Abstract
Background: Mobilization of maternal bone mineral partly supplies calcium for fetal and neonatal bone growth and development.
Objective: We investigated whether pregnant women with low calcium intakes may have a more extensive skeletal response postpartum that may compromise their short- or long-term bone health.
Design: In a subset of participants (n = 125) in a double-blind, randomized, placebo-controlled trial (International Trial Registry: ISRCTN96502494) in pregnant women in The Gambia, West Africa, with low calcium intakes (≈350 mg Ca/d), we measured bone mineral status of the whole body, lumbar spine, and hip by using dual-energy X-ray absorptiometry and measured bone mineral status of the forearm by using single-photon absorptiometry at 2, 13, and 52 wk lactation. We collected blood and urine from the subjects at 20 wk gestation and at 13 wk postpartum. Participants received calcium carbonate (1500 mg Ca/d) or a matching placebo from 20 wk gestation to parturition; participants did not consume supplements during lactation.
Results: Women who received the calcium supplement in pregnancy had significantly lower bone mineral content (BMC), bone area (BA), and bone mineral density (BMD) at the hip throughout 12 mo lactation (mean ± SE difference: BMC = −10.7 ± 3.7%, P = 0.005; BA = −3.8 ± 1.9%, P = 0.05; BMD = −6.9 ± 2.6%, P = 0.01). The women also experienced greater decreases in bone mineral during lactation at the lumbar spine and distal radius and had biochemical changes consistent with greater bone mineral mobilization.
Conclusions: Calcium supplementation in pregnant women with low calcium intakes may disrupt metabolic adaptation and may not benefit maternal bone health. Further study is required to determine if such effects persist long term or elicit compensatory changes in bone structure.
INTRODUCTION
In pregnancy, the calcium for fetal bone growth and mineralization is supplied by increases in maternal calcium absorption and mobilization of mineral from the maternal skeleton (1–3). Metabolic changes, including bone mineral mobilization and alterations in calcium-regulating hormones, also occur during lactation (2, 3). The replenishment of maternal bone mineral occurs in late lactation or after breastfeeding stops (2–4). From studies in women with calcium intakes close to recommended intakes, these changes appear to be independent of dietary intake and can be regarded as physiologic (1, 2, 5, 6). However, for many women, especially in traditional societies in Africa and elsewhere, calcium intakes are low, and the demands on calcium economy are high because of repeated cycles of pregnancy and lactation (2). It is possible that such low calcium intakes during pregnancy are insufficient to meet requirements and compromise the bone health of the mother or her infant.
In a double-blind, placebo-controlled trial of calcium supplementation (1500 mg Ca/d) in pregnant women in The Gambia, West Africa, with very low calcium intakes (≈350 mg Ca/d), we studied a subset of 125 mothers and their infants to investigate the effects of an increase in calcium intake during pregnancy on bone health. The calcium supplement was of no significant benefit to the infant in terms of birth weight, growth, and bone mineral status in the first year of life and had no significant effect on breast-milk calcium concentrations (7). In this article, we report the effects of the calcium supplement on maternal bone mineral status at the whole body, lumbar spine, hip, and forearm at 2, 13, and 52 wk postpartum and on maternal biochemistry at 13 wk postpartum.
SUBJECTS AND METHODS
Participants and study design
A subset of 125 participants in a trial of pregnancy calcium supplementation and blood pressure in rural Gambian women (International Trial Registry: ISRCTN96502494) was invited to participate in an investigation of bone outcomes for the mother and infant in the first year postpartum. This represented all women in the 2 villages of Keneba and Manduar, West Kiang, The Gambia, over a period of ≈3 y who completed the pregnancy phase of the main trial and subsequently delivered a healthy singleton baby. The women had been randomly assigned, double-blind and in permuted blocks of 4, to receive a supplement that contained 1500 mg Ca/d (3 calcium carbonate tablets; Calcichew; Nycomed Pharma AS, Asker, Norway; distributed by Shire Pharmaceuticals, Andover, United Kingdom) or matching placebo (microcrystalline cellulose and lactose; Nycomed Pharma AS) from 20 wk of pregnancy to parturition. The tablets were delivered daily to participants by fieldworkers and consumed between the midday and evening meals. The tablets were well accepted, with no reports of adverse effects, and compliance was high: 97% of participants consumed ≥95% of the tablets offered. The mean (±SD) supplementation period in pregnancy was 136 ± 15 d. The supplementation program was stopped at delivery, and no calcium supplements were consumed during lactation. Detailed descriptions of the inclusion criteria for the bone-outcomes study, the flowchart of recruitment, exclusions and losses of participants, the supplementation protocol, dietary assessment methods, and the results of the study of breast-milk calcium and infant outcomes were published (7). Mean (±SD) subject characteristics at 20 wk of pregnancy were as follows: age = 27.4 ± 7.5 y; weight = 56.3 ± 6.7 kg; height = 1.61 ± 0.05 m; dietary calcium intake = 355 ± 190 mg Ca/d; dietary phosphorus intake = 769 ± 291 mg P/d; and median parity = 3 (range: 0–10) (16% of subjects were primigravidae). There were no significant differences between the supplement groups before supplementation.
Bone absorptiometry and measurements of height and weight were scheduled at 2, 13, and 52 wk postpartum (7). All subjects attended each appointment, and no woman refused consent for any of the procedures. Blood and urine samples were collected at 13 wk postpartum. Blood and urine were collected at 20 wk of pregnancy before the start of supplementation, and samples were available for analysis. All mothers in the study were lactating throughout the first year postpartum, which was consistent with the custom in this region for women to breastfeed each child on demand for about 2 y with complementary foods introduced from around 4 to 6 mo of age. Descriptions of this population, with special reference to calcium intake and forearm bone mineral status, were published elsewhere (8, 9).
The study was approved by the joint Medical Research Council Gambia and the Gambian Government Ethics Committee. All subjects gave written informed consent to participate in the study after being given a verbal explanation in their local language.
