Abstract
BACKGROUND
The goal of this study was to investigate the surgical management and outcomes of patients with primary colorectal cancer (CRC) and synchronous liver metastasis (sCRLM).
STUDY DESIGN
Using a multi-institutional database, we identified 1,004 patients treated for sCRLM between 1982 and 2011. Clinicopathologic and outcomes data were evaluated with uni- and multivariable analyses.
RESULTS
A simultaneous CRC and liver operation was performed in 329 (33%) patients; 675 (67%) underwent a staged approach (“classic” staged approach, n = 647; liver-first strategy, n = 28). Patients managed with the liver-first approach had more hepatic lesions and were more likely to have bilateral disease than those in the other 2 groups (p < 0.05). The use of staged operative strategies increased over the time of the study from 58% to 75% (p < 0.001). Liver-directed therapy included hepatectomy (90%) or combined resection + ablation (10%). A major resection (>3 segments) was more common with a staged approach (39% vs 24%; p < 0.001). Overall, 509 patients (50%) received chemotherapy in either the preoperative (22%) or adjuvant (28%) settings, with 11% of patients having both. There were 197 patients (20%) who had a complication in the postoperative period, with no difference in morbidity between staged and simultaneous groups or major vs minor hepatectomies (p > 0.05). Ninety-day postoperative mortality was 3.0%, with no difference between simultaneous and staged approaches (p = 0.94). The overall median and 5-year survivals were 50.9 months and 44%, respectively; long-term survival was the same regardless of the operative approach (p > 0.05).
CONCLUSIONS
Simultaneous and staged resections for sCRLM can be performed with comparable morbidity, mortality, and long-term oncologic outcomes.
Colorectal cancer (CRC) accounts for more than 51,000 deaths each year in the United States, making it the second most common cause of cancer-related deaths.1 Approximately one-half of patients with CRC will develop liver metastasis during the course of their disease, with 15% to 42% presenting with synchronous primary CRC and colorectal liver metastasis (CRLM).2–4 Surgical therapy of CRLM remains the only therapeutic option with potential for cure.5,6 In modern series, the overall 5-year survival reported after hepatic resection with curative intent ranges from 35% to 58%.7–18
Traditionally, a staged approach (colorectal first) has been used in the management of patients with synchronous CRLM (sCRLM). This involves the initial extirpation of the primary CRC, often followed by systemic chemotherapy, followed later by a liver-directed operation to address the hepatic disease. The last 2 decades have brought an increased understanding of the biology of CRLM, resulting in more effective targeted therapies in addition to decreased mortality after liver-directed operations.4,11,19 These developments have led surgeons managing patients with sCRLM to consider other operative sequences such as a liver-first (reverse strategy) staged approach, in which the hepatic disease is addressed, followed by extirpation of the primary CRC at later date.20–22 In patients with clearly resectable CRLM, several investigators have advocated for a simultaneous resection of both the primary CRC and CRLM in the same operative setting.23,24 There have been limited data published comparing all 3 operative strategies for patients with sCRLM. In particular, previous studies have not focused especially on the degree of morbidity and mortality. In this study, we sought to determine the safety and efficacy of the 3 operative strategies for patients with sCRLM in a large, multi-institutional, international analysis. Specifically, we examined the short- and long-term outcomes of patients who were managed with curative intent liver-directed operations in patients with sCRLM. In addition, we identified factors predictive of complications and clinicopathologic characteristics associated with long-term survival after curative intent, liver-directed operations for patients with sCRLM in a large international multicenter cohort.
METHODS
Between October 1982 and June 2011, 1,004 patients treated with curative intent surgery for synchronous colo-rectal and CRLM were identified from 4 major hepatobiliary centers in the United States (Johns Hopkins School of Medicine, Baltimore, MD) and Europe (Hôpitaux Universitaires de Genève, Geneva, Switzerland; Unit of Hepato-Biliary-Pancreatic Surgery, Lisbon, Portugal; Ospedale San Raffaele, Milan, Italy); the study was approved by the institutional review boards of the respective institutions.
Patients were divided into 3 groups: those undergoing a staged procedure in which the primary CRC was extirpated first, followed at a later date by liver-directed therapy (“colorectal first” or “classic approach”); patients managed in a staged fashion in which the CRLM was addressed first followed at a later date by the CRC primary (“liver first” or “reverse strategy”); and those patients who were managed with a simultaneous resection of both the CRC primary and CRLM in the same operative setting (“simultaneous”). Additionally, patients were categorized as having either a “simultaneous” or a “staged” operation depending on the timing of the operations. Patients who underwent previous hepatic resections or ablations of the CRLM were excluded from this study. Only patients who had surgery with curative intent either with resection or combined resection plus ablation were included; patients undergoing ablative procedures only were excluded. If the patient had extrahepatic colorectal metastasis, the extrahepatic disease had to be surgically addressed with curative intent either at the time of the hepatic operation or at another date for the patient to be included in the study cohort.
Data collection
Standard demographic and clinicopathologic data for each patient as well as data on tumor characteristics of the primary CRC and of the CRLM were collected. Data were collected on the primary CRC location (rectal vs colon) and the date of CRC operation, if a staged procedure (ie, colorectal first or liver first). For the CRLM, data were collected on the number, size in centimeters, the number of CRLM treated, and the distribution of the hepatic metastasis (unilateral or bilateral) at the time of the liver-directed operation. Resection at the time of surgery was classified as less than a hemihepatectomy (eg, segmentectomy or subsegmentectomy), hemihepatectomy, or extended hepatectomy (≥5 liver segments) per the Brisbane standardized nomenclature.25 Additionally, the extent of resection was further classified as a “major” (≥3 segments) or “minor” (<3 segments) hepatectomy. The use of concomitant ablation was also noted. The use and timing of perioperative chemotherapy was also noted; the specific regimen was not defined because this differed greatly across the international patient spectrum. Hepatic resection margins were classified as R0 (microscopically negative), R1 (microscopically positive), and R2 (grossly positive). These data referred to the margin status of resected liver metastasis; if the patient underwent a combined resection plus ablation, the margin status referred to the resected specimen.
