Abstract
Stereotactic body radiation therapy (SBRT) achieves excellent local control for locally advanced pancreatic cancer (LAPC), but may increase late duodenal toxicity. Volumetric-modulated arc therapy (VMAT) delivers intensity-modulated radiation therapy (IMRT) with a rotating gantry rather than multiple fixed beams. This study dosimetrically evaluates the feasibility of implementing duodenal constraints for SBRT using VMAT vs IMRT. Non–duodenal sparing (NS) and duodenal-sparing (DS) VMAT and IMRT plans delivering 25 Gy in 1 fraction were generated for 15 patients with LAPC. DS plans were constrained to duodenal Dmax of <30 Gy at any point. VMAT used 1 360° coplanar arc with 4° spacing between control points, whereas IMRT used 9 coplanar beams with fixed gantry positions at 40° angles. Dosimetric parameters for target volumes and organs at risk were compared for DS planning vs NS planning and VMAT vs IMRT using paired-sample Wilcoxon signed rank tests. Both DS VMAT and DS IMRT achieved significantly reduced duodenal Dmean, Dmax, D1cc, D4%, and V20 Gy compared with NS plans (all p ≤ 0.002). DS constraints compromised target coverage for IMRT as demonstrated by reduced V95% (p = 0.01) and Dmean (p = 0.02), but not for VMAT. DS constraints resulted in increased dose to right kidney, spinal cord, stomach, and liver for VMAT. Direct comparison of DS VMAT and DS IMRT revealed that VMAT was superior in sparing the left kidney (p < 0.001) and the spinal cord (p < 0.001), whereas IMRT was superior in sparing the stomach (p = 0.05) and the liver (p = 0.003). DS VMAT required 21% fewer monitor units (p < 0.001) and delivered treatment 2.4 minutes faster (p < 0.001) than DS IMRT. Implementing DS constraints during SBRT planning for LAPC can significantly reduce duodenal point or volumetric dose parameters for both VMAT and IMRT. The primary consequence of implementing DS constraints for VMAT is increased dose to other organs at risk, whereas for IMRT it is compromised target coverage. These findings suggest clinical situations where each technique may be most useful if DS constraints are to be employed.
Keywords: Pancreatic cancer, Duodenal sparing, VMAT, IMRT
Introduction
Pancreatic cancer is expected to take more than 37,000 lives in 2012.1 Despite considerable advances in surgical, chemotherapeutic, and radiotherapeutic treatment modalities, the long-term survival of this disease remains dismal. The current standard of care for most cases of unresectable, locally advanced pancreatic cancer (LAPC) involves treatment with concurrent chemoradiation in the hope of downstaging or stabilizing the primary tumor, subsequently followed by maintenance chemotherapy to prevent development of metastatic disease.2,3
Multiple recent studies have demonstrated improved safety and efficacy of intensity-modulated radiation therapy (IMRT) in the treatment of pancreatic cancer.4–9 Of late, the use of stereotactic body radiation therapy (SBRT) has been actively investigated as an option for the delivery of radiation. Chang et al. demonstrated adequate local tumor control and acceptable toxicity for SBRT in the setting of unresectable pancreatic cancer.10,11 The major site of normal tissue toxicity in their study was the duodenum, with 12 of 73 patients (16%) experiencing grade 2 to 4 duodenal toxicity on extended follow-up. Duodenal toxicity manifested as ulceration in 9 patients (3 of whom also experienced gastrointestinal hemorrhage), stricture formation in 2 patients, and perforation in 2 patients (1 of whom also experienced ulceration).10,11 Based on the Quantitative Analysis of Normal Tissue Effects in the Clinic guidelines published in 2010, both the volume of the small bowel irradiated and the magnitude of the radiation dose received influence the probability that patients will develop acute and late toxicity of the small bowel.12 The dosimetric and volumetric limits for SBRT outlined in these guidelines recommended that the volume of the small bowel receiving greater than 12.5 Gy in a single fraction should ideally be kept to less than 30 cc, whereas the maximum point dose to the small bowel for a hypofractionated regimen should be less than 30 Gy.
Volumetric-modulated arc therapy (VMAT) represents an increasingly studied radiotherapeutic technique. VMAT involves the rotation of a gantry in 1 or 2 360° arcs around the patient, potentially reducing normal tissue toxicity by achieving more conformal dose distribution around the target volume. Although this technique has been studied in many anatomical sites, 4 relatively recent studies have specifically addressed utilization of VMAT in the treatment of pancreatic cancer.13–16 Eppinga et al. published a series of 10 cases assessing the delivery of VMAT as an alternative to standard 5-field IMRT in advanced pancreatic cancer.13 Despite being a small case series, it demonstrated a significant decrease in mean radiation dose to the small bowel using VMAT. Cai et al. compared VMAT with tomotherapy and specifically noted improved target coverage utilizing 15 MV photons and RapidArc technology.14 Vieillot et al. demonstrated improved bilateral kidney dose utilizing VMAT.17 Most recently, Ali et al. compared VMAT with IMRT and found improved monitor units (MU) and bilateral kidney dose with VMAT.16
These studies have demonstrated the proof of principle that VMAT planning for pancreatic cancer has the potential to provide significant dosimetric advantages, but they all have utilized conventional long-course dosing regimens (1.8 Gy per fraction). None have examined the utility of VMAT in delivering hypofractionated, high dose per fraction regimens as seen in SBRT. Furthermore, the specific question of whether duodenal sparing (DS) can be implemented as the primary constraint without compromising target coverage or increasing dose to other organs at risk (OARs) has not been investigated.
Our goal in this study, therefore, was to assess delivery characteristics as well as dose distribution to the target volume and normal tissues for pancreatic SBRT plans that incorporated DS as the primary constraint using VMAT vs IMRT techniques. We accomplished this through comparison of DS plans with standard nonsparing plans using VMAT and IMRT to analyze the effects of these constraints on dose distribution for each technique. We then performed a head-to-head dosimetric comparison of DS VMAT plans to DS IMRT plans to see which technique was more effective in sparing the duodenum while maintaining target coverage and minimizing the dose shunted to other OARs.