Data collection and procedures
Bone absorptiometry of the whole body, lumbar spine (lumbar vertebrae 1–4), and hip (total, shaft, trochanter, and neck) was conducted by dual-energy X-ray absorptiometry (DXA) (Lunar DPX+ software version 4.7b; Lunar Radiation Corporation, Madison, WI). The outcome variables were bone mineral content [BMC (g)], bone area [BA (cm2)], bone mineral density ([BMD (g/cm2) = BMC/BA], and size-adjusted BMC [SA-BMC = BMC adjusted for BA, weight, and height (10)]. All hip regions were included because the total hip is a composite of femoral neck, greater trochanter, and femoral shaft regions, and reliance on clinically relevant measurements at the total hip and femoral neck can mask physiologic changes in other regions of the hip (4). Bone measurements of the distal radius (5 mm intraosseous space) and midshaft radius (one-third of the forearm length measured from the distal tip of the ulna) were obtained by single-photon absorptiometry (SPA) (Lunar SP2; Lunar Radiation Corporation) (1, 7, 8). The outcome variables were BMC (g/cm), bone width [BW (cm)], BMD [(g/cm2) = BMC/BW] and SA-BMC [BMC adjusted for BW, weight, and height (10)]. Calibration and performance of the DXA (unpublished data, 2007) and SPA (7) were monitored regularly and showed satisfactory stability and precision. However, as described elsewhere (7), it was not possible to collect 3 sets of bone measurements for every participant (ie, one at each of the 3 time points) because DXA was not available at the start of the study and because of periodic technical problems with the instrumentation or scan quality. The total numbers of subjects in the calcium and placebo groups, respectively, who were measured by SPA were as follows: at 2 wk = 56 (92%) and 60 (94%) subjects; at 13 wk = 54 (89%) and 55 (86%) subjects; and at 52 wk = 48 (77%) and 45 (70%) subjects. The total numbers of subjects in the calcium and placebo groups, respectively, who were measured by DXA were as follows: at 2 wk = 24 (39%) and 27 (42%) subjects; at 13 wk = 29 (48%) and 29 (45%) subjects; and at 52 wk = 40 (66%) and 39 (61%) subjects. The proportions of subjects who were successfully measured on ≥1, ≥2, and all 3 occasions, respectively, were as follows: SPA = 94%, 91%, and 70% of subjects, and DXA = 66%, 47%, and 37% of subjects. The proportions of subjects were similar in the 2 groups.
Successful blood and urine collections were obtained from the majority of subjects (n = 123; except n = 115 for urine at 13 wk). Overnight-fasting blood samples were collected into precooled tubes containing lithium heparin for most analytes or potassium EDTA for parathyroid hormone (PTH). The tubes were immediately centrifuged at 4°C, and plasma was stored at −40°C before transportation to MRC Human Nutrition Research, Cambridge, United Kingdom, and storage at −80°C before analysis. Wherever possible, samples from the same individual and from subjects within the same randomization block were analyzed together. All samples were measured in duplicate; if results differed by >10%, the samples were reanalyzed. Intact PTH (1–84) and intact osteocalcin (1–49) were measured by immunoradiometric assay (Diasorin, Wokingham, United Kingdom). Radioimmunoassay was used for calcitonin (Calcitonin II; Diasorin), 25-hydroxyvitamin D (Diasorin) and 1,25-dihydroxyvitamin D [1,25(OH)2D] (Gamma B; IDS, Bolton, United Kingdom). Calcium, inorganic phosphate, creatinine, and total and bone-specific alkaline phosphatase were measured by colorimetry (Roche Diagnostics, Lewes, United Kingdom) on a centrifugal analyzer as follows: calcium was measured by the methyl thymol blue method (Roche Uni-Kit II; Roche Diagnostics); phosphate was measured by the ammonium molybdate method (Roche Uni-mate 7; Roche Diagnostics); creatinine was measured by the kinetic buffered Jaffe method without deproteinization; and total and bone-specific alkaline phosphatase were measured by the p-nitrophenyl phosphate method (Roche Alp MPR2; Roche Diagnostics) at 37°C before and after lectin precipitation, respectively. Albumin was measured with nephelometry by using anti-human albumin antibody (Diasorin). Accuracy and precision were monitored across the working range of the assays by using external quality-assurance reference materials (National External Quality Assessment Scheme, Department of Clinical Biochemistry, Royal Infirmary, Edinburgh, United Kingdom; Vitamin D External Quality Assessment Scheme, Endocrine/Oncology, Charing Cross Hospital, London, United Kingdom) or purchased commercially (Roche Human Control; Roche Diagnostics). In addition, an aliquot of a pooled plasma sample was assayed in each batch to monitor drift.
Urine was collected over 24 h as described previously (7). Titratable acidity was measured before urine processing (7). An aliquot was acidified to 0.3 mol/L with HCl, stored at −20°C, and transported to MRC Human Nutrition Research, Cambridge, United Kingdom, for calcium, phosphate, and creatinine analysis by using the plasma methods but with acidified standards and reference materials. An aliquot of unacidified urine was stored for the measurement of free deoxypyridinoline by enzyme-linked immunosorbent assay (Metra Systems, Wheatley, United Kingdom).
Statistical analyses
Statistical analyses were performed with DataDesk software (version 6.2.1; Data Description Inc, Ithaca, NY). Data were transformed to natural logarithms to normalize skewness and to investigate proportional effects (10). In natural logarithms, the effect of a variable × 100 corresponds closely to the effect expressed as a percentage (11). Descriptive characteristics are presented as mean (±SD) or geometric mean (−1 SD, +1 SD) depending on the distribution of the data. The effects of the pregnancy supplement are presented as mean (95% CI) percentage differences between the groups derived from coefficients in the statistical models described below.
We used regression analyses to determine the effect of the pregnancy supplement at 2 wk postpartum on BMC, BA (or BW for SPA measures), and BMD in each skeletal region of interest. Consideration of SA-BMC was achieved by using multiple linear regression analysis of BMC with BA (or BW), body weight, height, and supplement group (calcium coded as 1, placebo coded as 0) as independent variables (10). Nonsignificant variables (P > 0.05) were removed by backward elimination. Age, parity, tablet compliance, season, and dietary calcium intake were considered potential confounders, but they did not materially alter the supplement effect and, for simplicity, are not included in the models presented. Regression analysis was also used to examine the effect of supplement group on maternal biochemical indexes at 13 wk postpartum after adjustment for the value of each variable at 20 wk of pregnancy.
We examined the influence of the pregnancy supplement on changes in BMC, BA (or BW), and BMD from 2 to 13 and 52 wk postpartum by using repeated-measures analysis of variance or covariance with Scheffé post hoc tests. This was performed with the use of hierarchical linear models that included subject (nested by supplement group), time (2, 13, or 52 wk), supplement group (calcium or placebo) plus a time-by-group–interaction term. For SA-BMC, BA (or BW) and weight were also included, and parsimonious models were produced. Because in such analyses each subject acts as their own control, a full set of data per individual was not required, and the models were constructed with all available data. Similar results were obtained when the analyses were restricted to subjects with a complete dataset, although the statistical significance was reduced (data not presented).
RESULTS
The weight and bone mineral status of the 2 groups at 2 wk postpartum are compared in Tables 1 and 2. There were no significant differences in weight, BMC, BA (or BW), BMD, or SA-BMC at the whole body, lumbar spine, or radius. In contrast, there were differences consistent with lower bone mineral status at the hip in the calcium group. These differences were significant at the total hip and femoral shaft and were of similar magnitude but not significant at the other subregions. There was also a trend toward smaller BA in the calcium group at all hip sites, which was significant at the total hip.