Perioperative morbidity and mortality data were collected and graded according to the system proposed by Clavien and colleagues, which included the therapeutic consequences of complications within 90 days of the liver-directed operation.26 Severe complications were classified as ≥ Grade IIIa and included those complications treated with surgical, endoscopic, or radiologic interventions or resulting in death (Grade V). Dates of last follow-up, vital status, and recurrence of CRLM were collected on all patients. Hepatic recurrences of the CRLM were calculated only for patients who were alive at 90 days.27
Statistical analyses
Summary statistics were obtained using established methods and presented as percentages or median values with standard deviation. Fisher’s exact test was used for cells with n < 5 and the Bonferroni adjustment was used for comparisons of multiple proportions. Factors associated with complications were examined using logistic regression modeling. To identify variables for inclusion in the multivariate model, variables were selected using the Hosmer and Lemeshow criteria of p < 0.25 in combination with important clinical variables and confounders.28,29 The final multivariate model’s performance and discriminative ability were examined using the Hosmer and Lemeshow goodness-of-fit test and receiver operating characteristic curves (ROC). Survival was calculated from the date of the liver-directed operation to the date of last contact or death and was estimated using the nonparametric product limit method described by Kaplan and Meier.30 Differences in survival were examined using the log-rank (Mantel-Cox) test. Factors associated with survival were examined using univariate and multivariate Cox regression analyses. The multivariate model was built using purposeful direct entry based on the Wald statistic, with all variables with p < 0.25 included in the multivariate model. The hazard ratio (HR) and the 95% confidence intervals (CI) were estimated and a p < 0.05 was considered significant. All statistical analyses were 2-sided and were performed using Statistical Packages for the Social Sciences (SPSS, IBM, Version 20.0).
RESULTS
Patient, tumor, and operative details
Among the 4 institutions, there were 1,004 patients with CRC and sCRLM who underwent operative management of both the primary and metastatic liver disease. The majority of patients were men (n = 598; 59.6%) with a median age of 60.0 years (SD 22.5 years). Most patients presented with a colon primary (n = 726; 72.3%). Among patients with sCRLM, 38.4% (n = 380) had bilateral disease, the median number of CRLMs was 2.0 (SD 2.6), and median tumor size was 3.5 cm (SD 3.0 cm). Overall, 509 patients (50%) received chemotherapy; 22.2% of patients (n = 222) received preoperative chemotherapy only, 28.5% (n = 287) adjuvant therapy only, and 10.7% (n = 108) both pre- and postoperative chemotherapy.
Management of patients with staged operative procedures (ie, classic colorectal first and liver first) increased from 57.5% to 74.8% over the course of study period (p < 0.001; Fig. 1). This paralleled an increase in the burden of CRLM disease treated over time (1982 to 1996: median tumor number, 1.0 vs 1997 to 2011: median tumor number, 2.0; p < 0.001). Of the 675 patients (67.2%) whose disease was managed in a staged fashion, the vast majority had the CRC primary addressed first (colorectal first: n = 647; 64.4% vs liver first: n = 28; 2.8%). Only in the last 5 years was there an increased use of the reverse strategy approach (Fig. 1). A comparison of the preoperative characteristics of patients who underwent classic, liver first, or the simultaneous approach is detailed in Table 1. Although patients in the classic and simultaneous groups had a comparable number and size of CRLM, patients managed with a liver-first approach were more likely to have bilateral disease than those in the other 2 groups (p = 0.028). Patients managed with the liver-first approach also had more hepatic lesions treated at the time of the liver-directed operation (median 4.0, SD 3.4) compared with the other groups (p = 0.006). In addition, patients undergoing a liver-first approach were more likely to have a rectal primary tumor (n = 15; 53.6%) compared with patients undergoing either a colorectal-first or a simultaneous approach (p = 0.007). Overall, 117 patients (11.7%) had extrahepatic metastatic disease, with no difference between the 3 groups (p > 0.05). The majority of patients managed with a liver-first approach (n = 21; 75.0%) received preoperative treatment as compared with approximately one-third in the colorectal-first and simultaneous groups (p < 0.001). Conversely, patients managed with either colorectal-first or simultaneous resections were more likely to receive adjuvant chemotherapy than liver-first patients (p = 0.012).
Figure 1.
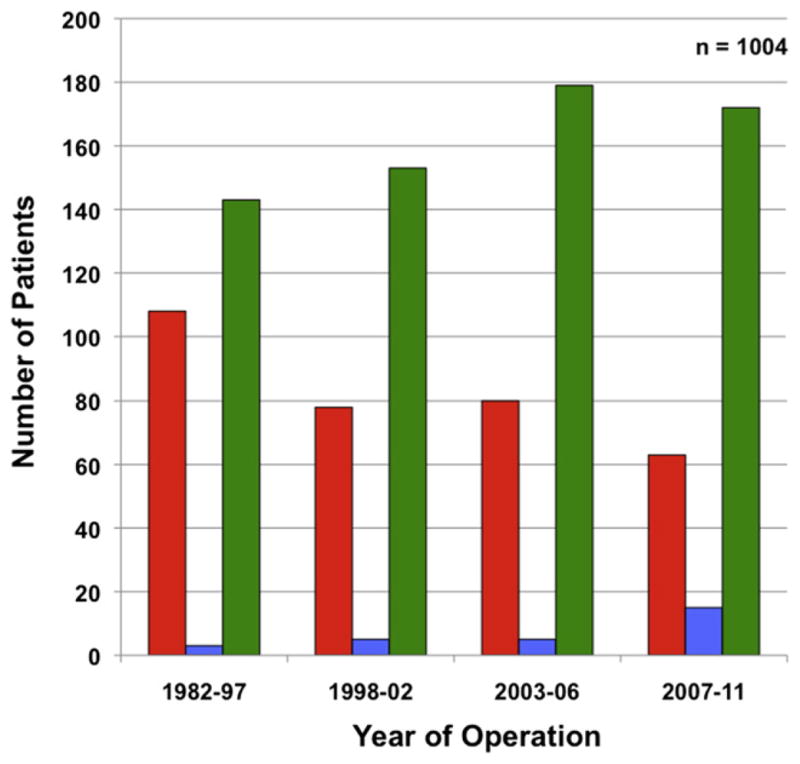
Surgical management of 1,004 patients with synchronous colorectal liver metastasis over time. The use of staged operative strategies (colorectal primary→liver, and liver→colorectal primary) increased over time as compared with simultaneous resections of both the primary and liver disease (p < 0.001). Operative strategy: red bar, simultaneous; blue bar, liver first (liver→colorectal cancer); green bar, colorectal cancer first (colorectal cancer→liver).