Methods and Materials
Patients
Fifteen patients with LAPC treated with definitive chemoradiation therapy using IMRT at our institution from 2006 to 2008 were identified. This cohort was a representative sample of the scope of disease typically treated with definitive chemoradiation at our institution. The median age was 64 years (range, 40 to 80 years) and 67% of patients were male. Tumors were typically located in the head of the pancreas (n = 13), though 2 were located distally in the body or tail. With respect to T classification, 2 patients were T3 and 13 were T4; nodal status was mostly unknown (NX; n = 12), but 3 patients were N1 as determined by radiologic evidence of regional nodal involvement. All patients were M0 at the time of imaging for treatment planning. Computed tomography simulation with oral contrast was performed in the supine position with immobilization using an alpha cradle. Non–duodenal sparing (NS) and DS VMAT and IMRT plans for SBRT to the pancreas were retrospectively generated for each patient for the purposes of this planning study (i.e., not for clinical treatment).
Radiation Planning
SBRT plans delivering 25 Gy in a single fraction were created for each patient using both VMAT and IMRT. Treatment planning was accomplished using computed tomography simulation data sets and Pinnacle version 9.0 (Philips), with utilization of the SmartArc function for VMAT planning. Prescription dose was set to 25 Gy in a single fraction normalized to the 80% isodose line. Four plans were created for each patient: a NS VMAT plan, a DS VMAT plan, a NS IMRT plan, and a DS IMRT plan. Constraints used during planning included the following: liver D50% < 5 Gy, right or left kidney D25% < 5 Gy, spinal cord Dmax < 5 Gy, and stomach D4% < 22.5 Gy; these were derived from the Stanford pancreatic SBRT experience.10 Furthermore, DS plans attempted to meet the additional constraint of duodenal Dmax <30 Gy at any point, as recommended in the Quantitative Analysis of Normal Tissue Effects in the Clinic guidelines.12 To avoid introduction of undue bias, identical contours were used for OARs in all plans, isocenters were placed at the same location for all 4 plan types, and highest priority was given to target coverage. The possibility of overplanning was minimized by using identical optimization procedures for both VMAT and IMRT plans. Once reasonable plans satisfying target coverage and normal tissue constraints were attained, they were reviewed and approved according to standard institutional regulations by an attending radiation oncologist who specializes in gastrointestinal malignancies (JMH).
For VMAT plans, 1 360° coplanar arc was used with 4° spacing between control points. For IMRT plans, 9 coplanar beams with fixed gantry positions spaced at 40° angles from one another were used. All treatments were planned for delivery at a rate of 602 MU per minute using 15-MV photons produced by a linear accelerator (Elekta Infinity System, Elekta, Stockholm, Sweden) equipped with multileaf collimators and capable of on-board kilovoltage orthogonal planar imaging.
Target Volumes and OARs
Delineation of the planning target volume (PTV) for this study included the internal target volume plus a 2-mm expansion. As per SBRT standards, regional lymph nodes were not included in the PTV. The relevant OAR volumes for this study were the duodenum, right and left kidneys, spinal cord, stomach, and liver. The duodenum was contoured throughout the superior-inferior extent of the PTV as well as 2 cm above and below the superior-most and inferior-most axial slices of the PTV, respectively. Other OARs were contoured as described in the Radiation Therapy Oncology Group consensus contouring atlas for pancreatic cancer.18 Contouring for all patients was performed by the same attending radiation oncologist (JMH) to minimize interphysician variability.
Plan Evaluation and Statistical Analysis
Evaluated volumes included the PTV, duodenum, right and left kidneys, spinal cord, stomach, and liver; all were reported as whole volumes. Dosimetric parameters were calculated using data from tabular cumulative dose-volume histograms (DVHs) with bin size set to 1 cGy. The mean value and range of each dosimetric parameter were reported (Table 1). By convention, DX% is defined as the dose received by X% of the volume of interest, VX% is defined as the percentage of the PTV receiving at least X% of the prescribed dose, and VX Gy is defined as the percentage of the volume of interest receiving at least X Gy. Mean dose received by a volume of interest is indicated by Dmean; minimum and maximum point doses are indicated by Dmin and Dmax, respectively. The homogeneity index (HI) was calculated by taking the ratio of Dmax/Dmin for the PTV. Conformity indices (CI) were calculated as follows: CIX% = ratio of the volume receiving X% of the prescription dose to the volume of the PTV. Averaged cumulative DVH plots for the PTV and each OAR were obtained by exporting tabular DVH data from Pinnacle at a bin size of 10 cGy. Integral dose to normal tissues excluding the PTV was calculated from the differential DVH data by exporting in tabular DVH form at 1 cGy bin size, multiplying each dose bin by the change in volume contained within that bin to yield a differential dose-volume value, and then summing these values. Normal tissues were defined by contouring the external surface of the body from the level of the tracheal bifurcation in the thorax (approximately T4 to T5) to the level of the superior-most portion of the sacroiliac joint in the pelvis (approximately S1); bowel gas and oral contrast contained within this region were then contoured and their densities overridden to 1 g/cm3, and the PTV was subtracted from the region of interest to generate the normal tissues region of interest. Nonparametric statistical comparison of the 4 different plan types was performed using the paired-sample Wilcoxon signed rank test, with an alpha level of ≤0.05 considered to be significant.
Table 1.