TABLE 1.
Effect of calcium supplementation in pregnancy on maternal weight and bone mineral status at the radius, spine, and whole body at 2 wk postpartum1
| Calcium vs placebo groups |
||||
| Calcium group | Placebo group | Percentage Δ (95% CI)2 | P | |
| Weight | ||||
| n | 61 | 64 | ||
| Weight (kg) | 55.6 ± 7.23 | 54.4 ± 6.7 | 2.0 (−2.4, 6.4) | 0.4 |
| Height | ||||
| n | 61 | 64 | ||
| Height (m) | 1.61 ± 0.05 | 1.61 ± 0.05 | 0.0 (−1.2, 1.2) | 0.9 |
| Whole body | ||||
| n | 24 | 27 | ||
| BMC (g) | 2252 ± 284 | 2248 ± 269 | 0.2 (−6.6, 7.0) | 0.9 |
| BA (cm2) | 2028 ± 171 | 2026 ± 187 | 0.2 (−4.6, 5.0) | 0.9 |
| BMD (g/cm2) | 1.108 ± 0.061 | 1.108 ± 0.057 | 0.0 (−3.0, 3.0) | 0.9 |
| SA-BMC (g) | 2237 ± 105 | 2237 ± 105 | 0.0 (−2.6, 2.6) | 0.9 |
| Lumbar spine | ||||
| n | 23 | 27 | ||
| BMC (g) | 48.7 ± 6.8 | 47.6 ± 8.7 | 3.1 (−6.3, 12.5) | 0.5 |
| BA (cm2) | 48.4 ± 4.0 | 47.1 ± 5.2 | 3.0 (−2.6, 8.6) | 0.3 |
| BMD (g/cm2) | 1.005 ± 0.101 | 1.006 ± 0.112 | 0.1 (−6.1, 6.3) | 0.9 |
| SA-BMC (g) | 47.5 ± 4.7 | 47.9 ± 5.0 | −0.8 (−6.8, 5.2) | 0.8 |
| Distal radius | ||||
| n | 53 | 60 | ||
| BMC (g/cm) | 0.706 ± 0.091 | 0.729 ± 0.102 | −3.2 (−8.3, 1.9) | 0.2 |
| BW (cm) | 2.326 ± 0.195 | 2.375 ± 0.152 | −2.2 (−5.0, 0.6) | 0.1 |
| BMD (g/cm2) | 0.305 ± 0.041 | 0.308 ± 0.042 | −1.1 (−6.2, 4.0) | 0.7 |
| SA-BMC (g/cm) | 0.708 ± 0.083 | 0.726 ± 0.096 | −2.4 (−7.2, 2.4) | 0.3 |
| Midshaft radius | ||||
| n | 56 | 60 | ||
| BMC (g/cm) | 0.803 ± 0.082 | 0.818 ± 0.074 | −2.0 (−5.6, 1.6) | 0.3 |
| BW (cm) | 1.204 ± 0.114 | 1.213 ± 0.119 | −0.7 (−4.3, 2.9) | 0.7 |
| BMD (g/cm2) | 0.668 ± 0.055 | 0.679 ± 0.064 | −1.4 (−4.8, 2.0) | 0.4 |
| SA-BMC (g/cm) | 0.801 ± 0.060 | 0.818 ± 0.062 | −2.1 (−4.9, 0.7) | 0.2 |
Whole-body and spine scans were conducted by dual-energy X-ray absorptiometry, and radius scans were conducted by single-photon absorptiometry (SPA). BMC, bone mineral content; BA, bone area; BMD, bone mineral density; SA-BMC, size-adjusted BMC [derived by including BA (for dual-energy X-ray absorptiometry) or bone width (BW; for SPA), body weight, and height in the logarithmic model; evaluating the residual for each subject; adding the residual to loge (mean BMC) value; and calculating the antilogarithm].
Differences between groups expressed as percentages derived by using regression analysis with continuous variables transformed to natural logarithms.
Mean ± SD (all such values).
TABLE 2.
Effect of calcium supplementation in pregnancy on maternal bone mineral status at the hip at 2 wk postpartum1
| Calcium vs placebo groups |
||||
| Calcium group (n = 20) | Placebo group (n = 23) | Percentage Δ (95% CI)2 | P | |
| Total hip | ||||
| BMC (g) | 27.4 ± 3.83 | 31.5 ± 5.5 | −13.1 (−23.4, −2.9) | 0.01 |
| BA (cm2) | 27.4 ± 1.8 | 29.0 ± 2.7 | −5.5 (−10.5, −0.5) | 0.03 |
| BMD (g/cm2) | 0.999 ± 0.106 | 1.087 ± 0.117 | −7.7 (−14.7, −0.7) | 0.03 |
| SA-BMC (g) | 28.7 ± 2.9 | 29.8 ± 2.9 | −3.8 (−10.1, 2.5) | 0.2 |
| Femoral shaft | ||||
| BMC (g) | 15.4 ± 1.8 | 17.6 ± 2.6 | −12.8 (−21.2, −4.4) | 0.004 |
| BA (cm2) | 13.3 ± 0.8 | 13.9 ± 1.2 | −3.7 (−8.3, 0.8) | 0.1 |
| BMD (g/cm2) | 1.161 ± 0.136 | 1.272 ± 0.149 | −9.1 (−16.8, −1.4) | 0.02 |
| SA-BMC (g) | 15.6 ± 1.7 | 17.4 ± 1.9 | −10.5 (−17.8, −3.2) | 0.006 |
| Trochanter | ||||
| BMC (g) | 7.75 ± 1.63 | 9.11 ± 2.40 | −14.9 (−31.9, 2.2) | 0.09 |
| BA (cm2) | 9.81 ± 1.45 | 10.63 ± 1.83 | −7.7 (−18.2, 2.8) | 0.1 |
| BMD (g/cm2) | 0.784 ± 0.093 | 0.845 ± 0.115 | −7.2 (−15.6, 1.2) | 0.09 |
| SA-BMC (g) | 8.02 ± 0.84 | 8.39 ± 0.73 | −4.7 (−10.7, 1.3) | 0.1 |
| Femoral neck | ||||
| BMC (g) | 4.25 ± 0.85 | 4.72 ± 0.94 | −10.6 (−23.6, 2.3) | 0.1 |
| BA (cm2) | 4.28 ± 0.59 | 4.52 ± 0.71 | −5.2 (−14.8, 4.3) | 0.3 |
| BMD (g/cm2) | 0.988 ± 0.112 | 1.042 ± 0.107 | −5.4 (−12.6, 1.8) | 0.1 |
| SA-BMC (g) | 4.33 ± 0.47 | 4.53 ± 0.46 | −4.6 (−11.7, 2.5) | 0.2 |
Hip scans were conducted by dual-energy X-ray absorptiometry. BMC, bone mineral content; BA, bone area; BMD, bone mineral density; SA-BMC, size-adjusted BMC [derived by including BA, body weight, and height in the logarithmic model; evaluating the residual for each subject; adding the residual to the loge (mean BMC) value; and calculating the antilogarithm].