Table 1.
Clinicopathologic Characteristics of 1,004 Patients Undergoing Simultaneous, Reverse Strategy (Liver First), and Colorectal Primary First Resections of Colorectal Liver Metastasis
| Variable | Total (n = 1,004) | Colorectal first (n = 647) | Liver first (n = 28) | Simultaneous (n = 329) | p Value |
|---|---|---|---|---|---|
| Demographics | |||||
| Median age ± SD, y | 60.0 ± 22.5 | 61.0 ± 17.8 | 58.0 ± 12.3 | 60.0 ± 30.1 | >0.05 |
| Sex, male | 598 (59.6) | 396 (61.2) | 17 (60.7) | 185 (56.2) | >0.05 |
| Primary colorectal cancer | |||||
| Location | |||||
| Colon | 726 (72.3) | 475 (73.6) | 13 (46.4) | 238 (72.3) | 0.007 |
| Rectum | 276 (27.5) | 170 (26.4) | 15 (53.6) | 91 (27.7) | |
| Colorectal liver metastasis | |||||
| Year of liver operation | |||||
| 1982–1997 | 254 (25.3) | 143 (22.1) | 3 (10.7) | 108 (32.8) | <0.001 |
| 1998–2002 | 236 (23.5) | 153 (23.6) | 5 (17.9) | 78 (23.7) | <0.001 |
| 2003–2006 | 264 (26.3) | 179 (27.7) | 5 (17.9) | 80 (24.3) | <0.001 |
| 2007–2011 | 250 (24.9) | 172 (26.6) | 15 (53.6) | 63 (19.1) | <0.001 |
| Bilateral hepatic disease | 380 (38.4) | 240 (37.5) | 16 (64.0) | 124 (38.3) | 0.028 |
| Median CRLM ± SD, n | 2.0 ± 2.6 | 2.0 ± 2.6 | 3.0 ± 3.6 | 2.0 ± 2.4 | 0.009 |
| > 2 hepatic metastases | 325 (34.2) | 199 (32.7) | 14 (58.3) | 112 (35.2) | 0.030 |
| Median size of CRLM ± SD, cm | 3.5 ± 3.0 | 3.5 ± 3.1 | 3.0 ± 2.4 | 3.0 ± 2.7 | 0.051 |
| Largest CRLM ≥ 3.5 cm | 433 (47.0) | 285 (48.6) | 7 (33.6) | 141 (44.9) | >0.05 |
| Presence of extrahepatic metastasis | 117 (11.7) | 69 (10.6) | 1 (3.6) | 47 (6.8) | >0.05 |
| Operative details | |||||
| Type of liver-directed therapy | |||||
| Resection only | 899 (89.5) | 575 (88.9) | 26 (92.9) | 298 (90.6) | >0.05 |
| Combined resection + ablation | 105 (10.5) | 71 (11.1) | 2 (7.1) | 31 (9.4) | >0.05 |
| Median no. of CRLM treated ± SD | 2.0 ± 2.7 | 2.0 ± 3.1 | 4.0 ± 3.4 | 2.0 ± 1.7 | 0.006 |
| Extent of liver resection | |||||
| Partial or bisegmentectomy | 596 (59.4) | 353 (54.6) | 13 (46.4) | 230 (69.9) | <0.001 |
| Hemihepatectomy | 204 (20.3) | 145 (22.4) | 6 (21.4) | 53 (16.1) | >0.05 |
| Extended hepatectomy | 134 (13.3) | 105 (16.2) | 4 (14.3) | 25 (7.6) | <0.001 |
| Unknown extent of hepatic resection | 70 (7.0) | 44 (6.8) | 5 (17.9) | 21 (6.4) | >0.05 |
| Major hepatectomy (>3 segments) | 338 (33.6) | 250 (38.6) | 10 (35.7) | 78 (23.7) | <0.001 |
| Treatment | |||||
| Preoperative chemotherapy only* | 222 (22.1) | 130 (20.1) | 21 (75.0) | 71 (21.6) | <0.001 |
| Adjuvant chemotherapy only* | 287 (28.5) | 216 (33.4) | 0 (0) | 71 (21.6) | <0.001 |
| Both preoperative and adjuvant chemotherapy* | 108 (10.7) | 59 (9.1) | 6 (21.4) | 43 (13.1) | <0.001 |
Unless otherwise indicated, data are presented as n (%).
Preoperative and adjuvant chemotherapy refers to treatment in relation to the time of liver-directed therapy.
CRLM, colorectal liver metastasis.
The vast majority of patients in all 3 groups were managed with hepatic resection alone (overall, n = 899; 89.5%; p > 0.05); only a minority of patients were treated with combined resection plus ablation (n = 105; 10.5%). There was no difference in the use of resection plus ablation among patients treated with either a staged or simultaneous approach (p = 0.454). A combined modality approach was more likely to be used in patients with bilateral hepatic disease (64.8% vs 35.3% in unilateral disease; p < 0.001) and in patients presenting with a higher number of CRLM (p < 0.001). Regarding the extent of hepatic resection, a minor hepatectomy (fewer than 3 segments) was performed in most patients (n = 596; 59.4%). Of note, patients in the simultaneous resection group were more likely to undergo a minor hepatic resection (n = 251; 76.2%) compared with patients in either the classic (n = 397; 61.4%) or liver-first (n = 18; 64.2%) groups (p < 0.001). Overall, 33.6% of patients (n = 338) underwent a major hepatectomy; a lower proportion of patients in the simultaneous group (n = 78; 25.3%) were managed with a major hepatectomy compared with the staged groups (p < 0.001). Similarly, extended hepatectomy was more commonly performed in the staged setting (colorectal first, 16.2% and liver first, 17.9% vs simultaneous, 7.6%; p < 0.001). The majority of patients had an R0 margin status (n = 732; 72.9%).
