Abstract
Purpose
We evaluate the operative outcome and oncologic outcome of laparoscopic liver resection for hepatocellular carcinoma (HCC), and compare with open liver resection.
Methods
From January 2004 to December 2012, clinical data of 70 patients who underwent laparoscopic liver resection for HCC (laparoscopic liver resection group, lapa-group) were collected and analyzed retrospectively. Control group (open liver resection group, open-group) were retrospectively matched, and compared with lapa-group.
Results
Laparoscopic major liver resections were performed in 4 patients. Laparoscopic anatomical resections and nonanatomical resections were performed in 39 patients, and 31 patients, respectively. Mean operative time was shorter in lapa-group (215.5 ± 121.84 minutes vs. 282.30 ± 80.34 minutes, P = 0.001), mean intraoperative transfusion rate and total amount were small in lapa-group (24.28%, 148.57 ± 3,354.98 mL vs. 40.78%, 311.71 ± 477.01 mL). Open conversion occurred in 6 patients (8.57%) because of bleeding, inadequate resection, invisible mass on intraoperative ultrasonography, and tumor rupture. In lapa-group and open-group, 3-year disease-free survival rates were 58.3% ± 0.08%, and 62.6% ± 0.06%, respectively (P = 0.773). In lapa-group and open-group 3-year overall survival rates were 65.3% ± 0.8%, and 65.7% ± 0.6%, respectively (P = 0.610).
Conclusion
Laparoscopic liver resection for HCC is feasible and safe in a large number of patients, with reasonable operative and oncologic results.
Keywords: Laparoscopy, Hepatectomy, Hepatocellular carcinoma
INTRODUCTION
Since the introduction of laparoscopic liver resection in 1991 [1], laparoscopic liver resection has been frequently proposed with improvements in technology and instruments. From benign disease to malignant disease, from nonanatomical minor resection to anatomical major resection, from hand-assisted surgery to pure laparoscopic surgery, laparoscopic surgery has been in wide spread use and refinement of technology has increased. Many studies have demonstrated the feasibility and safety of laparoscopic liver resection. And like other laparoscopic surgeries, short-term benefits such as less pain, less bleeding, less hospital stay have been reported [2-9]. But a few studies of oncological outcome in laparoscopic liver resection for hepatocellular carcinoma (HCC) have been reported and debated. The aim of this retrospective study is to compare the operative outcome and oncological outcome of open liver resection versus laparoscopic liver resection for HCC.
METHODS
From January 2004 to December 2012, clinical data of 70 patients who underwent laparoscopic liver resection for HCC (laparoscopic liver resection group, lapa-group) were collected and analyzed retrospectively.
Inclusion and exclusion criteria
Although the best indication for laparoscopic liver resection is peripherally located small lesions, larger size of tumor is not a contraindication for laparoscopic liver resection. In addition, tumor number was not a contraindication. Difficult tumor location is relative contraindication for laparoscopic liver resection, for example, located very closely to major vascular or biliary structures. Portal or hepatic vein involvement and invasion to adjacent organs was not an indication for laparoscopic liver resection. From January 2004 to December 2012, 77 patients underwent laparoscopic liver resection for HCC. Five patients did not reveal HCC on permanent biopsy, and so were excluded form this study. In addition, 2 patients who underwent laparoscopic microwave ablation or radiofrequency ablation were excluded, because it was not liver resection.
Control group
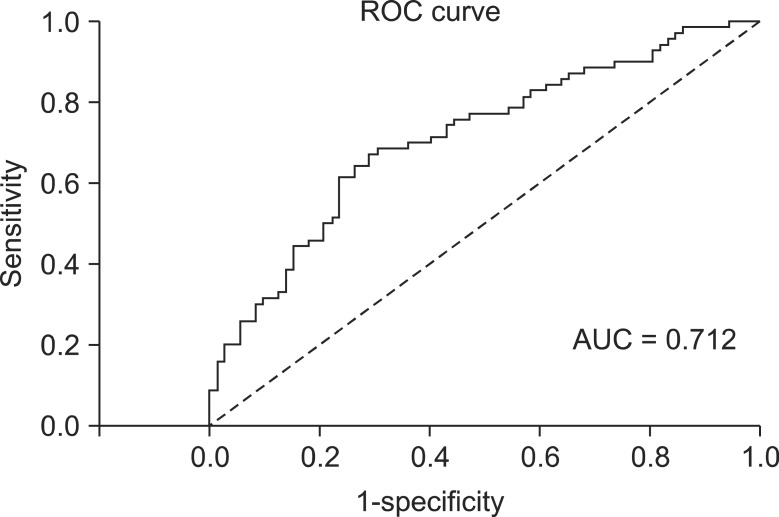
Control group (open liver resection group, open-group) patients were selected by propensity score matching method (AUC = 0.712, Fig. 1), and retrospectively matched for the following criteria: sex, age, international normalized ratio (INR), albumin, α-fetoprotein (AFP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, indocyanine green (ICG) R15, hepatitis, tumor size, tumor location, type of resection. Operative outcomes were analyzed by resection margin, operative time, rate of blood transfusion, total amount of intraoperative blood transfusion, postoperative complication. Oncological outcomes were analyzed by disease-free survival (DFS), overall survival (OS), and recurrence pattern.