Differences between groups expressed as percentages derived by using regression analysis with continuous variables transformed to natural logarithms.
Mean ± SD (all such values) with use of all available data.
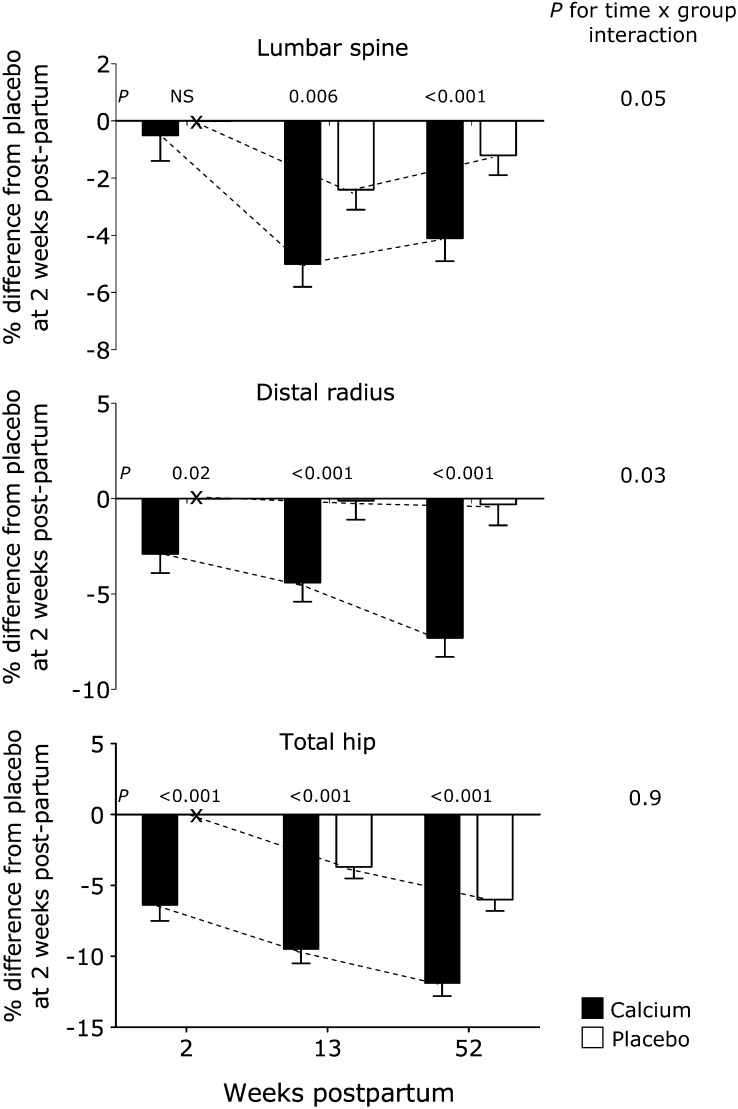
The changes in weight and bone outcomes in the 2 groups from 2 to 13 and 2 to 52 wk postpartum are presented in Tables 3 and 4. Weight decreased significantly with time, as did BMC, BMD, and SA-BMC at all skeletal sites except the midshaft radius. Values were lower at 52 wk than at 13 wk except at the lumbar spine (Figure 1). The decreases were seen in both groups except at the distal radius, where the time effect was not significant in the placebo group. However, the decreases at the lumbar spine and distal radius were greater in the calcium group, as shown by significant time-by-group interactions (Table 3, Figure 1). By 52 wk, BMC, BMD, and SA-BMC at these sites and in whole-body BMC were 2–4% lower in the calcium group than in the placebo group. There were no time-by-group interactions at the hip in either total or individual sub-regions, but the group differences seen at 2 wk remained at 13 and 52 wk (Table 4, Figure 1). The group differences at the total hip, averaged over the 3 time points, were substantial with BMC = −10.7%, BA = −3.8%, BMD = −6.9%, and SA-BMC = −6.0%. Significant differences of similar magnitude were noted at the hip subregions (Table 4). There were also significant decreases in BA at the whole body and lumbar spine that paralleled decreases in BMC but not at the forearm or hip. However, there were no significant time-by-group interactions for BA at any site, which indicated that the changes in BA were comparable in the 2 groups.
TABLE 3.
Changes in maternal body weight and bone mineral status at the whole body, lumbar spine, and radius at 13 and 52 wk postpartum1
| Percentage change from 2 wk2 |
|||||||
| 13 wk |
52 wk |
P3 |
|||||
| Calcium group | Placebo group | Calcium group | Placebo group | Time | Group | Time × group interaction | |
| Body weight | |||||||
| n | 61 | 62 | 60 | 60 | |||
| Weight | −1.0 ± 0.8 | −1.3 ± 0.8 | −4.8 ± 0.845 | −3.9 ± 0.846 | ≤0.001 | 0.4 | 0.5 |
| Whole body | |||||||
| n | 29 | 27 | 38 | 39 | |||
| BMC | −1.6 ± 0.7 | −1.8 ± 0.77 | −5.0 ± 0.745 | −2.9 ± 0.74 | ≤0.001 | 0.9 | 0.03 |
| BA | −0.7 ± 0.6 | −1.1 ± 0.6 | −2.5 ± 0.646 | −1.2 ± 0.6 | ≤0.001 | 0.5 | 0.1 |
| BMD | −0.9 ± 0.38 | −0.7 ± 0.3 | −2.6 ± 0.345 | −1.7 ± 0.346 | ≤0.001 | 0.4 | 0.1 |
| SA-BMC | −0.9 ± 0.37 | −0.7 ± 0.3 | −2.6 ± 0.345 | −1.7 ± 0.346 | ≤0.001 | 0.06 | 0.1 |
| Lumbar spine | |||||||
| n | 29 | 29 | 40 | 39 | |||
| BMC | −5.3 ± 0.94 | −2.1 ± 0.97 | −3.5 ± 1.08 | −0.2 ± 0.9 | ≤0.001 | 0.9 | 0.02 |
| BA | −0.5 ± 0.4 | 0.2 ± 0.4 | 0.5 ± 0.59 | 0.1 ± 0.4 | 0.006 | 0.7 | 0.5 |
| BMD | −4.8 ± 0.74 | −2.3 ± 0.78 | −4.1 ± 0.84 | −1.1 ± 0.7 | ≤0.001 | 0.6 | 0.01 |
| SA-BMC | −4.5 ± 0.74 | −2.4 ± 0.78 | −3.6 ± 0.84 | −1.2 ± 0.7 | ≤0.001 | 0.5 | 0.05 |
| Distal radius | |||||||
| n | 53 | 54 | 48 | 45 | |||
| BMC | −1.2 ± 1.0 | 0.1 ± 1.0 | −4.0 ± 1.189 | −0.2 ± 1.1 | 0.02 | 0.06 | 0.06 |
| BW | 0.7 ± 0.6 | 0.5 ± 0.6 | 1.2 ± 0.6 | 0.2 ± 0.6 | 0.2 | 0.8 | 0.4 |
| BMD | −1.9 ± 1.1 | −0.6 ± 1.1 | −5.2 ± 1.249 | −0.5 ± 1.2 | 0.004 | 0.2 | 0.02 |
| SA-BMC | −1.4 ± 1.0 | −0.1 ± 1.0 | −4.3 ± 1.149 | −0.3 ± 1.1 | 0.01 | 0.08 | 0.03 |
| Midshaft radius | |||||||
| n | 54 | 55 | 48 | 45 | |||
| BMC | 0.6 ± 0.8 | 0.3 ± 0.8 | −0.3 ± 0.8 | 0.4 ± 0.8 | 0.9 | 0.2 | 0.7 |
| BW | 0.2 ± 0.4 | 0.3 ± 0.4 | −0.2 ± 0.5 | 0.6 ± 0.5 | 0.7 | 0.6 | 0.5 |