Perioperative outcomes
Among the 947 patients in whom complete complication data were available, 197 patients (19.6%) had a complication in the postoperative period; the overall incidence of complications was similar among the 3 groups (p > 0.05; Fig. 2, Table 2). Of note, the overall proportion of patients who experienced a severe complication (Clavien Grades IIIa, IIIb, IVa, IVb, or V) did not differ between patients treated with the staged or simultaneous approach (p > 0.05). Patients who underwent a minor hepatectomy using a simultaneous approach had overall and severe complication incidences of 19.3% and 12.1%, respectively (Table 3). In comparison, simultaneous major hepatectomy was associated with similar overall and severe complication incidences (25.0% and 15.8%, respectively; both p > 0.05). Among patients who underwent a staged approach, the overall (16.5% and 23.7%) and severe (10.7% and 13.8%) complications were similar among patients undergoing a minor or major hepatectomy, respectively. Of note, for patients undergoing a major hepatectomy, there was no difference in overall complications, minor complications, or severe complications between patients managed in a staged vs simultaneous fashion (all p > 0.05). On univariate logistic regression analysis, major hepatectomy was associated with an increased odds of having a complication (p = 0.049) and this factor remained an independent predictor of morbidity on multivariate analyses (odds ratio 1.42; [95% CI, 1.01 to 2.00]; p = 0.044; Table 4).
Figure 2.
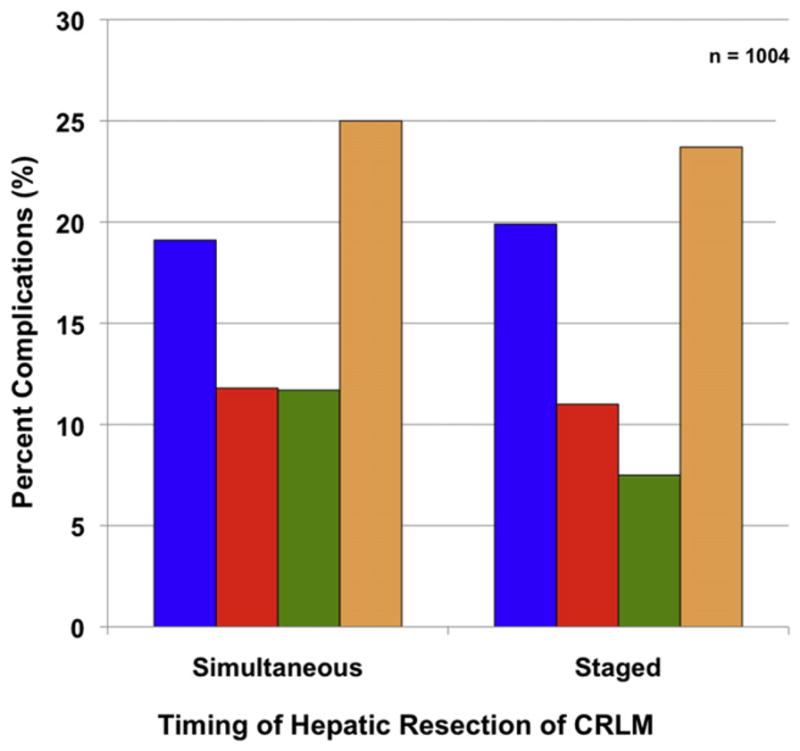
Postoperative complications (Clavien Grade) by simultaneous vs staged operative approaches in 1,004 patients with synchronous colorectal and hepatic metastasis. There was no difference between the groups (all p > 0.05). Clavien Grade ≥ IIIa indicates severe complication. Complications after major and minor resections refer to overall complications. CRLM, colorectal liver metastasis. Postoperative complications: blue bar, overall complications; red bar, severe complications; green bar, after minor resection; tan bar, after major resection.
Table 2.
Outcomes after Liver-Directed Management in 1,004 Patients Undergoing Simultaneous, Reverse Strategy (Liver First), and Colorectal Primary First Resections of Colorectal Liver Metastasis
| Variable | Patients (n = 1,004)
|
Colorectal first (n = 647)
|
Liver first (n = 28)
|
Simultaneous (n = 329)
|
p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Hepatic resection margins* | |||||||||
|
| |||||||||
| R0 | 732 | 72.9 | 465 | 71.9 | 8 | 28.6 | 259 | 78.7 | 0.019 |
|
| |||||||||
| R1 | 113 | 11.5 | 75 | 11.7 | 3 | 10.7 | 35 | 10.9 | >0.05 |
|
| |||||||||
| R2 | 2 | 0.2 | 1 | 0.2 | 0 | 0 | 1 | 3 | >0.05 |
|
| |||||||||
| Missing | 157 | 15.6 | 106 | 16.4 | 17 | 60.7 | 34 | 10.3 | <0.001 |
|
| |||||||||
| Any complication after liver-directed therapy† | 197 | 19.6 | 128 | 19.8 | 6 | 21.4 | 63 | 19.1 | >0.05 |
|
| |||||||||
| Clavien Grade of complications within 90 d† | |||||||||
|
| |||||||||
| No complications | 743 | 74.0 | 486 | 75.1 | 17 | 60.7 | 240 | 72.9 | >0.05 |
|
| |||||||||
| Grade I | 13 | 1.3 | 7 | 1.1 | 0 | 0 | 6 | 1.8 | >0.05 |
|
| |||||||||
| Grade II | 78 | 7.8 | 43 | 6.6 | 2 | 7.1 | 33 | 10.0 | >0.05 |
|
| |||||||||
| Grade IIIa | 45 | 4.5 | 29 | 4.5 | 0 | 0 | 16 | 4.9 | >0.05 |
|
| |||||||||
| Grade IIIb | 19 | 1.9 | 10 | 1.5 | 0 | 0 | 9 | 2.7 | >0.05 |
|
| |||||||||
| Grade IVa | 18 | 1.8 | 14 | 2.2 | 0 | 0 | 4 | 1.2 | >0.05 |
|
| |||||||||
| Grade IVb | 1 | 0.1 | 0 | 0 | 0 | 1 | 0.3 | >0.05 | |
|
| |||||||||
| Grade V‡ | 30 | 3.0 | 21 | 3.2 | 0 | 0 | 9 | 2.7 | >0.05 |
|
| |||||||||
| Unknown | 57 | 5.7 | 37 | 5.7 | 9 | 32.1 | 11 | 3.3 | <0.001 |
|
| |||||||||
| Severe complications (Clavien Grade ≥ IIIa) | 113 | 11.2 | 74 | 11.4 | 0 | 0 | 39 | 11.8 | >0.05 |
|
| |||||||||
| 90-d mortality‡ | 30 | 3.0 | 21 | 3.2 | 0 | 0 | 9 | 2.7 | >0.05 |
|
| |||||||||
| Recurrence of CRLM at last follow-up§ | 556 | 57.0 | 373 | 59.6 | 12 | 42.9 | 171 | 53.4 | >0.05 |
Unless otherwise indicated, data are presented as n (%). Comparison of multiple proportions performed using Bonferroni adjustment.
