SUMMARY
We devised a high-throughput, cell-based assay to identify compounds to treat Group3 medulloblastoma (G3 MB). Mouse G3 MBs neurospheres were screened against a library of approximately 7,000 compounds including U.S. Food and Drug Administration (FDA)-approved drugs. We identified pemetrexed and gemcitabine that preferentially inhibited G3 MB proliferation in vitro compared to control neurospheres and substantially inhibited G3 MB proliferation in vivo. When combined, these two drugs significantly increased survival of mice bearing cortical implants of mouse and human G3 MBs that overexpress MYC compared to each agent alone, while having little effect on mouse MBs of the Sonic Hedgehog (SHH) subgroup. Our findings strongly suggest that combination therapy of pemetrexed and gemcitabine is a promising treatment for G3 MBs.
Keywords: Group3 and Sonic Hedgehog medulloblastoma, decitabine, pemetrexed, gemcitabine, high throughput screen, FDA-approved compounds, C-MYC (MYC), Trp53
INTRODUCTION
Medulloblastoma(MB), a tumor of the posterior fossa, is primarily a pediatric disease, although it occasionally occurs in adults (Ellison et al., 2011). MB is classified into four major subgroups based on their clinical and molecular profiles (Taylor et al, 2012). Two subgroups exhibit constitutive activation of the Sonic Hedgehog (SHH) or Wingless (WNT) developmental pathways. The other two are referred to as Group 3 (G3) and G4. Importantly, the molecular subgrouping of these tumors also relates to distinct patient demographics, histologic classification, somatic genetic variations, and clinical outcome. For example, patients with WNT MB tend to be older, female, and to uniformly survive with current therapy (Northcott et al., 2012). In contrast, patients with G3 MB tend to be younger, male, have anaplastic histology, exhibit a higher incidence of metastatic disease, and have a poor prognosis (Dubuc et al., 2013; Kool et al., 2012; Parsons et al., 2011; Pugh et al., 2012; Rausch et al., 2012; Robinson et al., 2012). One characteristic feature of G3 MB is their high MYC expression in >75% of cases. Indeed, one study shows that MYC expression is elevated in 20/26 (77%) G3 MB (named Group C), compared to 1/35 (3%) G4 MB (named Group D) (Northcott et al., 2011). Whole genome sequencing studies on 17 G3 MBs reveals only 1 tumor (5.9%) with true MYC amplification while 2 other tumors display an aberrant copy number gain of the MYC gene. However 15 of these 17 (88%) G3 MBs demonstrate high MYC expression (Robinson et al., 2012). Despite these pronounced differences, patients affected with MB are typically treated with uniform surgery, radiotherapy and adjuvant chemotherapy including vincristine, cisplatin and cyclophosphamide (Packer et al., 2013) or lomustine (CCNU) and carboplatin (Massimino et al., 2012). These therapies fail to cure one-third of all patients and carry widespread morbidities that impair survivors’ quality of life.
Molecular subgrouping has the potential to improve risk stratification and tailor therapy to reduce toxicities to potential survivors. Paramount to this strategy is the development of accurate models that recapitulate the subgroups for preclinical therapeutic testing. We developed a mouse model of G3 MB through the orthotopic transplantation of transgenic cerebellar granule neuronal progenitors (GNPs) in the cortices of naïve recipient animals. GNPs were purified by percoll density gradient from the cerebella of 5–7 days old Trp53−/−; Cdkn2c−/− mice and infected with retroviruses encoding MYC (Kawauchi et al., 2012). MB tumors develop within 30 days of transplantation with as few as 100 tumor cells that recapitulate the high level of tri-methylation of histone H3 at lysine 27 (H3K27me3) seen in human G3 and G4 (Robinson et al., 2012). Tumors grow as neurospheres that, when transplanted into cortices of recipient mice, induce secondary MBs that mimic the primary tumors (Kawauchi et al., 2012). Because neurospheres can be passaged repeatedly while maintaining their functional and molecular properties, they provide a unique platform to conduct screens of compounds to identify those with therapeutic potential against human G3 MB. We here report the outcome of screening a library of compounds that included FDA-approved drugs and candidate compounds in development.