| BMD | 0.5 ± 0.8 | 0.0 ± 0.8 | −0.7 ± 0.9 | −0.2 ± 0.9 | 0.8 | 0.5 | 0.9 |
| SA-BMC | 0.6 ± 0.8 | 0.2 ± 0.8 | −0.2 ± 0.8 | 0.3 ± 0.8 | 0.7 | 0.9 | 0.8 |
Radius scans were conducted by single-photon absorptiometry (SPA), and spine and whole-body scans were conducted by dual-energy X-ray absorptiometry. BMC, bone mineral content; BA, bone area; BMD, bone mineral density; SA-BMC, size-adjusted BMC derived by including bone width (BW; for SPA) or BA (for dual-energy X-ray absorptiometry) and body weight in the hierarchical ANCOVA model. Body weight was a significant independent variable of bone mineral status at the lumbar spine only.
All values are means ± SEs over time expressed as percentages derived from hierarchical ANOVA and ANCOVA models with continuous variables in natural logarithms that involve subjects nested by group, time, and time × group interaction terms.
P values for each component from the interaction model.
Significance of change within each group from 2 wk to 13 or 52 wk: 4P ≤ 0.001, 7P ≤ 0.05, 8P ≤ 0.01.
Significance of change within each group from 13 to 52 wk: 5P ≤ 0.001, 6P ≤ 0.01, 9P ≤ 0.05.
TABLE 4.
Changes in maternal bone mineral status at the hip at 13 and 52 wk postpartum1
| Percentage change from 2 wk2 |
|||||||
| 13 wk |
52 wk |
P3 |
|||||
| Calcium group (n = 25) | Placebo group (n = 27) | Calcium group (n = 39) | Placebo group (n = 37) | Time | Group | Overall calcium effect4 | |
| Total hip | |||||||
| BMC | −4.0 ± 1.25 | −6.2 ± 1.56 | −7.0 ± 1.66 | −8.3 ± 1.56 | ≤0.001 | 0.005 | −10.7 (−18.1, −3.4) |
| BA | −0.7 ± 1.1 | −2.0 ± 1.0 | −1.3 ± 1.1 | −1.9 ± 1.1 | 0.08 | 0.05 | −3.8 (−7.6, 0.0) |
| BMD | −3.3 ± 0.97 | −4.2 ± 0.86 | −5.8 ± 0.968 | −6.4 ± 0.969 | ≤0.001 | 0.01 | −6.9 (−12.1, −1.7) |
| SA-BMC | −3.1 ± 0.97 | −3.7 ± 0.86 | −5.5 ± 0.968 | −6.0 ± 0.869 | ≤0.001 | 0.05 | −6.0 (−12.0, 0.0) |
| Femoral shaft | |||||||
| BMC | −2.6 ± 1.5 | −5.6 ± 1.46 | −5.2 ± 1.57 | −7.0 ± 1.46 | ≤0.001 | 0.003 | −9.3 (−15.3, −3.3) |
| BA | 0.3 ± 1.0 | −1.7 ± 1.0 | 0.0 ± 1.0 | −1.0 ± 1.0 | 0.6 | 0.3 | −1.6 (−4.7, 1.5) |
| BMD | −2.9 ± 0.87 | −3.9 ± 0.86 | −5.2 ± 0.868 | −6.0 ± 0.869 | ≤0.001 | 0.01 | −7.7 (−13.4, −1.9) |
| SA-BMC | −2.9 ± 0.87 | −3.6 ± 0.86 | −5.2 ± 0.868 | −5.8 ± 0.869 | ≤0.001 | 0.02 | −7.3 (−13.3, −1.3) |
| Trochanter | |||||||
| BMC | −6.1 ± 2.9 | −6.9 ± 2.75 | −10.4 ± 2.97 | −10.3 ± 2.87 | ≤0.001 | 0.02 | −15.2 (−27.7, −2.7) |
| BA | −2.0 ± 2.5 | −2.1 ± 2.4 | −3.6 ± 2.5 | −2.4 ± 2.4 | 0.2 | 0.05 | −7.7 (−15.6, 0.2) |
| BMD | −4.1 ± 1.37 | −4.8 ± 1.36 | −6.8 ± 1.36 | −7.9 ± 1.369 | ≤0.001 | 0.02 | −7.5 (−13.7, −1.3) |
| SA-BMC | −4.1 ± 1.47 | −4.7 ± 1.37 | −6.7 ± 1.46 | −7.8 ± 1.369 | ≤0.001 | 0.02 | −7.1 (−13.1, −1.1) |
| Femoral neck | |||||||
| BMC | −5.1 ± 2.05 | −6.9 ± 1.97 | −8.8 ± 2.06 | −8.9 ± 2.06 | ≤0.001 | 0.07 | −8.5 (−17.7, 0.7) |
| BA | −1.0 ± 1.7 | −2.6 ± 1.6 | −1.1 ± 1.7 | −2.8 ± 1.6 | 0.2 | 0.6 | −2.1 (−9.1, 4.9) |
| BMD | −4.2 ± 1.17 | −4.3 ± 1.16 | −7.6 ± 1.169 | −6.1 ± 1.16 | ≤0.001 | 0.02 | −6.4 (−11.6, −1.2) |
| SA-BMC | −4.2 ± 1.27 | −4.2 ± 1.16 | −7.6 ± 1.269 | −6.1 ± 1.16 | ≤0.001 | 0.02 | −6.0 (−11.4, −0.6) |
Hip scans were conducted by dual-energy X-ray absorptiometry. BMC, bone mineral content; BA, bone area; BMD, bone mineral density; SA-BMC, size-adjusted BMC derived by including BA and body weight in the hierarchical ANCOVA model. Body weight was not a significant independent variable of bone mineral status at the hip and was removed from the models presented.