Margin status of resected liver metastasis; if combined resection+ablation, the margin status refers to the resected specimen.
For simultaneous resections this represents any complication related to primary or liver resection and for staged resections this represents complications after liver-directed operation.
No reporting of perioperative mortality in the liver-first group because of inclusion of patients who underwent both a colorectal and hepatic operation.
Recurrences calculated only for 974 patients who were alive at 90 days.
CRLM, colorectal liver metastasis.
Table 3.
Complications after Major and Minor Hepatectomies for 897 Patients Undergoing Liver-Directed Operations for Management of Colorectal Liver Metastasis
| Variable | Staged (n = 598) | Simultaneous (n = 299) | p Value |
|---|---|---|---|
| Major hepatectomy (≥3 segments), n = 329, n (%) | |||
| No complications | 192 (75.9) | 51 (67.1) | >0.05 |
| Overall complications after liver-directed therapy | 60 (23.7) | 19 (25.0) | 0.818 |
| Minor complication (Clavien Grade I or II) | 26 (10.3) | 13 (17.1) | >0.05 |
| Severe complication (Clavien Grade ≥ IIIa) | 35 (13.8) | 12 (15.8) | >0.05 |
| Mortality within 90 d (Clavien Grade V) | 8 (3.2) | 35 (6.6) | >0.05 |
| Total, n | 253 | 76 | |
| Minor hepatectomy (<3 segments), n = 568, n (%) | |||
| No complications | 282 (81.7) | 170 (76.2) | >0.05 |
| Overall complications after liver-directed therapy | 57 (16.5) | 43 (19.3) | 0.399 |
| Minor complication (Clavien Grade I or II) | 26 (7.5) | 26 (11.7) | >0.05 |
| Severe complications (Clavien Grade ≥ IIIa) | 37 (10.7) | 27 (12.1) | >0.05 |
| Mortality within 90 days (Clavien Grade V) | 13 (3.8) | 4 (1.8) | 0.214 |
| Total, n | 345 | 223 | |
For simultaneous resections this represents any complication related to primary or liver resection and for staged resections this represents complications after liver-directed operation. Fisher’s exact test used for cells with n < 5.
Table 4.
Logistic Regression Analyses of Variables Associated with Complications after Completion of Both the Primary Colorectal and Liver-Directed Operation in 1,004 Patients with Synchronous Colorectal Liver Metastasis
| Prognostic factor | Univariate
|
Multivariate
|
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p Value | Odds ratio | 95% CI | p Value | |
| Year of liver-directed operation | ||||||
|
| ||||||
| 1982–1997 | Reference | Reference | ||||
|
| ||||||
| 1998–2002 | 0.79 | 0.51–1.23 | 0.303 | 0.77 | 0.49–1.22 | 0.270 |
|
| ||||||
| 2003–2006 | 0.92 | 0.61–1.40 | 0.707 | 0.81 | 0.51–1.26 | 0.344 |
|
| ||||||
| 2007–2011 | 0.74 | 0.47–1.15 | 0.173 | 0.62 | 0.38–1.02 | 0.057 |
|
| ||||||
| Age > 60 y | 1.17 | 0.86–1.60 | 0.312 | – | – | – |
|
| ||||||
| Male sex | 1.08 | 0.79–1.48 | 0.629 | – | – | – |
|
| ||||||
| Rectal primary | 0.74 | 0.52–1.06 | 0.101 | 0.77 | 0.53–1.12 | 0.108 |
|
| ||||||
| >2 Colorectal liver metastases | 0.93 | 0.67–1.30 | 0.686 | – | – | – |
|
| ||||||
| Largest CRLM >3.5 cm | 1.39 | 1.01–1.90 | 0.045 | 1.26 | 0.91–1.75 | 0.168 |
|
| ||||||
| Bilateral hepatic disease | 1.24 | 0.91–1.71 | 0.176 | 1.41 | 1.00–2.00 | 0.050 |
|
| ||||||
| Simultaneous operation | 1.30 | 0.94–1.79 | 0.112 | 1.35 | 0.96–1.91 | 0.084 |
|
| ||||||
| Major hepatic resection | 1.38 | 1.00–1.90 | 0.049 | 1.42 | 1.01–2.00 | 0.044 |
|
| ||||||
| Combined resection + ablation | 1.02 | 0.62–1.68 | 0.950 | – | – | – |
CRLM, colorectal liver metastasis.