Dosimetric comparison of non–duodenal sparing (NS) vs duodenal-sparing (DS) plans. Numerical values are given as: mean value (range). Bolded text indicates a statistically significant difference (p ≤ 0.05) in the dosimetric parameter between the 2 techniques indicated at the top of each p-value column as assessed by the paired-sample Wilcoxon signed rank test
| Volume | Parameter | NS VMAT | p (NS VMAT vs DS VMAT) | DS VMAT | p (DS VMAT vs DS IMRT) | DS IMRT | p (DS IMRT vs NS IMRT) | NS IMRT |
|---|---|---|---|---|---|---|---|---|
| PTV 135 cm3 (58.4 to 320 cm3) | V95% (%) | 97.9 (91.1 to 100) | 0.43 | 97.5 (92.7 to 100) | 0.09 | 98.6 (94.8 to 99.9) | 0.01 | 99.6 (98.6 to 100) |
| Dmean (Gy) | 28.8 (26.8 to 31.4) | 0.13 | 29.3 (27.6 to 31.2) | 0.13 | 28.8 (27.3 to 30.0) | 0.02 | 29.3 (27.5 to 31.4) | |
| Dmax (Gy) | 32.5 (30.2 to 34.8) | 0.009 | 33.3 (30.5 to 35.5) | 0.04 | 32.8 (30.3 to 36.4) | 0.65 | 32.9 (30.7 to 36.3) | |
| Dmin (Gy) | 21.2 (19.9 to 23.9) | 0.003 | 19.2 (16.4 to 23.4) | 0.004 | 20.6 (18.8 to 22.1) | 0.002 | 22.8 (18.8 to 24.9) | |
| CI50% | 4.3 (3.1 to 5.9) | 0.02 | 4.7 (3.7 to 6.1) | 0.11 | 4.5 (3.3 to 5.4) | 0.98 | 4.5 (3.6 to 5.3) | |
| CI80% | 1.7 (1.3 to 2.2) | 0.006 | 1.9 (1.6 to 2.4) | 0.13 | 1.8 (1.4 to 2.3) | 0.89 | 1.8 (1.5 to 2.2) | |
| CI95% | 1.2 (0.9 to 1.5) | 0.02 | 1.3 (1.0 to 1.7) | 0.17 | 1.2 (0.9 to 1.6) | 0.41 | 1.3 (1.0 to 1.6) | |
| HI | 1.5 (1.4 to 1.7) | 0.001 | 1.8 (1.4 to 2.1) | 0.002 | 1.6 (1.4 to 1.9) | 0.003 | 1.4 (1.3 to 1.7) | |
| Duodenum 82.9 cm3 (40.0 to 160 cm3) | Dmean (Gy) | 14.0 (7.8 to 22.8) | 0.002 | 12.8 (7.7 to 22.1) | 0.46 | 13.3 (7.3 to 21.6) | <0.001 | 15.4 (9.3 to 23.2) |
| Dmax(Gy) | 29.9 (25.9 to 32.4) | 0.002 | 28.3 (24.8 to 31.5) | 0.65 | 28.5 (25.2 to 31.6) | <0.001 | 31.2 (29.5 to 36.3) | |
| D1cc (Gy) | 27.2 (22.8 to 31.5) | <0.001 | 24.7 (18.9 to 29.2) | 0.53 | 25.6 (21.4 to 28.9) | <0.001 | 28.9 (23.0 to 33.9) | |
| D4% (Gy) | 25.1 (17.4 to 30.8) | <0.001 | 22.6 (14.5 to 28.8) | 0.16 | 23.5 (16.4 to 28.5) | <0.001 | 26.7 (20.5 to 30.3) | |
| V20 Gy (%) | 23.9 (2.0 to 77.9) | <0.001 | 18.8 (0.6 to 76.4) | 0.09 | 21.9 (1.8 to 78.0) | <0.001 | 30.5 (4.5 to 80.5) | |
| Right kidney 167 cm3 (103 to 242 cm3) | Dmean (Gy) | 6.2 (3.4 to 11.1) | 0.01 | 6.8 (3.2 to 11.8) | 0.12 | 6.0 (3.6 to 9.7) | 0.39 | 6.2 (3.6 to 10.9) |
| Dmax(Gy) | 15.8 (8.0 to 24.2) | 0.001 | 17.9 (9.1 to 26.7) | 0.10 | 17.2 (9.6 to 25.2) | 0.23 | 16.6 (10.1 to 26.9) | |
| D25% (Gy) | 8.3 (4.9 to 14.4) | 0.02 | 9.1 (4.8 to 14.4) | 0.14 | 8.4 (5.8 to 12.2) | 0.25 | 8.6 (6.0 to 13.6) | |
| V10 Gy (%) | 19.6 (0.0 to 74.9) | 0.05 | 20.9 (0.0 to 71.0) | 0.33 | 17.0 (0.0 to 49.2) | 0.53 | 13.6 (0.05 to 51.3) | |
| Left kidney 178 cm3 (128 to 231 cm3) | Dmean (Gy) | 2.7 (1.7 to 3.9) | 0.73 | 2.7 (1.7 to 4.7) | < 0.001 | 3.3 (2.5 to 4.5) | 0.82 | 3.3 (2.1 to 5.0) |
| Dmax(Gy) | 7.6 (4.6 to 15.7) | 0.31 | 7.9 (4.8 to 16.1) | 0.23 | 8.4 (5.5 to 13.8) | 0.57 | 8.6 (5.1 to 14.2) | |
| D25% (Gy) | 3.6 (2.2 to 4.9) | 0.73 | 3.5 (2.6 to 5.4) | < 0.001 | 4.9 (3.7 to 11.0) | 0.53 | 4.4 (3.3 to 5.2) | |
| V5 Gy (%) | 6.2 (0.0 to 23.6) | 0.60 | 7.6 (0.0 to 37.2) | 0.009 | 17.6 (1.0 to 34.3) | 0.91 | 15.3 (0.03 to 45.5) | |
| Spinal cord 17.8 cm3 (4.6 to 39.0 cm3) | Dmean (Gy) | 2.2 (0.6 to 1.2) | 0.05 | 2.3 (1.3 to 3.0) | 0.36 | 2.5 (1.3 to 7.8) | 0.32 | 2.5 (1.2 to 8.2) |
| Dmax (Gy) | 11.0 (7.4 to 17.0) | 0.07 | 11.6 (7.0 to 17.5) | 0.69 | 11.7 (8.6 to 15.9) | 0.002 | 10.9 (8.3 to 13.5) | |