Fig. 1.
Receiver operating characteristic (ROC) curve. AUC, area under the curve.
Surgical technique
All procedures were performed under general anesthesia. Patients were positioned in supine or left lateral decubitus position according to the tumor location. The pneumoperitoneum with carbon dioxide was introduced, and then intraabdominal pressure was monitored and maintained below 14 mmHg. Four or five trocars were used, depending on the surgical requirements. The trocar insertion sites were determined by the tumor locations. A flexible laparoscopic ultrasound probe was used to localize the tumor and determine the transection line before parenchymal dissection. If anatomical resection was planned such as segmentectomy, hepatic vein for resection margin was firstly recognized with intraoperative ultrasound, then portal pedicle was divided intraparenchymally, then liver parenchyma is dissected along the areas of demarcation of the liver surface. One to 2 cm depth of liver parenchyma was transected using a Harmonic Scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). An ultrasonic dissector (CUSA Excel, Integra Lifesciences Co., Plainsboro, NJ, USA) was used to dissect deep portions of the liver to skeletonize the portal and hepatic vein. An endoclip was used to control larger structures. We did not routinely apply the Pringle maneuver to control blood flow to the liver, but in selected cases, we used selective extraglissonian pedicle clamping with laparoscopic bulldog clamp for bleeding control or anatomical resection. The specimen was extracted using a vinyl bag. An argon beam coagulator was used on the cut liver surface to control bleeding with ventilation due to the risk of air embolism. Finally, fibrin glue was applied to the cut surface.
Postoperative management
After liver resection operation, all patients were provided intensive care unit care routinely. On principle of early enteral feeding, patients were allowed sips of water at postoperative day 1 and soft diet at postoperative day 2 if no other problems were found. All patients in these study groups were checked by abdomen computed tomography (CT) after 7 days from operation. If no other problems were found in abdomen CT, discharge and outpatient department follow-up was done. Tumor markers (AFP, PIVKA-II) are checked every 2-3 months. And ultrasonography or CT was checked every 6 months.
Statistical analysis
Statistical analysis was carried out with IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA) Control groups were selected by propensity score match with medcalcs 12.7.2 (MedCalc, Ostend, Belgium). Comparisons between categorical variables were analyzed with chi-square test and comparisons of continuous variables were analyzed with Student t-test. DFS and OS rates were calculated using the Kaplan-Meier method, and the log-rank test was used to compare differences in survival between the groups. Values of P < 0.05 were considered statistically significant. The 2 groups were compared on an intention-to-treat basis.
RESULTS
Demographics
Demographics features and tumor characteristics are summarized in Table 1. As defined by the matching criteria, both group patients were matched for sex, age, INR, albumin, AFP, AST/ALT, total bilirubin, ICG R15, hepatitis, tumor characteristics (size, location). Mean age was 59.3 years (standard deviation [SD], ±9.43) in the lapa-group and 57.41 years (SD, ±8.64) in the open-group (P = 0.566). Female to male ratio was similar between two groups (lapa-group, 12 [17.14%]:58 [82.86%]; open-group, 18 [23.68%]:58 [76.32%]; P = 0.220). The mean INR was 1.19 (SD, ±1.72) in the lapa-group and 1.04 (SD, ±0.29) in the open-group (P = 0.20). Moreover, additional parameters like albumin, AFP, ICG R15, tumor size and location, liver function test, etiology of hepatitis were similar between the two groups. Therefore, lapa-group and open-group were considered well matched.
Table 1.
