Lancaster RT, Mukhopadhyay S, Aranson NJ, Conrad MF, LaMuraglia GM, et al. Impact of chronic kidney disease on outcomes after abdominal aortic aneurysm repair. J Vasc Surg. 2012;56:1206–1213. doi: 10.1016/j.jvs.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 2.EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–2186. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 3.EVAR trial participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005;365:2187–2192. doi: 10.1016/S0140-6736(05)66628-7. [DOI] [PubMed] [Google Scholar]
- 4.Pitoulias GA, Schulte S, Donas KP, Horsch S. Secondary endovascular and conversion procedures for failed endovascular abdominal aortic aneurysm repair: can we still be optimistic? Vascular. 2009;17:15–22. doi: 10.2310/6670.2009.00004. [DOI] [PubMed] [Google Scholar]
- 5.Mehta M, Sternbach Y, Taggert JB, Kreienberg PB, Roddy SP, Paty PS, et al. Long-term outcomes of secondary procedures after endovascular aneurysm repair. J Vasc Surg. 2010;52:1442–1449. doi: 10.1016/j.jvs.2010.06.110. [DOI] [PubMed] [Google Scholar]
- 6.Venermo MA, Arko FR, 3rd, Salenius JP, Saarinen JP, Zvaigzne A, Zarins CK. EVAR may reduce the risk of aneurysm rupture despite persisting type Ia endoleaks. J Endovasc Ther. 2011;18:676–682. doi: 10.1583/11-3432.1. [DOI] [PubMed] [Google Scholar]
- 7.Veith FJ, Baum RA, Ohki T, Amor M, Adiseshiah M, Blankensteijn JD, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35:1029–1035. doi: 10.1067/mva.2002.123095. [DOI] [PubMed] [Google Scholar]
- 8.Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, van Marrewijk C, Laheij RJ. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32:739–749. doi: 10.1067/mva.2000.109990. [DOI] [PubMed] [Google Scholar]
- 9.Naughton PA, Garcia-Toca M, Rodriguez HE, Keeling AN, Resnick SA, Morasch MD, et al. Endovascular treatment of delayed type 1 and 3 endoleaks. Cardiovasc Intervent Radiol. 2011;34:751–757. doi: 10.1007/s00270-010-0020-y. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura JS, Moore WS. Clinical consequences of periprosthetic leak after endovascular repair of abdominal aortic aneurysm. Endovascular Technologies Investigators. J Vasc Surg. 1998;27:606–613. doi: 10.1016/s0741-5214(98)70224-1. [DOI] [PubMed] [Google Scholar]
- 11.Buth J, Harris PL, van Marrewijk C, Fransen G. The significance and management of different types of endoleaks. Semin Vasc Surg. 2003;16:95–102. doi: 10.1016/s0895-7967(03)00007-3. [DOI] [PubMed] [Google Scholar]
- 12.Cronenwett JL, Johnston KW. Rutherford's vascular surgery. 7th ed. Philadelphia: Saunders; 2009. [Google Scholar]
- 13.Broeders IA, Blankensteijn JD, Gvakharia A, May J, Bell PR, Swedenborg J, et al. The efficacy of transfemoral endovascular aneurysm management: a study on size changes of the abdominal aorta during mid-term follow-up. Eur J Vasc Endovasc Surg. 1997;14:84–90. doi: 10.1016/s1078-5884(97)80202-x. [DOI] [PubMed] [Google Scholar]
- 14.Sampaio SM, Panneton JM, Mozes GI, Andrews JC, Bower TC, Karla M, et al. Proximal type I endoleak after endovascular abdominal aortic aneurysm repair: predictive factors. Ann Vasc Surg. 2004;18:621–628. doi: 10.1007/s10016-004-0100-z. [DOI] [PubMed] [Google Scholar]
- 15.Zayed HA, Attia R, Modarai B, Clough RE, Bell RE, Carrell T, et al. Predictors of reintervention after endovascular repair of isolated iliac artery aneurysm. Cardiovasc Intervent Radiol. 2011;34:61–66. doi: 10.1007/s00270-010-9876-0. [DOI] [PubMed] [Google Scholar]
- 16.Sampaio SM, Shin SH, Panneton JM, Andrews JC, Bower TC, Cherry KJ, et al. Intraoperative endoleak during EVAR: frequency, nature, and significance. Vasc Endovascular Surg. 2009;43:352–359. doi: 10.1177/1538574409333581. [DOI] [PubMed] [Google Scholar]
- 17.Zayed HA, Bell RE, Clough RE, Thomas S, Sabharwal T, Reidy JF, et al. Results of endovascular repair of abdominal aortic aneurysms with an unfavorable proximal neck using large stent-grafts. Cardiovasc Intervent Radiol. 2009;32:1161–1164. doi: 10.1007/s00270-009-9557-z. [DOI] [PubMed] [Google Scholar]