| V5 Gy (%) | 21.6 (13.7 to 34.7) | 0.08 | 22.2 (14.9 to 34.2) | < 0.001 | 23.8 (7.2 to 74.5) | 0.21 | 24.1 (14.3 to 74.5) | |
| Stomach 251 cm3 (52.0 to 580 cm3) | Dmean (Gy) | 2.4 (0.2 to 7.7) | 0.08 | 2.6 (0.2 to 7.1) | 0.06 | 2.5 (0.2 to 6.3) | 0.25 | 2.4 (0.2 to 6.4) |
| Dmax (Gy) | 14.3 (1.3 to 29.2) | 0.03 | 15.5 (1.8 to 27.5) | 0.78 | 15.7 (2.3 to 27.2) | 0.31 | 15.4 (1.5 to 26.5) | |
| D30% (Gy) | 3.1 (0.3 to 12.0) | 0.20 | 3.3 (0.3 to 10.9) | 0.07 | 3.2 (0.2 to 9.8) | 0.22 | 3.0 (0.18 to 9.8) | |
| V5 Gy (%) | 15.5 (0.0 to 49.9) | 0.01 | 17.5 (0.0 to 49.3) | 0.31 | 17.4 (0.0 to 49.1) | 0.20 | 15.8 (0.0 to 48.4) | |
| Liver 1140 cm3 (406 to 1550 cm3) | Dmean (Gy) | 2.7 (0.2 to 4.2) | 0.36 | 2.8 (0.2 to 4.8) | 0.003 | 2.5 (0.3 to 4.3) | 0.33 | 2.6 (0.3 to 4.3) |
| Dmax (Gy) | 24.8 (5.8 to 31.4) | 0.08 | 25.6 (5.2 to 33.0) | 0.99 | 25.7 (6.3 to 32.1) | 0.63 | 25.6 (7.5 to 33.3) | |
| D50% (Gy) | 0.9 (0.1 to 1.5) | 0.03 | 1.0 (0.1 to 2.2) | < 0.001 | 0.8 (0.1 to 1.6) | 0.62 | 0.8 (0.1 to 1.5) | |
| V10 Gy (%) | 5.5 (0.0 to 14.4) | 0.36 | 5.9 (0.0 to 17.3) | 0.88 | 7.3 (0.0 to 31.3) | 0.83 | 5.8 (0 to 16.2) | |
| Normal tissues | Integral Dose (Gy cm3 10−5) | 0.35 (0.18 to 0.48) | 0.03 | 0.37 (0.20 to 0.50) | 0.04 | 0.35 (0.21 to 0.48) | 0.33 | 0.35 (0.21 to 0.50) |
| Treatment delivery characteristics | MU | 5286 (4747 to 5801) | 0.03 | 5437 (4798 to 6093) | < 0.001 | 6894 (5255 to 9354) | 0.05 | 6521 (5007 to 8083) |
| Beam-on time (s) | 527 (473 to 578) | 0.03 | 542 (578 to 607) | < 0.001 | 687 (524 to 932) | 0.05 | 650 (499 to 806) |
Results
Dose to Target Volumes
DS VMAT vs NS VMAT
Implementation of DS constraints with VMAT did not result in inferior target coverage, as demonstrated by equivalent V95% and Dmean to the PTV for both DS and NS VMAT plans (p = 0.43 and 0.13, respectively; see Table 1 for all numerical dosimetric data). However, DS VMAT plans were less homogeneous, with higher Dmax (p = 0.009) and lower Dmin (p = 0.003) to the PTV, producing an inferior HI (p = 0.001). DS VMAT plans were also less conformal than their NS VMAT counterparts (p < 0.05 for CI50%, CI80%, and CI95%).
DS IMRT vs NS IMRT
In contrast to the results observed with VMAT, implementation of DS constraints with IMRT resulted in compromised target coverage, evidenced by a reduced V95% (p = 0.01) and Dmean (p = 0.02) to the PTV for DS compared with NS IMRT plans. DS IMRT plans were also less homogeneous than NS IMRT plans, with lower Dmin to the PTV (p = 0.002) and an inferior HI (p = 0.003). Similar conformity, however, was achieved with both plan types (p * 0.05 for CI50%, CI80%, and CI95%).
DS VMAT vs DS IMRT
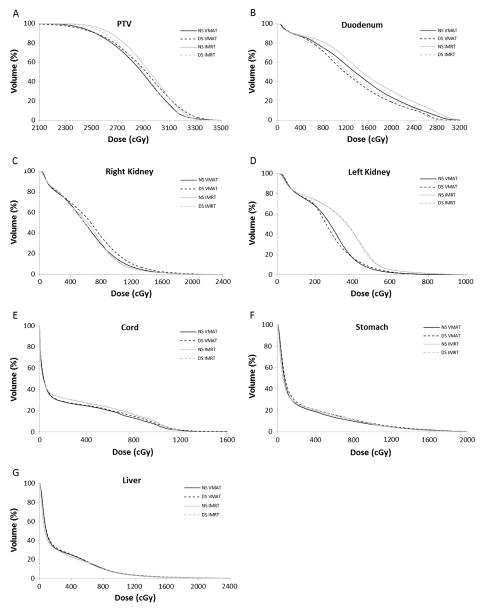
Similar target coverage was obtained with DS VMAT and DS IMRT plans, as measured by V95% and Dmean to the PTV, which were not different between the 2 plan types (p = 0.09 and 0.13, respectively). DS VMAT plans were less homogeneous than DS IMRT plans with a higher Dmax (p = 0.04) and lower Dmin (p = 0.004) to the PTV and, accordingly, an inferior HI (p = 0.002). However, no difference was observed in CI of the 2 plan types (p > 0.05 for CI50%, CI80%, and CI95%). An averaged cumulative DVH curve for the PTV is shown in Fig. 1A and suggests similar rates of dose falloff for DS VMAT and DS IMRT plans.
Fig. 1.
Averaged cumulative dose-volume histograms for (A) PTV, (B) duodenum, (C) right kidney, (D) left kidney, (E) spinal cord, (F) stomach, and (G) liver for NS VMAT (black solid line), DS VMAT (black dashed line), NS IMRT (gray solid line), and DS IMRT (gray dashed line) plan types.