Demographic characteristics and tumors and liver characteristics
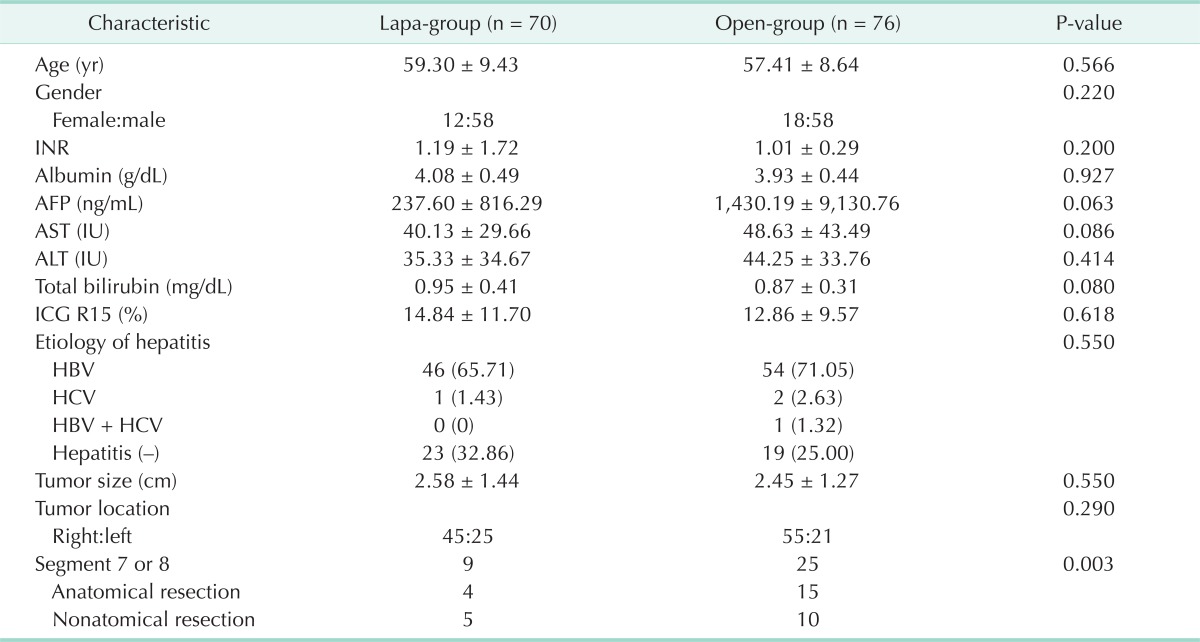
Values are presented as mean ± standard deviation or number (%).
Lapa-group, laparoscopic liver resection group; open-group, open liver resection group; INR, international normalized ratio; AFP, α-fetoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ICG, indocyanine green; HBV, hepatitis B virus; HCV, hepatitis C virus.
Operative outcome
The operative outcomes are summarized in Table 2. No difference of the type of resection was found between the two groups (P = 0.673). The mean operation time was shorter in the lapa-group than open-group (215.5 vs. 282.30, P = 0.001). The rate of intraoperative transfusion in lapa-group was smaller than that of open-group (24.28 % vs. 40.78 %, P = 0.001). The total amount of intraoperative transfusion in lapa-group was significantly less than the open-group (148.57 vs. 311.71, P = 0.001). In all patients, HCC was confirmed pathologically. Tumor differentiation (Ednomson-steiner grade) and pathologic staing (Union for International Cancer Control staging) were similar between two groups (P = 0.173 and P = 0.683, respectively). Patients were monitored for development of ascites, pleural effusion, bleeding, infection, etc. There was similar rate of postoperative complications between two groups (7.14% vs.14.47%, P = 0.450). No bleeding, wound infection, and pneumonia were observed in the lapa-group, but 3 cases of bleeding, 4 cases of wound infection and 1 case of pneumonia were observed in the open-group. There was 1 postoperative acute renal failure (ARF) in the lapa-group, but no ARF in the open-group. All complications were resolved by conservative treatments. In conclusion, there were no significant differences in complication rates between the two groups, but in the lapa-group there were less than in the open-group. There were six cases (8.57%) of laparoscopic to open conversion; three cases were due to bleeding, one case was due to inadequate resection, one case was due to invisible mass in ultrasonography, one case was due to tumor rupture. We reviewed histological reports focused on resection margin. In the lapa-group mean resection margin was 1.07 cm (SD, ± 1.37) and in the open-group mean resection margin was 0.80 cm (SD, ± 0.82). No significant difference between two groups was found (P = 0.147).
Table 2.