All values are means ± SEs from hierarchical ANOVA and ANCOVA models with continuous variables in natural logarithms that involve subjects nested by group, time, and time × group interaction terms.
P values for each component from the model without the interaction term. The time × group interaction term was not significant at any site in the hip.
Overall magnitude of group differences expressed as mean (95% CI) percentage differences obtained from hierarchical models without interaction terms and representing the average calcium effect over all 3 time points.
Significance of differences from 2 to 13 and 52 wk within each group: 5P ≤ 0.05, 6P ≤ 0.001, 7P ≤ 0.01.
Significance of differences from 13 to 52 wk within each group: 8P ≤ 0.01, 9P ≤ 0.05.
FIGURE 1.
Effect of the calcium supplement in pregnancy on size-adjusted bone mineral content (SA-BMC) of the lumbar spine, distal radius, and total hip at 2, 13, and 52 wk postpartum. SA-BMC = bone mineral content adjusted for bone area (or bone width), weight, and height. Bars and error bars represent the mean ± SE percentage differences in SA-BMC relative to the placebo group at 2 wk postpartum in the calcium group (solid bars) and placebo group (open bars). Dotted lines represent the apparent time trend within each group. An “x” on the x axes denotes placebo value at 2 wk postpartum and is used as the reference and set at zero. Results were obtained from Scheffé post hoc tests for time × group interaction terms in hierarchical repeated-measures ANOVA models that included subject (nested by group), time, group, and time × group interaction. The P values depicted are for the comparison of calcium and placebo groups at each time point. The numbers of subjects at 2, 13, and 52 wk, respectively, were as follows—for the lumbar spine: 23, 29, and 40 in the calcium group and 27, 29, and 39 in the placebo group; for the distal radius: 53, 53, and 48 in the calcium group and 60, 54, and 45 in the placebo group; and for the total hip: 20, 25, and 39 in the calcium group and 23, 27, and 37 in the placebo group.
The data for the plasma and urinary indexes are presented in Table 5. Significant group differences were observed at 13 wk postpartum in urinary mineral outputs and plasma concentrations of vitamin D metabolites, other calciotropic hormones, and bone turnover markers. The pattern of biochemical differences was suggestive of a greater turnover of minerals between the maternal skeleton and the extracellular pool in the calcium group with a net bone mineral loss (ie, greater urinary calcium and phosphorus excretion, lower plasma PTH and 1,25(OH)2D concentrations, higher plasma calcitonin and 25-hydroxyvitamin D concentrations, and lower plasma bone-specific alkaline phosphatase concentrations with trends toward lower plasma osteocalcin concentrations and greater urinary deoxypyridinoline excretions). There were no significant group differences in plasma concentrations of minerals and albumin (Table 5) or in urinary titratable acidity and creatinine clearance (data not presented). The effects of the pregnancy supplement were superimposed on the changes in biochemistry from 20 wk of pregnancy to 13 wk postpartum as experienced by both groups of women (ie, significant reductions in urinary calcium excretion and plasma concentrations of vitamin D metabolites plus increases in plasma concentrations of PTH and bone turnover markers) (Table 5).
TABLE 5.
Effect of calcium supplementation in pregnancy on maternal biochemistry at 13 wk postpartum1
| 20 wk pregnancy2 |
13 wk postpartum2 |
Group effect |
||||
| Calcium group | Placebo group | Calcium group | Placebo group | Percentage Δ (95% CI)3 | P | |
| Urinary mineral outputs (mg/d) | ||||||
| Calcium | 67.0 (30.4, 148) | 66.0 (26.2,166) | 44.0 (19.7, 98.5)4 | 31.8 (12.6, 0.3)4 | +31.6 (0.2, 62.9) | 0.05 |
| Phosphorus | 327 (193, 554) | 348 (213, 569) | 406 (248, 665)5 | 343 (230, 511) | +15.6 (0.0, 31.1) | 0.05 |
| Vitamin D metabolites and other calciotropic hormones | ||||||
| 25(OH)D (nmol/L) | 101 (80.1, 127) | 100 (76.8, 130) | 76.4 (61.0, 95.6)4 | 68.9 (53.7, 88.4)4 | +9.8 (3.7, 15.9) | 0.0026 |
| 1,25(OH)2D (pmol/L) | 397 (298, 530) | 371 (278, 495) | 229 (163, 322)4 | 243 (186, 317)4 | −9.4 (−19.0, 0.2) | 0.056 |
| PTH (ng/L) | 18.3 (11.7, 28.7) | 20.7 (12.4, 34.6) | 32.2 (20.6, 50.3)5 | 39.4 (23.1, 67.1)5 | −17.0 (−34.2, 0.3) | 0.05 |
| Calcitonin (ng/L) | 46.7 (32.7, 66.7) | 46.8 (33.8, 64.9) | 49.7 (34.6, 71.6) | 44.6 (28.4, 70.1) | +11.1 (−0.3, 22.6) | 0.066 |
| Bone markers | ||||||
| Bone alkaline phosphatase (U/L) | 32.6 (20.4, 52.0) | 30.4 (16.7, 55.5) | 78.3 (52.8, 116)5 | 84.8 (57.5, 125)5 | −11.0 (−22.1, 0.2) | 0.056 |
| Total alkaline phosphatase (U/L) | 117 (86.7, 158) | 109 (74.8, 160) | 201 (164, 247)5 | 196 (144, 267)5 | +0.8 (−6.6, 8.1) | 0.8 |
| Osteocalcin (μg/L) | 4.05 (2.50, 6.56) | 3.45 (1.68, 7.09) | 10.5 (6.61, 16.6)5 | 11.0 (6.81, 17.7)5 | −11.4 (−25.6, 2.8) | 0.16 |
| Deoxypyridinoline output (nmol/d) | 48.2 (28.9, 80.4) | 47.6 (27.1, 83.8) | 72.1 (47.8, 109)5 | 62.5 (37.6, 104)5 | +9.1 (−4.7, 22.9) | 0.2 |
| Plasma minerals and albumin | ||||||
| Calcium (mg/L) | 89.1 (85.3, 93.0) | 88.6 (84.1, 93.4) | 93.9 (88.8, 99.4)5 | 93.6 (89.4, 98.0)5 | +0.2 (−1.6, 2.1) | 0.8 |
| Phosphate (mg/L) | 33.8 (29.8, 38.4) | 34.4 (30.1, 39.4) | 39.9 (34.1, 46.5)5 | 39.4 (34.0, 45.8)5 | +1.5 (−3.9, 6.8) | 0.6 |
| Albumin (g/L) | 31.4 (27.5, 35.8) | 31.6 (27.9, 35.8) | 40.3 (35.8, 45.4)5 | 40.3 (36.0, 45.1)5 | +0.4 (−3.1, 3.8) | 0.8 |
Numbers of subjects with biochemical data: 61 subjects in the calcium group and 62 subjects in the placebo group (except for urinary outputs at 13 wk for which there were 57 subjects in the calcium group and 58 subjects in the placebo group). 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; PTH, parathyroid hormone. There were no significant differences in biochemical analytes between the calcium and placebo groups at 20 wk of pregnancy.