Dose to OARs
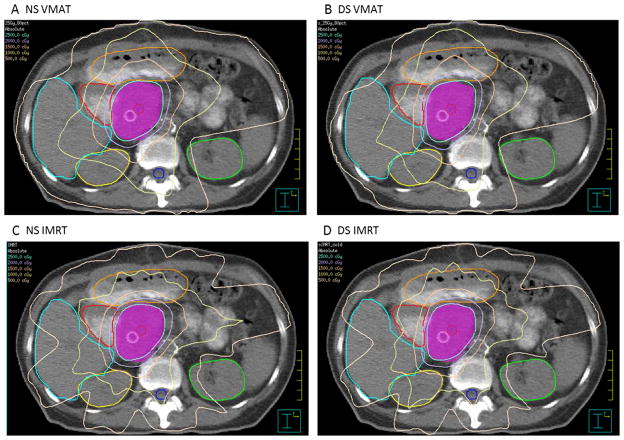
Averaged cumulative DVH curves for each OAR are portrayed in Fig. 1B–G. Representative axial slices showing contoured OARs and isodose distributions for NS VMAT, DS VMAT, NS IMRT, and DS IMRT plans at the same level for 1 patient are depicted in Figure 3A–D.
Fig. 3.
Representative axial slices showing contoured ROIs and isodose distributions for (A) NS VMAT, (B) DS VMAT, (C) NS IMRT, and (D) DS IMRT plans. As delineated by the legend in the top left corner of each image, isodose lines are shown every 500 cGy from 500–2500 cGy. Contoured ROIs shown include the liver, duodenum, jejunum (not dosimetrically evaluated in this study), PTV, right kidney, spinal cord, and left kidney. (Color version of figure is available online.)
DS VMAT vs NS VMAT
Using VMAT, implementation of DS planning resulted in reduction of Dmean and Dmax to the duodenum, as well as decreases in D1 cc, D4%, and V20 Gy (all p ≤ 0.002). On average, DS VMAT planning reduced Dmean to the duodenum by 9.8% (standard deviation [SD], 8.7), Dmax by 5.4% (SD, 4.4), and V20 Gy by 40.7 (SD, 31.1). These improvements in duodenal dose parameters did cause slight increases in dose to some OARs. Shunting of dose occurred most overtly to the right kidney, for which all 4 dosimetric parameters measured (Dmean, Dmax, D25%, and V10 Gy) significantly increased. The mean magnitude of these increases, however, was less than 15% for all 4 parameters and the increase in mean V10 Gy from 19.6% to 20.9% was on the borderline of statistical significance (p = 0.05). In contrast, there was no increase in dose received by the left kidney in DS plans, as shown by the lack of any differences in Dmean, Dmax, D25%, and V10 Gy between DS and NS plans (all p * 0.05). DS planning caused a slight increase in Dmean to the spinal cord from 2.2 to 2.3 Gy (p = 0.05), but no differences in Dmax (p = 0.07) or V5 Gy (p = 0.08). Dose to the stomach increased in DS plans as measured by Dmax (p = 0.03) and V5 Gy (p = 0.01), though there was no increase in Dmean (p = 0.08) or D30% (p = 0.20). D50% to the liver increased in DS plans from 0.9 to 1.0 Gy (p = 0.03), but no difference was seen in Dmean (p = 0.36), Dmax (p = 0.08), or V10 Gy (p = 0.36). Mean integral dose to normal tissues increased from 0.35 to 0.37 Gy cm3 10−5 (p = 0.03) for DS plans, representing a difference of 5.7%.
DS IMRT vs NS IMRT
Using IMRT, DS planning likewise was able to achieve reductions in all 5 dosimetric parameters relating to the duodenum (Dmean, Dmax, D1cc, D4%, and V20 Gy; all p < 0.001). Implementation of DS planning resulted in average reductions in Dmean, Dmax, and V20 Gy of 8.4% (SD, 5.3), 14.3% (SD, 7.9), and 40.2% (SD, 22.0), respectively. As mentioned before, the primary consequence of these improvements in duodenal dosimetric parameters was compromised target coverage, as represented by decreases in V95% (p = 0.01), Dmean (p = 0.02), and Dmin (p = 0.002) to the PTV. For the most part, DS planning did not result in shunting of dose to other OARs when using IMRT. No differences in dosimetric parameters were noted between DS and NS plans for the kidneys, stomach, or liver (all p > 0.05). Dmax to the spinal cord did increase from 10.9 to 11.7 Gy (p = 0.002), but Dmean (p = 0.32) and V5 Gy (p = 0.21) remained similar. Integral dose to normal tissues was no different for the 2 plan types (p = 0.33).
DS VMAT vs DS IMRT
Dosimetric parameters relating to the duodenum were similar for the 2 DS plan types (all p > 0.05), although there was a trend toward improved V20 Gy (18.8 vs 21.9%) with DS VMAT (p = 0.09). No differences in dose to the right kidney were observed (p > 0.05 for all parameters), yet DS VMAT plans were superior in sparing the left kidney, as measured by Dmean (p < 0.001), D25% (p < 0.001), and V5 Gy (p = 0.009). DS VMAT plans were also advantageous in regard to the spinal cord, achieving a decreased V5 Gy (p < 0.001) compared with DS IMRT plans, although Dmean and Dmax were no different (p = 0.36 and 0.69, respectively). Dosimetric parameters for the stomach were superior for DS IMRT compared with DS VMAT, with a lower Dmean (p = 0.05) and a trend toward decreased D50% (p = 0.07). DS IMRT was superior in ability to spare the liver, as measured by a lower Dmean (p = 0.003) and D50% (p < 0.001) compared with DS VMAT, whereas Dmax (p = 0.99) and V20 Gy (p = 0.88) were similar between the 2 DS techniques. Integral dose was slightly greater for DS VMAT plans compared with DS IMRT (0.37 vs 0.35 Gy cm3 10−5, respectively; p = 0.04).