Operative outcomes
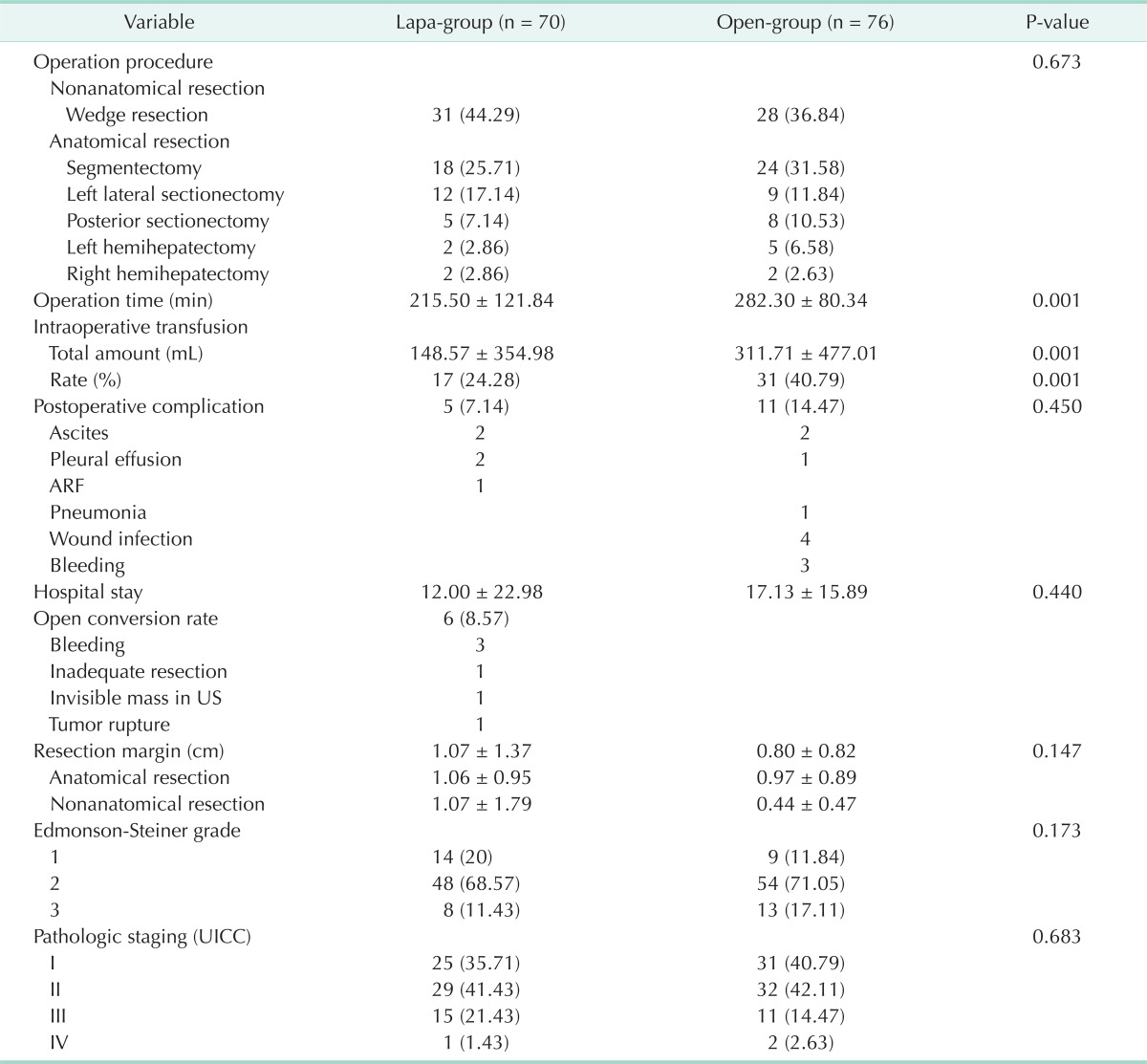
Values are presented as number (%) or mean ± standard deviation.
Lapa-group, laparoscopic liver resection group; open-group, open liver resection group; ARF, acute renal failure; US, ultrasonography; UICC, Union for International Cancer Control.