There were 30 patient deaths, for a 90-day mortality of 3.0%. Mortality was comparable when comparing patients treated with a simultaneous (2.7%) vs staged (3.1%) approach (p = 0.743). Patients who died within 90 days of the liver-directed operation were similar with regard to patient age, location of the CRC primary, tumor size, number, and distribution of the hepatic disease compared with patients who survived beyond 90 days (all p > 0.05). In addition, when comparing simultaneous vs staged approaches, there was no difference in the 90-day postoperative mortality of patients undergoing a partial hepatectomy (1.7% vs 3.6%) or hemihepatectomy (5.7% vs 3.3%; both p > 0.05). The mortality associated with an extended hepatectomy performed simultaneously was 8.0% vs 2.8% when done as part of a staged approach (p > 0.05).
Recurrence and predictors of survival
Among the 974 patients who did not die within 90 days, at a median follow-up of 34 months, 556 (57.0%) patients suffered recurrence. Recurrence was similar when comparing the simultaneous (59.6%) vs staged (57.2%) approaches (p = 0.171). Although a simultaneous or staged approach was not associated with the risk of recurrence, on univariate logistic regression, history of a rectal primary tumor and more than 2 CRLM were associated with an increased odds of recurrence (both p < 0.05); on multivariate analysis, a rectal primary tumor site remained significant (odds ratio 2.14; 95% CI 1.47 to 2.99; p < 0.001).
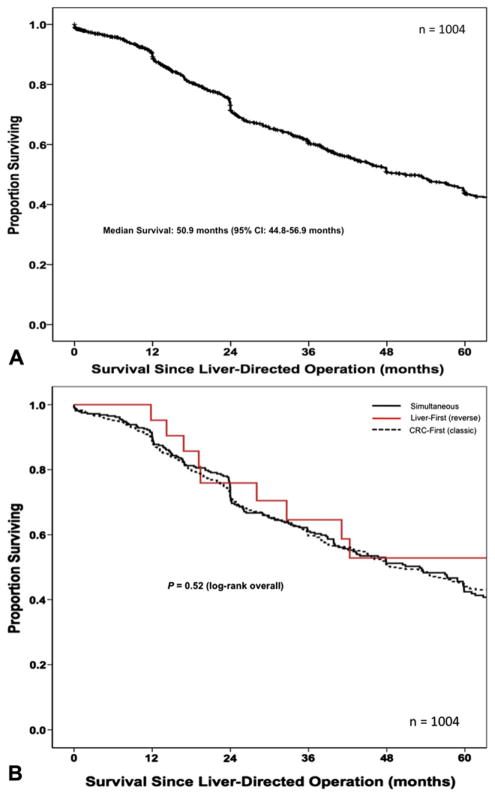
The median overall survival after the liver-directed operation was 50.9 months (95% CI 44.8 to 56.9 months). The 1-, 3-, and 5-year overall survivals were 89%, 60%, and 44%, respectively (Fig. 3a). Patients managed with simultaneous or staged approaches had a similar 5-year survivals (42% vs 44%; p = 0.688); in fact, there were no differences in overall survival when comparing the colorectal-first, liver-first, or simultaneous approaches (p = 0.526; Fig. 3b). On univariate analyses, factors associated with survival included male sex, rectal primary, more than 2 CRLM, and combined resection plus ablation (all p < 0.05). Specifically, patients who had more than 2 CRLM had a worse survival (43.2 months) compared with patients who underwent hepatectomy for 2 or fewer lesions (59.8 months; p = 0.019). In addition, the median survival for patients undergoing a combined resection plus ablation was 35.8 months compared with 54.2 months for patients managed with resection alone (p = 0.004). After controlling for competing risk factors with multivariate analysis, male sex (HR 1.25; [95% CI 1.03 to 1.51]; p = 0.024), a rectal primary (HR 1.22; [95% CI 1.00 to 1.50]; p = 0.050), and combined resection plus ablation (HR 1.57; [95% CI 1.14 to 2.16]; p = 0.006) remained independently associated with a worse survival (Table 5).
Figure 3.
(A) Overall Kaplan-Meier survival of 1,004 patients with synchronous primary colorectal (CRC) and colorectal liver metastasis (CRLM) managed with resection of both primary and liver disease (median survival 50.9 months; 95% CI 44.8 to 56.9 months). (B) Overall survival of 1,004 patients after surgical management of synchronous colorectal liver metastasis stratified by timing and sequence of operations (p = 0.520, log-rank overall).
Table 5.
Cox Regression Analyses of Variables Associated with Survival after Time Liver-Directed Operation
| Prognostic factor | Univariate
|
Multivariate
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p Value | Hazard ratio | 95% CI | p Value | |
| Year of liver-directed operation | ||||||
|
| ||||||
| 1982–1997 | Reference | Reference | ||||
|
| ||||||
| 1998–2002 | 0.92 | 0.74–1.13 | 0.414 | 0.95 | 0.75–1.20 | 0.669 |
|
| ||||||
| 2003–2006 | 0.67 | 0.53–0.85 | 0.001 | 0.70 | 0.54–0.92 | 0.010 |
|
| ||||||
| 2007–2011 | 0.85 | 0.61–1.19 | 0.343 | 0.82 | 0.57–1.20 | 0.305 |
|
| ||||||
| Age > 60 y old | 1.07 | 0.90–1.27 | 0.445 | – | – | – |
|
| ||||||
| Male sex | 1.26 | 1.05–1.51 | 0.011 | 1.25 | 1.03–1.51 | 0.024 |
|
| ||||||
| Rectal primary | 1.22 | 1.01–1.47 | 0.039 | 1.22 | 1.00–1.50 | 0.050 |
|
| ||||||
| >2 Colorectal liver metastases | 1.26 | 1.04–1.53 | 0.019 | 1.13 | 0.90–1.43 | 0.297 |
|
| ||||||
| Largest CRLM >3.5 cm | 0.91 | 0.75–1.09 | 0.281 | – | – | – |
|
| ||||||
| Bilateral hepatic disease | 1.17 | 0.97–1.41 | 0.096 | 1.11 | 0.89–1.35 | 0.364 |
|
| ||||||
| Receipt of preoperative chemotherapy | 1.11 | 0.92–1.34 | 0.275 | – | – | – |
|
| ||||||
| Receipt of postoperative chemotherapy | 1.05 | 0.88–1.27 | 0.579 | – | – | – |
|
| ||||||
| Simultaneous operation | 1.04 | 0.87–1.24 | 0.688 | 1.08 | 0.88–1.31 | 0.472 |
|
| ||||||
| Minor hepatic resection | 1.26 | 1.04–1.53 | 0.020 | 1.23 | 1.00–1.50 | 0.047 |
|
| ||||||
| Combined resection + ablation | 1.52 | 1.15–2.00 | 0.004 | 1.57 | 1.14–2.16 | 0.006 |
|
| ||||||
| Margin status of hepatectomy | ||||||
|
| ||||||
| R0 | Reference | Reference | ||||
|
| ||||||
| R1/R2 | 1.13 | 0.86–1.48 | 0.387 | 1.04 | 0.79–1.34 | 0.764 |
|
| ||||||
| Unknown | 1.47 | 1.17–1.84 | 0.001 | 1.13 | 0.81–1.59 | 0.408 |
CRLM, colorectal liver metastasis.