RESULTS
High-throughput screen using mouse G3 MBneurospheres
Tumor cells purified from several independently-derived primary mouse G3 MBs were grown as neurospheres for 4–5 passages providing lines with comparable cell proliferation characteristics. Two lines, derived from independent tumors and infections, were hereafter referred to as “Myc1” and “Myc2”. Neurospheres from the cerebellum of 7-day (P7) old Trp53−/−; Cdkn2c−/− mice (hereafter referred as Trp53-null) were used as control. To determine the number of cells necessary for exponential growth 4 days after plating, mouse Trp53-null and Myc1 were plated at different densities (Figure S1A). Other control cells included TERT− human fibroblasts (BJ) to identify compounds with non-specific toxicities and HepG2 (a human hepatocellular carcinoma cell line) to eliminate highly cytotoxic compounds.
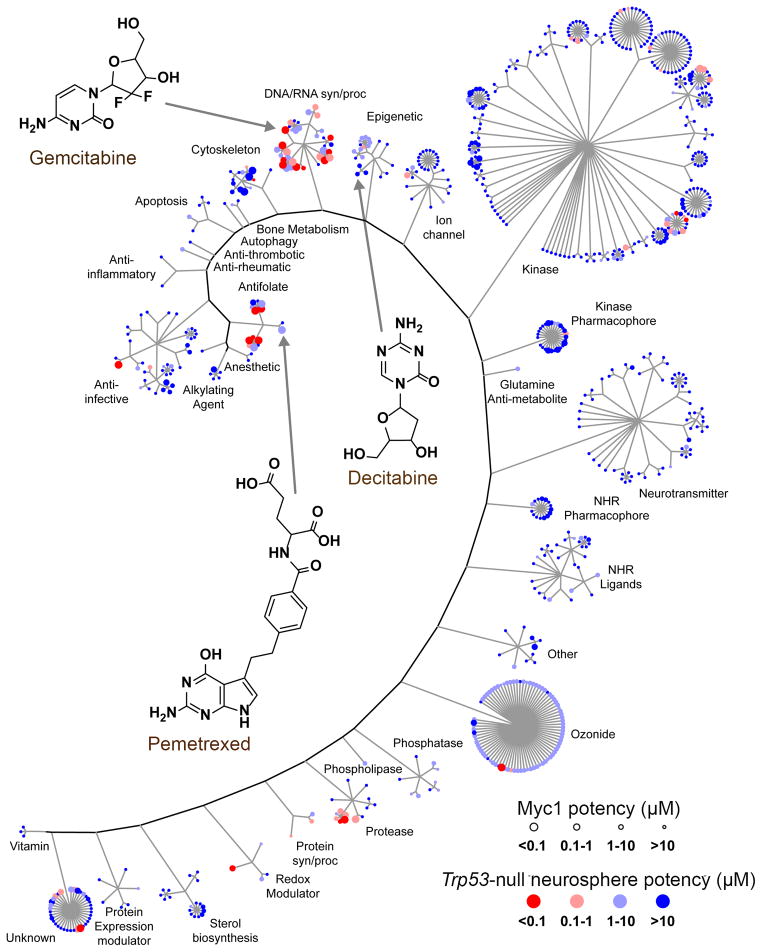
We performed a primary screen of a ‘bioactive’ library using a luminescence-based assay that measures cell proliferation via ATP. The library contained 7389 compounds (6568 unique) obtained from different sources, including 830 FDA-approved drugs (Figure S1B; see Supplemental Information for details). Compounds were tested at a single concentration (10 μM) in triplicate. Z-prime and other assay diagnostics were acceptable (Figure S1C) and the scatterplot of controls and compound activities showed adequate separation between signal and noise for both Myc1 and Trp53-null (Figure S1D, left panels). Receiver operator characteristic (ROC) analysis indicated that the assay demonstrated acceptable discriminatory power between true positives and true negatives, with the Area Under the Curve (AUC) > 0.8 for both lines and that an assay cutoff of > 50% returned ca.70% of all true positives for Myc1 (Figure S1D, right panels).690 of the 7389 compounds, including all with inhibition >50% in the primary screen with Myc1, analogs of these hits, and other compounds of interest, were tested in dose-response experiments in triplicate using concentrations ranging from 4 nM to 10 μM. Of the 690 hits, we identified 65 unique compounds with potency <1 μM against Myc 1 (Figure 1, Table S1).