Plasma concentrations or urinary daily outputs expressed as the geometric mean (−1 SD, +1 SD) derived by taking the antilogarithm of the mean ± 1 SD value of the data transformed to logarithms.
Differences between calcium and placebo groups at 13 wk lactation expressed as percentages derived from regression analysis with adjustment for values at 20 wk of pregnancy.
Paired t test of time effect within groups that showed a significant decrease in value from 20 wk of pregnancy (P ≤ 0.001).
Paired t test of time effect within groups that showed a significant increase in value from 20 wk of pregnancy (P ≤ 0.001), except for urinary phosphorus output in the calcium group (P = 0.02).
Group × time interaction by repeated-measures ANOVA (P ≥ 0.05), except for calcitonin and bone alkaline phosphatase (P = 0.06).
DISCUSSION
This study was designed to test whether a greater calcium intake in pregnancy has a beneficial effect on maternal bone outcomes after delivery and during the first year of lactation for women with low calcium intakes. It was conducted in a subset of participants (7) in a trial that investigated the putative effects (12, 13) of calcium supplementation on maternal blood pressure and preeclampsia risk reduction. As described elsewhere, the pregnancy supplement had no significant effect on breast-milk calcium concentrations in the subsequent lactation period, and there was no evidence of beneficial infant outcomes in terms of weight, length, and BMC in the first year of life (7). The expectation was that the study of maternal bone outcomes would show either no effect of the calcium supplement or an increase in bone mineral status and a diminution of lactational bone mobilization. However, we observed the opposite effect. Those women who had received the calcium carbonate supplement during pregnancy had a significantly lower bone mineral status at the hip, which was consistent throughout the 12 mo of the study, and there was evidence of greater bone mineral mobilization at the lumbar spine and distal radius during lactation. The possibility of an accentuated metabolic response to lactation (2, 3) was supported by the biochemical data at 13 wk postpartum, which suggested that there was a greater release of bone mineral into the extracellular pool in those who received the calcium supplement in pregnancy. The calcium supplement had no significant effect on maternal weight or bone size except at the hip, where a smaller BA was observed.
A possible explanation for the unexpected results is that the calcium supplement disrupted the processes of metabolic adaptation to a low calcium intake that was previously noted in rural Gambian women (8, 14). A reduced capability to increase intestinal calcium absorption and/or renal conservation of minerals during pregnancy and lactation might lead to a greater skeletal response and, hence, to greater mobilization of calcium from bone. The latter possibility is supported by the greater urinary calcium excretion of the calcium group at 13 wk postpartum. The classical response to the withdrawal of a calcium supplement is a rise in PTH secretion that promotes renal calcium reabsorption and 1,25(OH)2D-mediated intestinal calcium absorption and bone resorption, with a new steady-state urinary calcium excretion established within a few days (15). The continued difference in urinary calcium output between the groups 3 mo after supplementation ended suggests that the pregnancy calcium supplement may have altered the mothers' ability to conserve calcium. Furthermore, the smaller hip BA in the calcium group suggests diminished expansion of the hip during pregnancy (1), possibly through the suppression of pregnancy-related bone remodeling by calcium (2, 3). However, differences in bone edge detection because of the lower BMC may also be partly responsible (16). We are undertaking further analysis of the DXA scans to provide insights into the effect of the calcium supplement on maternal hip geometry.
The volume of breast milk consumed by the infant is a recognized predictor of the magnitude of the bone mineral changes experienced by the lactating mother (5), and PTH-related peptide produced in the mammary gland in response to suckling is regarded as a principal regulator of the maternal skeletal response to lactation (2, 3). Therefore, another plausible mechanism for the results of this study is that the calcium supplement increased the infant demand for breast milk or altered suckling behavior, which resulted in greater mammary PTH-related peptide production and enhanced maternal bone mobilization to support greater breast-milk calcium secretion. However, this explanation is unlikely given the lack of any significant increase in the calcium content of breast milk or in offspring size and BMC associated with the calcium supplementation (7).
This study was limited by practical difficulties in a challenging field environment of obtaining serial measurements from individuals at specific times. Nevertheless, the findings were robust regardless of whether the outcomes were analyzed with all available data or restricted to women with bone measurements on all 3 occasions. Also, no bone measurements were made during pregnancy before supplementation, and no prepregnancy bone measurements were available. However, the randomization procedure plus the magnitude and statistical significance of the observed differences and the corroborating biochemical data make it unlikely that the observed supplementation effects on maternal bone outcomes were due to recruiting bias or chance.
The changes in bone mineral status by 13 wk lactation that we observed in these Gambian mothers were similar to those reported in breastfeeding women with calcium intakes closer to recommended intakes (2–5, 17–24). However, unlike other studies (4, 25, 26), there was little evidence of replenishment of bone mineral by 12 mo lactation. Indeed the pattern was for further decreases at the hip and, in those who had received the calcium supplement during pregnancy, at the distal radius and whole body. The results may reflect continuation of breastfeeding or prolonged lactational amenorrhea beyond 12 mo, as is common in many traditional societies. Alternatively, the low calcium intakes of the Gambian mothers and/or other aspects of their marginal diet may have been insufficient to enable the restoration of bone mineral during lactation. We are conducting a follow-up study to determine whether the effects of the calcium supplement were temporary or long lasting.
In conclusion, this study suggests that calcium supplementation during pregnancy in women with low calcium intakes leads to lower maternal bone mineral in the hip and to greater bone mobilization from the lumbar spine and distal radius during lactation. The effects are in the opposite direction to those that would be considered beneficial for skeletal health, but long-term follow-up is required to confirm this conclusion. This finding, coupled with our previous report of a lack of benefit of the calcium supplement in terms of fetal and infant growth and bone mineral accretion (7), questions the appropriateness of global recommendations for calcium intake and cautions against the need for calcium supplements in pregnancy unless there is evidence of a benefit for maternal blood pressure and reproductive health.
Acknowledgments
We thank the participants in the study and the staff of Medical Research Council (MRC) Keneba and MRC Human Nutrition Research who contributed to the successful completion of this work.