Oncological outcome
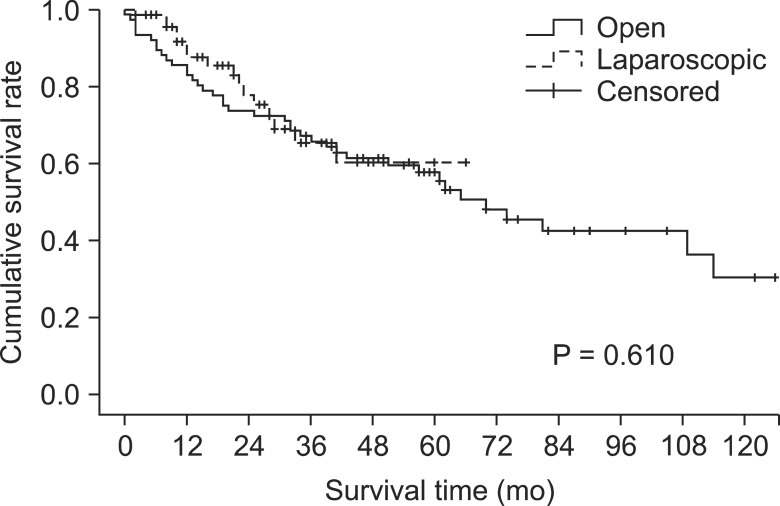
The mean follow-up time was 48.64 months (range, 2-66 months) in the lapa-group and 78.33 months (range, 1-150 months) in the open-group. The OS rate after laparoscopic surgery was 65.3% at 3 years, and 60.3% at 5 years and, respectively, 65.7%, 57.7% after open surgery. Overall survival rate was similar between the two groups (Fig. 2) (P = 0.610).
Fig. 2.
Overall survival rate.
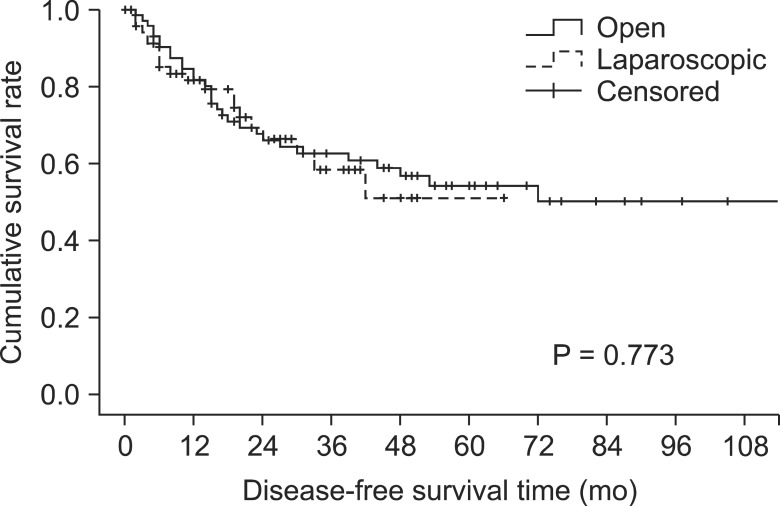
The DFS rate after laparoscopic surgery was 58.3% at 3 years, and 51.0% at 5 years and, respectively, 62.6% and 54.3% after open surgery. DFS rate was similar between the two groups (Fig. 3) (P = 0.773).
Fig. 3.
Disease-free survival rate.
Recurrence types of each group are summarized in Table 3. In the lapa-group, 30 patients developed recurrence (resection marginal recur, n = 6; one nodular recur, n = 16; multinodular diffuse recur, n = 7; lymph node metastasis, n = 1). One of 7 multinodular diffuse recurrence patients was found with lung metastasis and another one was found with lymph node metastasis. In the open-group, 21 patients developed recurrence (resection marginal recur, n = 3; one nodular recur, n = 9; multinodular diffuse recur, n = 8; lymph node metastasis, n = 1). One of 9 one-nodular recurrence patients was found with other distant metastasis. Disease recurrence rate was similar between the two groups (42.86% vs. 27.63%, P = 0.406).
Table 3.
Recurrence type
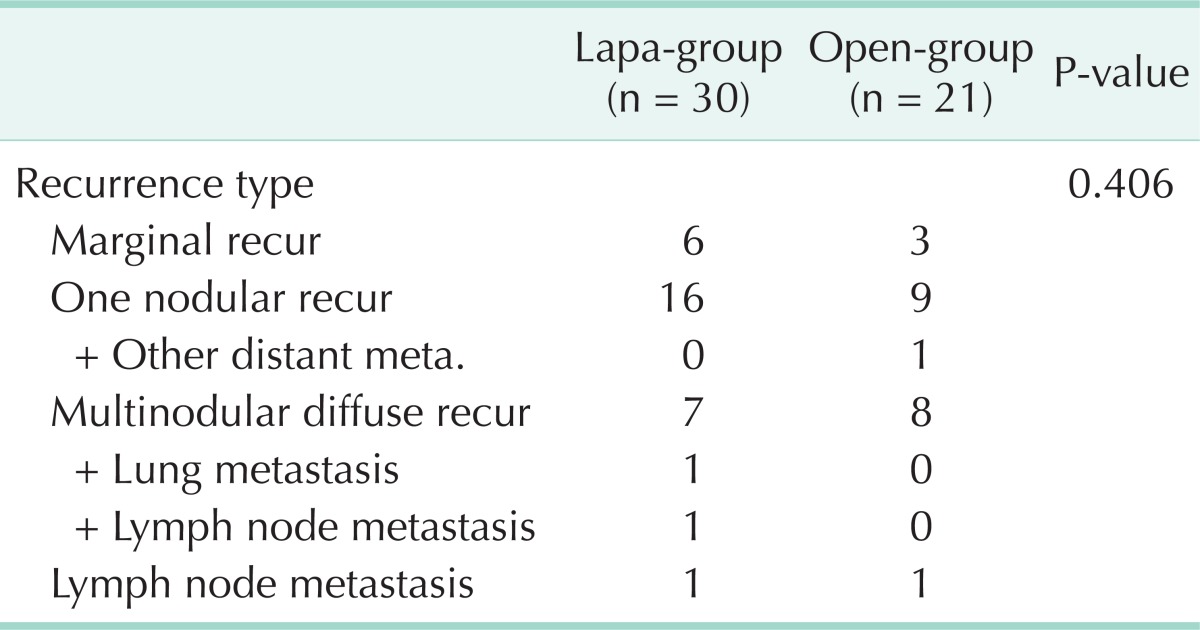
Lapa-group, laparoscopic liver resection group; open-group, open liver resection group.