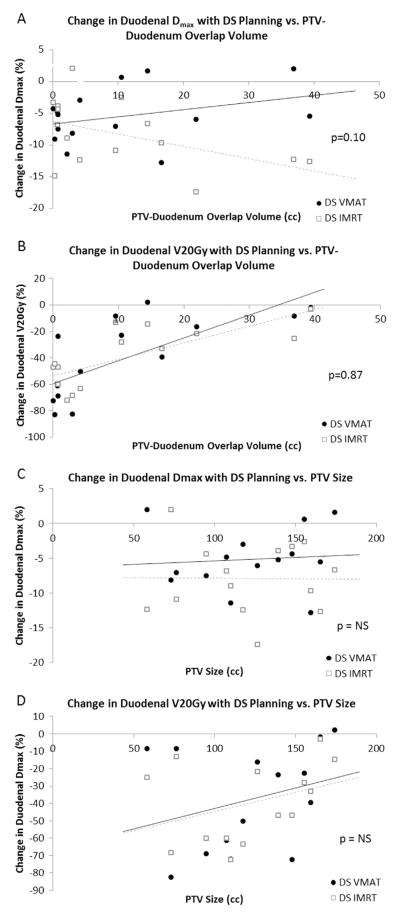
As the overlap volume between PTV and duodenum increased, there was a trend toward lesser ability to spare the duodenum from a high point dose (as shown by a lesser percent reduction in duodenal Dmax) for DS VMAT as compared with DS IMRT (p = 0.10) (Fig. 2A). This suggests that DS VMAT planning may be preferable for patients with a smaller region (<10 cc) of PTV-duodenal overlap and DS IMRT for patients with a larger region (>10 cc) of overlap. Both DS VMAT and DS IMRT, however, showed similar decreased ability to spare the duodenum volumetrically (as demonstrated by a lesser percent reduction in duodenal V20 Gy) as PTV-duodenal overlap increased (p = 0.87) (Fig. 2B). We further examined the relationship between PTV size and duodenal dosimetric parameters to investigate whether a larger PTV compromised DS ability. Interestingly, a larger PTV did not appear to substantially alter the ability of either DS VMAT or DS IMRT to spare the duodenum from a high point dose (Fig. 2C); however, a larger PTV hindered both DS VMAT and DS IMRT similarly in sparing the duodenum volumetrically (Fig. 2D).
Fig. 2.
(A) Graphical representation of the relationship between PTV-duodenal overlap and the percentage change in duodenal Dmax achieved with DS planning. There is a trend toward inferior ability of DS VMAT plans (black circles, solid black line) to spare the duodenum a high point dose as PTV-duodenum overlap volume increases compared with DS IMRT plans (open boxes, dotted gray line), especially when the overlap volume exceeds 10 cc (p = 0.10). (B) Graphical representation of the relationship between PTV-duodenal overlap and duodenal V20 Gy for DS VMAT (black circles, solid black line) and DS IMRT plans (open boxes, dotted gray line). DS VMAT and DS IMRT plans demonstrate a similar impaired ability to spare the duodenum volumetrically as PTV-duodenum overlap volume increases, with no significant difference between the 2 distributions (p = 0.87). (C) Graphical representation of the relationship between PTV size and percentage change in duodenal Dmax with DS planning for DS VMAT (black circles) and DS IMRT plans (open boxes). A larger PTV does not appear to correlate with an impaired ability to spare the duodenum a high point dose for either DS VMAT (R2 = 0.0064) or DS IMRT (R2 = 0.0002). One patient with a much larger PTV (320 cc) was excluded from this graph to allow for a scale that more clearly represents the other data points. (D) Graphical representation of the relationship between PTV size and percentage change in duodenal V20 Gy with DS planning for DS VMAT (black circles) and DS IMRT plans (open boxes). Both DS VMAT and DS IMRT plans show a similar trend toward impaired ability to spare the duodenum volumetrically as PTV size increases (p = NS). One patient with a much larger PTV (320 cc) was excluded from this graph to allow for a scale that more clearly represents the other data points.
Treatment Delivery Characteristics
MU and beam-on times required to deliver the 25-Gy single fraction were calculated for each patient by the planning software. For both VMAT and IMRT, mean number of MU required and mean beam-on time were greater for DS vs NS plans (p = 0.003 and 0.05, respectively), but the temporal differences amounted to only an additional 33 seconds on average for VMAT and 37 seconds for IMRT. Comparison of DS VMAT and DS IMRT plans showed that DS VMAT could deliver the treatment more quickly for all 15 patients, with a beam-on time 2.4 minutes shorter on average than that for DS IMRT (p < 0.001) and requiring 1457 (21%) fewer MU than DS IMRT (p < 0.001). These beam-on times do not include the time required for beam and patient setup.
Discussion
Our study had 2 main purposes: (a) to separately determine for VMAT and IMRT techniques the dosimetric consequences of implementing DS constraints in terms of target coverage and dose to other OARs; and (b) to then directly compare DS VMAT and DS IMRT plans to assess which technique achieved greater DS while maintaining adequate target coverage and minimizing dose shunting to other OARs. This study is the first to examine the dosimetric feasibility of implementing DS constraints when administering SBRT for unresectable pancreatic tumors. Furthermore, it is the first to investigate the utility of VMAT for the delivery of SBRT to the pancreas as well as to compare the efficacy of VMAT vs IMRT in sparing the duodenum. Our study used a dose of 25 Gy × 1 fraction for stereotactic treatment, a regimen previously implemented by Chang et al.10 Although the current trend in the clinical setting is to use hypofractionated (e.g., 5 Gy × 5 fractions or 6.6 Gy × 5 fractions) rather than single-fraction SBRT regimens, we believe the results of our comparative dosimetric analysis serve as a proof of concept generalizable to other stereo-tactic regimens for pancreatic cancer.