The authors' responsibilities were as follows—LMAJ and AP: were the principal investigators and were responsible for the study design, data collection and analysis, interpretation of results, and drafting the manuscript; AP: conceived the study, supervised LMAJ who conducted the work as part of his PhD program, and had final responsibility for the decision to submit the manuscript for publication; MAL and YS: were responsible for DXA; AP, LMAJ, and YS: were responsible for SPA; MAL: was responsible for the scrutiny and interpretation of DXA scans; GRG: contributed to preliminary data analysis and was responsible for drafting and critical review of the manuscript; and TJC: was responsible for expert statistical input and critical review of the manuscript. The biochemical analyses were conducted by the staff of the Bone Indices Laboratory at MRC Human Nutrition Research. All authors had full access to the data. The sources of funding and donation had no role in the study design, collection, analysis, and interpretation of the data or decision to publish. None of the authors reported a financial or personal conflict of interest.
REFERENCES
- 1.Olausson H, Laskey MA, Goldberg GR, Prentice A. Changes in bone mineral status and bone size during pregnancy, and the influences of body weight and calcium intake. Am J Clin Nutr 2008;88:1032–9 [DOI] [PubMed] [Google Scholar]
- 2.Prentice A. Micronutrients and the bone mineral content of the mother, fetus and newborn. J Nutr 2003;133:1693S–9S [DOI] [PubMed] [Google Scholar]
- 3.Prentice A. Pregnancy and lactation. : Pettifor J, Juppner H, Glorieux F, Pediatric bone: biology and diseases. London, United Kingdom: Academic Press, 2003:249–69 [Google Scholar]
- 4.Laskey MA, Prentice A. Bone mineral changes during and after lactation. Obstet Gynecol 1999;94:608–15 [DOI] [PubMed] [Google Scholar]
- 5.Laskey MA, Prentice A, Hanratty LA, et al. Bone changes after 3 mo of lactation: influence of calcium intake, breast-milk output, and vitamin D-receptor genotype. Am J Clin Nutr 1998;67:685–92 [DOI] [PubMed] [Google Scholar]
- 6.Prentice A. Calcium supplementation during breast-feeding. N Engl J Med 1997;337:558–9 [DOI] [PubMed] [Google Scholar]
- 7.Jarjou LMA, Prentice A, Sawo Y, et al. Randomized, placebo-controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr 2006;83:657–66 [DOI] [PubMed] [Google Scholar]
- 8.Prentice A, Jarjou LMA, Cole TJ, Stirling DM, Dibba B, Fairweather-Tait S. Calcium requirements of lactating Gambian mothers: effects of a calcium supplement on breast-milk calcium concentration, maternal bone mineral content, and urinary calcium excretion. Am J Clin Nutr 1995;62:58–67 [DOI] [PubMed] [Google Scholar]
- 9.Prentice A, Laskey MA, Shaw J, et al. The calcium and phosphorus intakes of rural Gambian women during pregnancy and lactation. Br J Nutr 1993;69:885–96 [DOI] [PubMed] [Google Scholar]
- 10.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr 1994;60:837–42 [DOI] [PubMed] [Google Scholar]
- 11.Cole TJ. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med 2000;19:3109–25 [DOI] [PubMed] [Google Scholar]
- 12.Villar J, Merialdi M, Gulmezoglu AM, et al. Nutritional interventions during pregnancy for the prevention or treatment of maternal morbidity and preterm delivery: an overview of randomized controlled trials. J Nutr 2003;133:1606S–25S [DOI] [PubMed] [Google Scholar]
- 13.Villar J, Abdel-Aleem H, Merialdi M, et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am J Obstet Gynecol 2006;194:639–49 [DOI] [PubMed] [Google Scholar]
- 14.Fairweather-Tait S, Prentice A, Heumann KG, et al. Effect of calcium supplements and stage of lactation on the calcium absorption efficiency of lactating women accustomed to low calcium intakes. Am J Clin Nutr 1995;62:1188–92 [DOI] [PubMed] [Google Scholar]
- 15.Bringhurst FR, Strewler GJ. Renal and skeletal actions of parathyroid hormone (PTH) and PTH-related protein. : Bilezikian JP, Raisz LG, Rodan G, Principles of bone biology. San Diego, CA: Academic Press, 2002:483–514 [Google Scholar]
- 16.Laskey MA, Murgatroyd PR, Prentice A. Comparison of narrow-angle fan-beam and pencil-beam densitometers: in vivo and phantom study of the effect of bone density, scan mode, and tissue depth on spine measurements. J Clin Densitom 2004;7:341–8 [DOI] [PubMed] [Google Scholar]
- 17.Pearson D, Kaur M, San P, Lawson N, Baker P, Hosking D. Recovery of pregnancy mediated bone loss during lactation. Bone 2004;34:570–8 [DOI] [PubMed] [Google Scholar]
- 18.Karlsson C, Obrant KJ, Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int 2001;12:828–34 [DOI] [PubMed] [Google Scholar]
- 19.Hopkinson JM, Butte NF, Ellis K, Smith EO. Lactation delays postpartum bone mineral accretion and temporarily alters its regional distribution in women. J Nutr 2000;130:777–83 [DOI] [PubMed] [Google Scholar]
- 20.Kolthoff N, Eiken P, Kristensen B, Nielsen SP. Bone mineral changes during pregnancy and lactation: a longitudinal cohort study. Clin Sci (Lond) 1998;94:405–12 [DOI] [PubMed] [Google Scholar]
- 21.Kalkwarf HJ, Specker BL. Bone mineral loss during lactation and recovery after weaning. Obstet Gynecol 1995;86:26–32 [DOI] [PubMed] [Google Scholar]
- 22.Cross NA, Hillman LS, Allen SH, Krause GF. Changes in bone mineral density and markers of bone remodeling during lactation and postweaning in women consuming high amounts of calcium. J Bone Miner Res 1995;10:1312–20 [DOI] [PubMed] [Google Scholar]
- 23.Akesson A, Vahter M, Berglund M, Eklof T, Bremme K, Bjellerup P. Bone turnover from early pregnancy to postweaning. Acta Obstet Gynecol Scand 2004;83:1049–55 [DOI] [PubMed] [Google Scholar]
- 24.Krebs NF, Reidinger CJ, Robertson AD, Brenner M. Bone mineral density changes during lactation: maternal, dietary, and biochemical correlates. Am J Clin Nutr 1997;65:1738–46 [DOI] [PubMed] [Google Scholar]
- 25.Sowers M, Corton G, Shapiro B, et al. Changes in bone density with lactation. JAMA 1993;269:3130–5 [PubMed] [Google Scholar]
- 26.Polatti F, Capuzzo E, Viazzo F, Colleoni R, Klersy C. Bone mineral changes during and after lactation. Obstet Gynecol 1999;94:52–6 [DOI] [PubMed] [Google Scholar]