Types of treatment of first recurrence are summarized in Table 4. In the lapa-group, 14 patients were treated by transarterial chemoembolisation (TACE), one patient was treated by intra-arterial chemotherapy (IA CTx), and one patient was treated by radiotherapy. The remaining 14 patients were treated in other ways (Per-oral chemotherapy, conservative treatment, etc.). In the open-group, 13 patients were treated by TACE, two patients were treated by IA CTx, two patients were treated by reoperation, and four patients were treated by other ways. Distributions of treatment after recurrence were similar between two groups (P = 0.360).
Table 4.
Type of treatment the first recurrence
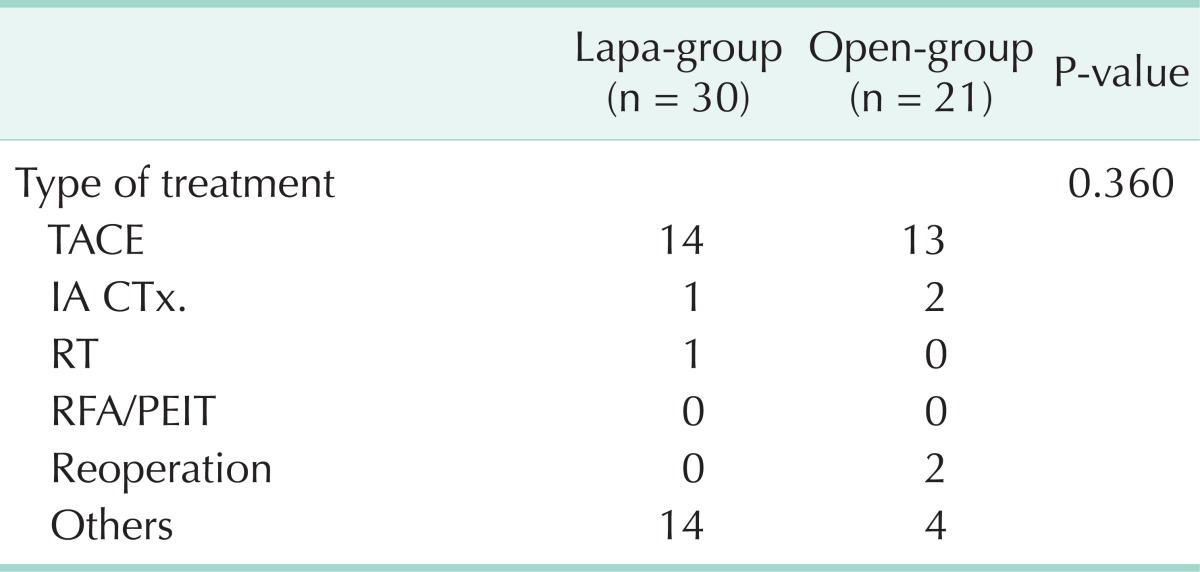
Lapa-group, laparoscopic liver resection group; open-group, open liver resection group; TACE, transarterial chemoembolization; IA CTx, intra-arterial chemotherapy; RT, radiotherapy; RFA, Radiofrequency ablation; PEIT, percutaneous ethanol injection therapy.