Separate comparisons of DS to NS VMAT plans and of DS to NS IMRT plans were performed to assess the feasibility of incorporating DS constraints in each technique. On the whole, our results demonstrate that both VMAT and IMRT allow for successful implementation of DS constraints at only minor expense to target coverage and other OARs. DS VMAT and DS IMRT achieved similar reductions in duodenal Dmax, Dmean, and V20 Gy compared with their nonsparing counterparts, with the largest reduction occurring in V20 Gy for both. Notably, however, the resulting effect on dose to target volumes and other OARs was distinct between the 2 techniques. Although introduction of DS constraints to VMAT planning did not compromise target coverage, it did cause an increase in dose to some OARs, namely the right kidney, spinal cord, stomach, and liver. In an opposite fashion, inclusion of DS constraints in IMRT planning compromised target coverage, but resulted in almost no shunting of dose to other OARs, with only dose to the spinal cord showing a significant increase. In a clinical setting, this information may guide the radiation planning team toward the use of VMAT to spare the duodenum when the patient’s anatomy causes difficulty in obtaining adequate tumor coverage, though IMRT may be favored when it is the normal tissue dose constraints that prove more challenging to meet. Implementation of DS caused decreased dose homogeneity for both VMAT and IMRT plans, which is expected given the close proximity of the duodenum to the head of the pancreas where most tumors in this study were located and the consequent redistribution of dose to spare the duodenum. This decreased homogeneity may result in an increased number of underdosed “cold spots” in the PTV when DS constraints are incorporated.
Head-to-head comparison of DS VMAT to DS IMRT was used to evaluate which technique could more effectively spare the duodenum while maintaining dose to target volumes and minimizing dose shunting to other OARs. Both DS VMAT and DS IMRT plans were found to be similarly effective in sparing the duodenum, although DS VMAT showed a trend toward improved duodenal V20 Gy. To investigate how the anatomy of individual patients influenced the effectiveness of DS, we evaluated how DS in both point dose and volumetric fashions varied with PTV-duodenum overlap as well as with PTV size (Fig. 2A/B and C/D, respectively). DS IMRT appeared to better accommodate greater PTV-duodenum overlap, as shown by a trend toward improved point dose sparing compared with DS VMAT when overlap volume exceeded 10 cc. Encouragingly, greater PTV size did not affect the ability of either DS VMAT or DS IMRT to spare the duodenum from a high point dose, although it did hinder both techniques in sparing the duodenum volumetrically. Of interest regarding OARs, DS VMAT achieved superior dosimetric parameters for the kidneys and spinal cord, whereas DS IMRT was dosimetrically superior for the liver and stomach. Integral dose to normal tissues was slightly greater for DS VMAT plans, but treatment time was shorter and required fewer MU.
Although the dosimetric OAR profiles of the 2 techniques offer unique advantages, DS VMAT delivery clearly engenders improved efficiency for this application. Our findings indicate that DS VMAT plans require 21% fewer MU and beam-on times that are 2.4 minutes shorter on average than for DS IMRT. These beam-on times do not take into account setup time required, which is generally longer for IMRT, where 9 beams must be accurately positioned, than for VMAT, where a single gantry must be properly aligned and then simply moved in an arc around the patient. Therefore, in practice DS VMAT plans may achieve an even greater temporal advantage over DS IMRT. We expect the reduced time required for treatment to generate higher machine throughput as well as enhanced patient comfort due to a shorter on-table, treatment immobilization time. Moreover, shorter treatment times are likely to decrease the effects of intrafraction motion.
Given the theoretical design of this planning comparison study, we cannot exclude the possibility of the introduction of bias. Yet, considering the relatively high number of patients treated for this disease at our institution and the consequently substantial experience in IMRT planning of pancreatic cancer SBRT cases by our team of dosimetrists, physicists, and physicians, we feel strongly that the VMAT plans were compared with high-quality IMRT plans. Furthermore, to avoid undue introduction of bias, VMAT plans were created with the primary goal to generate clinically acceptable plans in an expedient manner rather than to maximize potential dosimetric differences. Identical optimization parameters and weightings were used for both VMAT and IMRT to avoid overplanning for either plan type.
Through review of the literature, we were able to identify 4 other studies addressing the question of VMAT in pancreatic cancer.13–16 These studies were performed for conventionally fractionated radiation regimens and were designed to address the efficacy of VMAT in comparison with standard 5-field IMRT. Eppinga and colleagues reported improved CI with VMAT treatment, reduced dose to OARs, and decreased treatment delivery times.13 However, it must be noted that they used a 5-field IMRT plan and briefly mentioned that a 7-field plan may lead to similar or better outcomes in comparison with VMAT. In our study, we implemented a 9-field IMRT plan, which likely explains why we found CI to be similar for DS VMAT and DS IMRT plans.
Our study compares favorably to those of Cai et al., Vieillot et al., and Ali et al.14–16 In each of these studies, the VMAT technique was studied for conventionally fractionated treatment of pancreatic cancer (1.8-Gy fractions to 50.4 or 54 Gy, or 2.5-Gy fractions to 50 Gy). Cai et al. note that use of 15 MV photons as opposed to 6 MV may result in fewer required MU and shorter treatment time when employing VMAT for pancreatic cancer. For this reason, we utilized 15-MV photons in this study and found that despite incorporation of DS constraints VMAT plans still resulted in fewer MU and shorter treatment time required than when delivered using IMRT. We additionally showed that the decreased kidney dose previously reported for VMAT vs IMRT15,16 remains achievable when DS constrains are implemented.
One disadvantage of DS VMAT planning that our study identified is an increase in integral dose compared with both NS VMAT and DS IMRT planning. Although an important finding to be aware of, this increase in integral dose was small (5.7%) and it is unlikely that any change in clinical outcomes, such as second malignancy rate, would be detectable as a consequence. The effect of integral dose is further minimized by utilizing fractionated SBRT as opposed to 1 fraction. Our finding of an increased integral dose with VMAT compared with IMRT when implementing DS constraints adds another data point to the literature, which is currently undecided on the question of whether VMAT or IMRT generally produces superior integral dose parameters. Studies of malignancies at other sites have shown integral dose to be increased,19 similar,20 or reduced21,22 for VMAT relative to IMRT. This heterogeneity in findings is likely dependent on the anatomy of the tumor site as well as planning techniques and approaches.