DISCUSSION
Colorectal cancer remains the most common indication for hepatic resection in patients with metastatic disease.1,31 Up to 25% to 30% of patients with CRC will present with sCRLM. The optimal timing for surgical resection for patients with sCRLM has been a topic of much debate, and data on the topic continue to evolve. Given that morbidity and mortality associated with hepatectomy have decreased substantially over the past 20 years, the classic paradigm of a staged operation for sCRLM has been questioned. Specifically, given the improved feasibility and safety of performing major hepatic resections, some investigators have suggested that the simultaneous approach to sCRLM may be preferable.21,32,33 Past data have been limited, with previous studies being from a single institution,21,32 having relatively few patients in each of the different operative strategy categories,21,32,34,35 or failing to include the liver-first reverse strategy in their analyses.23 This study is important because it analyzed a large, multicenter, international cohort of patients who underwent surgical management of sCRLM. As such, the results of the study are more likely to be representative and generalizable than previous single-center studies. In addition, all 3 operative approaches (simultaneous, classic staged, and liver-first) were evaluated in the treatment of patients with sCRLM. We found that simultaneous and staged resections for sCRLM can be performed with comparable morbidity and mortality, as well as similar long-term oncologic outcomes.
In this study, the overall morbidity and mortality were 19.6% and 3.0%, respectively. Perioperative outcomes did not differ among patients treated with a staged or simultaneous approach. In addition, morbidity and mortality were comparable among patients who underwent a minor or major hepatectomy, regardless of the operative strategy used. In a study of patients with sCRLM, Martin and colleagues33 similarly reported no difference in morbidity or mortality comparing the staged vs simultaneous approaches. Broquet and associates21 also did not note a difference in the postoperative morbidity between staged and simultaneous operations, although these data were not stratified by major compared with minor hepatectomy. Although Reddy and coworkers23 noted no difference in perioperative outcomes with minor hepatectomy, the authors did report an increase in morbidity and mortality with the simultaneous approach and major hepatectomy. In the present study, we noted an increase in mortality among patients who underwent an extended hepatic resection during a simultaneous (8.0%) vs staged (2.8%) approach. This trend was not statistically significant, but the small number of patients who underwent an extended hepatic resection may have contributed to a type II statistical error. In the majority of cases, however, both minor and major hepatectomy appeared to be equally safe as part of either a staged or a simultaneous approach.
Regarding the long-term oncologic outcomes, the median and 5-year survivals after resection of both the primary CRC and CRLM in patients with sCRLM were 51 months and 44%, respectively. In addition, we found that 57% of patients experienced a recurrence, with a median follow-up of 34 months. Perhaps more interesting was our finding that the operative strategy for sCRLM had no impact on long-term outcomes (Fig. 3). In a much smaller, single institution cohort, Broquet and colleagues21 had similarly reported comparable overall survival among patients treated with the classic, simultaneous, or liver-first approach. Rather than operative strategy, tumor-specific factors were more associated with long-term oncologic success. For example, the need to use combined resection plus ablation for patients with more extensive disease was associated with a worse long-term survival, regardless of whether the timing of the operation was simultaneous or staged. These data serve to underscore the importance of biology and not technique in the prognosis of patients with sCRLM.
Historically the debate surrounding the management of patients with sCRLM has included only the classic staged vs simultaneous approach, but the liver-first/ reverse strategy staged approach recently has gained interest among surgeons. First reported by Mentha and colleagues36 in 2006, the reverse strategy approach was proposed as a possible means to minimize the delay in treating CRLM in patients with sCRLM. In this study, the majority of patients treated with the liver-first approach had a rectal primary tumor. The reverse strategy may be particularly beneficial in this group because these patients often require chemotherapy, radiotherapy, and a complex pelvic operation. As such, the classic staged approach (ie, rectal tumor followed by liver at a later date) can result in a significant delay in the treatment of the CRLM. To avoid this delay in treating the liver disease, a liver-first strategy may be more applicable to patients with sCRLM and a rectal primary.22 In this series, more than 50% of the liver-first operations were performed in the last 5 years of our study. Despite liver-first patients having a greater hepatic disease burden and undergoing major resection more often, the reverse strategy was safe and had long-term outcomes comparable to those of the other groups.
This study had several limitations. The data were retrospective in nature, and accurate collection of all complications may have been problematic and might have led to under-reporting of overall morbidity. We reported an overall morbidity of 19.6% after surgery for sCRLM, which was consistent with morbidity data reported from some large hepatobiliary centers,35,37,38 but lower than others.23,32,33 Given that our cohort included only patients who underwent both resection of the primary colorectal tumor and the liver metastasis, there may have been a reporting bias. Specifically, data on patients who were intended to undergo the staged approach but who never underwent the second operation would have been under-represented in our data set. Therefore, it is possible that an intention-to-treat analysis may have revealed an even higher incidence of morbidity or mortality in the staged group.
CONCLUSIONS
In conclusion, sCRLM patients managed with either a staged or simultaneous approach had similar perioperative and long-term outcomes. Both minor and major hepatectomy can be performed safely with low morbidity and mortality as part of either a simultaneous or a staged operative strategy. Although not statistically different, patients who underwent an extended hepatic resection did seem to have a higher perioperative mortality. As such, caution should still be exercised when considering an extended hepatectomy as part of a simultanteous approach to sCRLM. Oncologically, patients managed with a staged or simultaneous approach had similar recurrence and overall survival. These data indicated that long-term outcomes among patients with sCRLM are dictated by biology, not surgical strategy.