DISCUSSION
In the Louisville statement, in the year 2008 [10], the best indication for laparoscopic liver resection for HCC seems to be smaller than 5 cm, single nodule, peripheral lesion (segment 2-6). So, simple comparison between laparoscopic surgery and open surgery could have selection bias. Therefore, this study describes the results of case-matched comparative analysis between laparoscopic and open liver resections for HCC. Two groups were matched for patients' demographics, features, and tumor characteristics (location, size, number) to minimize the selection bias.
As the technique has developed recently and experience has been accumulated, these indications seem to gradually be developed, so tumor size and location seemed to be not important. In our case, lesser than that of the open group, but there were nine cases for the segment 7, 8 operation. Recently, the intercostal approach operation has been adopted for posterosuperior located HCC; therefore, limitations due to tumor location seems likely to gradually disappear in the future [11]. Furthermore, tumor size itself may not be a contraindication for laparoscopic liver resection [12]. There were 3 cases of operations for tumors bigger than 5 cm.
There are several advantages of laparoscopic surgery such as less pain, less wound, less bleeding, less adhesion, etc. These advantages correspond with Enhanced Recovery After Surgery, in order to reduce the possibility of inflammatory, humoral and immune response after surgery. Thus, this surgery can decrease complications, including postoperative ileus, making patients' hospital stay decreased [13-15]. Especially, the lack of adhesion is able to enhance the possibility of repeated resection such as HCC, which can easily recur [16]. In the future, planning of liver transplantation can be positively influenced by the surgery [17]. Moreover, this study has shown a significant difference in operation time, which can be shortened in lapa-group.
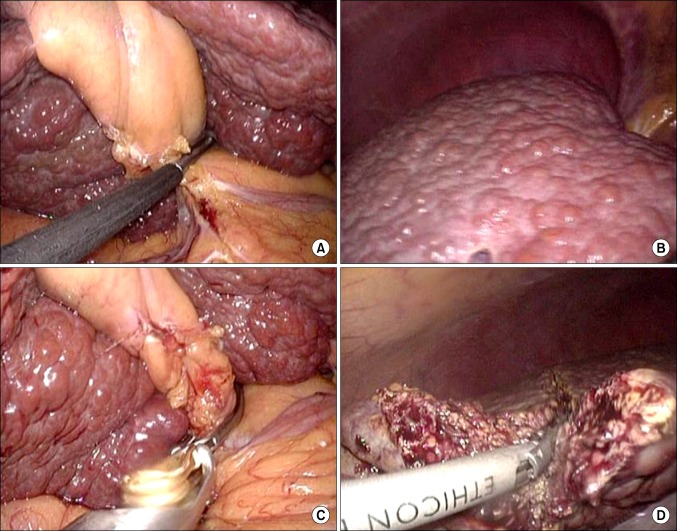
Laparoscopic surgery also has the difficulty of bleeding control. In this study, one of the significant reasons of open conversion is failure to control bleeding. However, there are several other methods to prevent it; laparoscopic Pringle method or selective glissonian pedicle clamping/stapling. Several ways which have been carried out anatomical liver resection are shown using extrahepatic glissonian pedicle approach or intrahepatic glissonian pedicle approach [18,19]. In our case, we did not use the Pringle method, but there were some cases using laparoscopic bulldog clamp to control selective inflow control (Fig. 4). This case contributes not only bleeding control but also anatomical resection through color demarcation lines. And, in our study, rate and amount of intraoperative blood transfusion were smaller in lapa-group than in open-group.
Fig. 4.
Right glissonian pedicle was clamped by laparoscopic intestinal clamp (A), then demarcation line is noted (B). Right glissonian pedicle is clamped by laparoscopic bulldog (C), then liver parenchyma is transected using an energy device (D).
While taking the laparoscopic operation, there are many operative benefits; however, one of the reasons why the laparoscopic operation is not generally accepted is because of the oncologic outcome. In many studies, they indicated that there are no differences in DFS rate and OS rate between laparoscopic surgery and open surgery [5,9,20,21]. As our results show, we have not found significant differences of DFS, OS, and recurrence pattern between the two groups. A large-scale, controlled randomized study seems clinically difficult, but it seems to be necessary as long-term results can be well designed at a large scale.
Other limitations of laparoscopic surgery are tumor cell seeding or tumor recurrence through port site. Tumor seeding can occur in open surgery, though we have only one case of tumor seeding. We can anticipate and prevent tumor seeding by careful manipulation.
In conclusion, laparoscopic liver resection for HCC is feasible and safe in a large number of patients, and not inferior to open liver resection in regard to operative outcome. And oncologic outcome of laparoscopic liver resection for HCC is not inferior to open liver resection. To prevent tumor seeding, we must manipulate tumor masses carefully.