Although we are pleased with the results of this dosimetric analysis and its implications for SBRT planning, it must be further studied to assess its clinical utility. At our institution, we have begun to utilize DS VMAT and DS IMRT treatment planning approaches in select patients undergoing SBRT for locally advanced pancreatic tumors. It is possible that this approach may reduce long-term duodenal toxicity following SBRT, though we do not yet have adequate data to evaluate this hypothesis.
Conclusions
For the radiotherapeutic treatment of LAPC, implementation of DS constraints produces significant reductions in point dose and volumetric dose parameters for the duodenum using both VMAT and IMRT. When using VMAT, the primary consequence of incorporating these constraints is shunting of dose to other OARs, whereas when using IMRT the primary consequence is compromised target coverage. Direct comparison of DS VMAT and IMRT plans reveals that kidney and spinal cord doses are improved with VMAT, whereas stomach and liver doses are more favorable with IMRT. These findings suggest clinical situations where 1 technique may be preferred over the other if DS constraints are to be employed during SBRT planning for LAPC.
Acknowledgments
The authors thank the Claudio X. Gonzalez Family Foundation, the Simpkins Family Foundation, and the Flannery Family Foundation for their generous support in making this project possible.
Footnotes
J.M.H. receives speaking honoraria from the Elekta Co.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: A qualitative systematic review. J Clin Oncol. 2009;27:2269–77. doi: 10.1200/JCO.2008.19.7921. [DOI] [PubMed] [Google Scholar]
- 3.Willett CG, Czito BG, Bendell JC, et al. Locally advanced pancreatic cancer. J Clin Oncol. 2005;23:4538–44. doi: 10.1200/JCO.2005.23.911. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454–9. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Bortfeld T, Webb S. Single-arc IMRT? Phys Med Biol. 2009;54:N9–20. doi: 10.1088/0031-9155/54/1/N02. [DOI] [PubMed] [Google Scholar]
- 6.Landry JC, Yang GY, Ting JY, et al. Treatment of pancreatic cancer tumors with intensity-modulated radiation therapy (IMRT) using the volume at risk approach (VARA): Employing dose-volume histogram (DVH) and normal tissue complication probability (NTCP) to evaluate small bowel toxicity. Med Dosim. 2002;27:121–9. doi: 10.1016/s0958-3947(02)00094-8. [DOI] [PubMed] [Google Scholar]
- 7.Milano MT, Chmura SJ, Garofalo MC, et al. Intensity-modulated radio-therapy in treatment of pancreatic and bile duct malignancies: Toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445–53. doi: 10.1016/j.ijrobp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Tinkl D, Grabenbauer GG, Golcher H, et al. Downstaging of pancreatic carcinoma after neoadjuvant chemoradiation. Strahlenther Onkol. 2009;185:557–66. doi: 10.1007/s00066-009-1977-9. [DOI] [PubMed] [Google Scholar]
- 9.Brown MW, Ning H, Arora B, et al. A dosimetric analysis of dose escalation using two intensity-modulated radiation therapy techniques in locally advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:274–83. doi: 10.1016/j.ijrobp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–72. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 11.Murphy JD, Christman-Skieller C, Kim J, et al. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:1420–6. doi: 10.1016/j.ijrobp.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 12.Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:S101–7. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 13.Eppinga W, Lagerwaard F, Verbakel W, et al. Volumetric modulated arc therapy for advanced pancreatic cancer. Strahlenther Onkol. 2010;186:382–7. doi: 10.1007/s00066-010-2094-5. [DOI] [PubMed] [Google Scholar]
- 14.Cai J, Yue J, McLawhorn R, et al. Dosimetric comparison of 6 MV and 15 MV single arc rapidarc to helical tomotherapy for the treatment of pancreatic cancer. Med Dosim. 2010;11:317–20. doi: 10.1016/j.meddos.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Vieillot S, Azria D, Riou O, et al. Bilateral kidney preservation by volumetric-modulated arc therapy (RapidArc) compared to conventional radiation therapy (3D-CRT) in pancreatic and bile duct malignancies. Radiat Oncol. 2011;6:147. doi: 10.1186/1748-717X-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali AN, Dhabaan AH, Jarrio CS, et al. Dosimetric comparison of volumetric modulated arc therapy and intensity-modulated radiation therapy for pancreatic malignancies. Med Dosim. 2012;37:271–5. doi: 10.1016/j.meddos.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Vieillot S, Azria D, Lemanski C, et al. Plan comparison of volumetric-modulated arc therapy (RapidArc) and conventional intensity-modulated radiation therapy (IMRT) in anal canal cancer. Radiat Oncol. 2010;5:92. doi: 10.1186/1748-717X-5-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrams RA, Regine WF, Goodman KA, et al. Consensus panel contouring atlas for the delineation of the clinical target volume in the postoperative treatment of pancreatic cancer. Radiat Ther Oncol Group. 2012 doi: 10.1016/j.ijrobp.2012.01.022. Available at: http://www.rtog.org/CoreLab/ContouringAtlases/PancreasAtlas.aspx. [DOI] [PMC free article] [PubMed]
- 19.Yoo S, Wu QJ, Lee WR, et al. Radiotherapy treatment plans with RapidArc for prostate cancer involving seminal vesicles and lymph nodes. Int J Radiat Oncol Biol Phys. 2009;76:935–42. doi: 10.1016/j.ijrobp.2009.07.1677. [DOI] [PubMed] [Google Scholar]
- 20.Clivio A, Fogliata A, Franzetti-Pellanda A, et al. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: A treatment planning comparison with fixed field IMRT. Radiother Oncol. 2009;92:118–24. doi: 10.1016/j.radonc.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Vanetti E, Clivio A, Nicolini G, et al. Volumetric modulated arc radio-therapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: A treatment planning comparison with fixed field IMRT. Radiother Oncol. 2009;92:111–7. doi: 10.1016/j.radonc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Cozzi L, Dinshaw KA, Shrivastava SK, et al. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol. 2008;89:180–91. doi: 10.1016/j.radonc.2008.06.013. [DOI] [PubMed] [Google Scholar]