Abbreviations and Acronyms
- CRC
colorectal cancer
- CRLM
colorectal liver metastasis
- HR
hazard ratio
- sCRLM
synchronous colorectal liver metastasis
Footnotes
Disclosure Information: Authors have nothing to disclose. Timothy J Eberlein, Editor-in-Chief, has nothing to disclose.
Presented at the Southern Surgical Association 124th Annual Meeting, Palm Beach, FL, December 2012.
Author Contributions
Study conception and design: Mayo, Pawlik
Acquisition of data: Mayo, Pulitano, Marques, Lamelas, Wolfgang, de Saussure, Choti, Gindrat, Aldrighetti, Barrosso, Mentha, Pawlik
Analysis and interpretation of data: Mayo, Pulitano, Marques, Lamelas, Wolfgang, de Saussure, Choti, Gindrat, Aldrighetti, Barrosso, Mentha, Pawlik
Drafting of manuscript: Mayo, Pawlik
Critical revision: Mayo, Pulitano, Marques, Lamelas, Wolfgang, de Saussure, Choti, Gindrat, Aldrighetti, Barrosso, Mentha, Pawlik
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Blumgart LH, Fong Y. Surgical options in the treatment of hepatic metastasis from colorectal cancer. Curr Probl Surg. 1995;32:333–421. doi: 10.1016/s0011-3840(05)80012-7. [DOI] [PubMed] [Google Scholar]
- 3.Norstein J, Silen W. Natural history of liver metastases from colorectal carcinoma. J Gastrointest Surg. 1997;1:398–407. doi: 10.1016/s1091-255x(97)80126-6. [DOI] [PubMed] [Google Scholar]
- 4.Mayo SC, Heckman JE, Shore AD, et al. Shifting trends in liver-directed management of patients with colorectal liver metastasis: a population-based analysis. Surgery. 2011;150:204–216. doi: 10.1016/j.surg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 6.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11:1057–1077. doi: 10.1007/s11605-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 7.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110:13–29. [PubMed] [Google Scholar]
- 9.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes KS, Rosenstein RB, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis Colon Rectum. 1988;31:1–4. doi: 10.1007/bf02552560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adson MA, van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg. 1984;119:647–651. doi: 10.1001/archsurg.1984.01390180015003. [DOI] [PubMed] [Google Scholar]
- 13.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703–710. discussion 710–711. [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins LT, Millikan KW, Bines SD, et al. Hepatic resection for metastatic colorectal cancer. Am Surg. 1997;63:605–610. [PubMed] [Google Scholar]
- 15.Jamison RL, Donohue JH, Nagorney DM, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg. 1997;132:505–510. doi: 10.1001/archsurg.1997.01430290051008. discussion 511. [DOI] [PubMed] [Google Scholar]
- 16.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong MC, Mayo SC, Pulitano C, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13:2141–2151. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- 18.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 19.Asiyanbola B, Chang D, Gleisner AL, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–851. doi: 10.1007/s11605-008-0494-y. [DOI] [PubMed] [Google Scholar]
- 20.Mentha G, Roth AD, Terraz S, et al. ‘Liver first’ approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg. 2008;25:430–435. doi: 10.1159/000184734. [DOI] [PubMed] [Google Scholar]
- 21.Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934–941. doi: 10.1016/j.jamcollsurg.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 22.Andres A, Toso C, Adam R, et al. A survival analysis of the liver-first reversed management of advanced simultaneous colorectal liver metastases: a LiverMetSurvey-based study. Ann Surg. 2012;256:772–779. doi: 10.1097/SLA.0b013e3182734423. [DOI] [PubMed] [Google Scholar]
- 23.Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 24.Bilchik AJ, Poston G, Curley SA, et al. Neoadjuvant chemotherapy for metastatic colon cancer: a cautionary note. J Clin Oncol. 2005;23:9073–9078. doi: 10.1200/JCO.2005.03.2334. [DOI] [PubMed] [Google Scholar]
- 25.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 26.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayo SC, Shore AD, Nathan H, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford) 2011;13:473–482. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley and Sons, Inc; 2000. [Google Scholar]
- 29.Lemeshow S, Hosmer DW., Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 31.Dimick JB, Wainess RM, Cowan JA, et al. National trends in the use and outcomes of hepatic resection. J Am Coll Surg. 2004;199:31–38. doi: 10.1016/j.jamcollsurg.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197:233–241. doi: 10.1016/S1072-7515(03)00390-9. discussion 241–242. [DOI] [PubMed] [Google Scholar]
- 33.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 34.Vogt P, Raab R, Ringe B, Pichlmayr R. Resection of synchronous liver metastases from colorectal cancer. World J Surg. 1991;15:62–67. doi: 10.1007/BF01658964. [DOI] [PubMed] [Google Scholar]
- 35.Weber DM. Laparoscopic surgery: an excellent approach in elderly patients. Arch Surg. 2003;138:1083–1088. doi: 10.1001/archsurg.138.10.1083. [DOI] [PubMed] [Google Scholar]
- 36.Mentha G, Majno PE, Andres A, et al. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872–878. doi: 10.1002/bjs.5346. [DOI] [PubMed] [Google Scholar]
- 37.de Santibanes E, Lassalle FB, McCormack L, et al. Simultaneous colorectal and hepatic resections for colorectal cancer: postoperative and longterm outcomes. J Am Coll Surg. 2002;195:196–202. doi: 10.1016/s1072-7515(02)01235-8. [DOI] [PubMed] [Google Scholar]
- 38.Jatzko G, Wette V, Muller M, et al. Simultaneous resection of colorectal carcinoma and synchronous liver metastases in a district hospital. Int J Colorectal Dis. 1991;6:111–114. doi: 10.1007/BF00300206. [DOI] [PubMed] [Google Scholar]