ACKNOWLEDGEMENTS
This research was supported by grant of Yeungnam University Medical Center (2010).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol. 1991;78(5 Pt 2):956–958. [PubMed] [Google Scholar]
- 2.Chen HY, Juan CC, Ker CG. Laparoscopic liver surgery for patients with hepatocellular carcinoma. Ann Surg Oncol. 2008;15:800–806. doi: 10.1245/s10434-007-9749-1. [DOI] [PubMed] [Google Scholar]
- 3.Dagher I, Lainas P, Carloni A, Caillard C, Champault A, Smadja C, et al. Laparoscopic liver resection for hepatocellular carcinoma. Surg Endosc. 2008;22:372–378. doi: 10.1007/s00464-007-9487-2. [DOI] [PubMed] [Google Scholar]
- 4.Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg. 2003;138:763–769. doi: 10.1001/archsurg.138.7.763. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, et al. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg. 2005;189:190–194. doi: 10.1016/j.amjsurg.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, et al. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg. 2006;243:499–506. doi: 10.1097/01.sla.0000206017.29651.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belli G, Fantini C, D'Agostino A, Cioffi L, Langella S, Russolillo N, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc. 2007;21:2004–2011. doi: 10.1007/s00464-007-9503-6. [DOI] [PubMed] [Google Scholar]
- 8.Shimada M, Hashizume M, Maehara S, Tsujita E, Rikimaru T, Yamashita Y, et al. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc. 2001;15:541–544. doi: 10.1007/s004640080099. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S, Nagano H, Marubashi S, Kawamoto K, Wada H, Eguchi H, et al. Hepatectomy based on the tumor hemodynamics for hepatocellular carcinoma: a comparison among the hybrid and pure laparoscopic procedures and open surgery. Surg Endosc. 2013;27:610–617. doi: 10.1007/s00464-012-2499-6. [DOI] [PubMed] [Google Scholar]
- 10.Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 11.Ishizawa T, Gumbs AA, Kokudo N, Gayet B. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg. 2012;256:959–964. doi: 10.1097/SLA.0b013e31825ffed3. [DOI] [PubMed] [Google Scholar]
- 12.Lurje G, Lesurtel M, Clavien PA. Multimodal treatment strategies in patients undergoing surgery for hepatocellular carcinoma. Dig Dis. 2013;31:112–117. doi: 10.1159/000347205. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann H, Kettelhack C. Fast-track surgery: conditions and challenges in postsurgical treatment: a review of elements of translational research in enhanced recovery after surgery. Eur Surg Res. 2012;49:24–34. doi: 10.1159/000339859. [DOI] [PubMed] [Google Scholar]
- 14.Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, et al. Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg. 2002;236:759–766. doi: 10.1097/01.SLA.0000036269.60340.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The FO, Buist MR, Lei A, Bennink RJ, Hofland J, van den Wijngaard RM, et al. The role of mast cell stabilization in treatment of postoperative ileus: a pilot study. Am J Gastroenterol. 2009;104:2257–2266. doi: 10.1038/ajg.2009.268. [DOI] [PubMed] [Google Scholar]
- 16.Shafaee Z, Kazaryan AM, Marvin MR, Cannon R, Buell JF, Edwin B, et al. Is laparoscopic repeat hepatectomy feasible? A tri-institutional analysis. J Am Coll Surg. 2011;212:171–179. doi: 10.1016/j.jamcollsurg.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Laurent A, Tayar C, Andreoletti M, Lauzet JY, Merle JC, Cherqui D. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:310–314. doi: 10.1007/s00534-009-0063-0. [DOI] [PubMed] [Google Scholar]
- 18.Cho A, Yamamoto H, Nagata M, Takiguchi N, Shimada H, Kainuma O, et al. Safe and feasible inflow occlusion in laparoscopic liver resection. Surg Endosc. 2010;24:246. doi: 10.1007/s00464-009-0667-0. [DOI] [PubMed] [Google Scholar]
- 19.Machado MA, Makdissi FF, Galvao FH, Machado MC. Intrahepatic Glissonian approach for laparoscopic right segmental liver resections. Am J Surg. 2008;196:e38–e42. doi: 10.1016/j.amjsurg.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, et al. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc. 2010;24:1170–1176. doi: 10.1007/s00464-009-0745-3. [DOI] [PubMed] [Google Scholar]
- 21.Belli G, Limongelli P, Fantini C, D'Agostino A, Cioffi L, Belli A, et al. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2009;96:1041–1048. doi: 10.1002/bjs.6680. [DOI] [PubMed] [Google Scholar]