Figure 1. Summary of the HTS on mouse Myc1 and Trp53-null neurospheres.
Distribution of 690 active compounds from dose-response in Myc1 and Trp53-null neurospheres according to their mechanism of action. Each compound is represented as a colored circle, with potency against Myc1 depicted by size and potency against Trp53-null shown by blue-red color. Potent compounds selective for Myc1 are represented by large, blue circles. See also Figure S1 and Table S1.
Screening results of FDA-approved drugs
To accelerate the transition of potential therapeutics into the clinic, we prioritized the FDA-approved drugs with oncology indications and potencies below or near 1 μM for further study. We conducted dose-response experiments on 35 FDA-approved drugs in triplicate on Myc1, Trp53-null, Hep-G2 and BJ cell lines (Figure S2A, Table 1). Drugs were grouped into nine activity classes: folate pathway inhibitors, other inhibitors of DNA/RNA synthesis, purine anti-metabolites, microtubule, sterol biosynthesis and topoisomerase inhibitors, epigenetic regulators, and proteasome and RNA polymerase inhibitors. Myc1 was extremely sensitive to folate pathway inhibitors with pemetrexed, methotrexate and raltitrexed being the most selective. The DNA/RNA synthesis inhibitor gemcitabine was highly potent in both Myc1 and Trp53-null, but achieved 100% efficacy in only Myc1. The purine anti-metabolite cladribine showed promising activity against Myc1, but a narrower therapeutic window against the control cell lines HepG2 and BJ compared to other drugs. The proteasome inhibitor bortezomib and the RNA polymerase inhibitor dactinomycin were equipotent and equally efficacious against both Myc1 and Trp53-null. Microtubule inhibitors, including vincristine and vinblastine, and topoisomerase inhibitors, such as doxorubicin, etoposide, and topotecan, were active in G3 MB. Because they are already used in the clinic for the treatment of MB, they were not considered further. Cerivastatin and fluvastatin, two sterol biosynthesis inhibitors targeting HMG-CoA reductase had high efficacy but low potency relative to the other compound classes. Decitabine, a drug that causes both DNA damage and alterations in DNA methylation, was moderately potent, and had much higher efficacy for Myc1 over Trp53-null.
Table 1.
EC50 values for FDA-approved drugs on Myc1, Trp53-null, and control cell lines.
| Compound | Myc1 (μM) | Trp53-null (μM) | HepG2 (μM) | BJ (μM) |
|---|---|---|---|---|
| Amsacrine | 0.16 (0.097–0.27) | 0.55 (0.18–1.7) | 11 (5.6–23) | 13 (7.6–21) |
| Ancitabine | 0.031 (0.024–0.04) | 0.55 (0.29–1) | ND | ND |
| Bortezomib | 0.0063 (0.0051–0.0078) | 0.0027 (0.0018–0.004) | 0.026 (0.0076–0.087) | 0.023 (0.0086–0.061) |
| Cerivastatin | 0.77 (0.65–0.9) | 2.5 (1.3–4.7) | 0.92 (0.55–1.6) | ND |
| Cladrabine | 0.0043 (0.0024–0.0078) | 0.25 (0.026–2.4) | 3.3 (2.6–4.3) | 1.4 (0.13–17) |
| Clofarabine | 0.15 (0.13–0.18) | 0.34 (0.28–0.4) | ND | ND |
| Cycloguanil | 0.11 (0.059–0.21) | 0.18 (0.14–0.23) | ND | ND |
| Cytarabine | 0.083 (0.066–0.1) | 0.2 (0.092–0.42) | 0.078 (0.041–0.15)‡ | ND |
| Dactinomycin | 9e-04 (0.00016–0.005) | 0.0024 (0.0012–0.005) | 0.0084 (0.005–0.014) | ND |
| Daunorubicin | 0.0075 (0.0059–0.0097) | 0.036 (0.016–0.079) | 1.7 (0.11–25) | 0.1 (0.02–0.52)‡ |
| Decitabine | 1.3 (0.72–2.2) | 0.032 (0.014–0.073)‡ | ND | ND |
| Doxorubicin | 0.028 (0.015–0.052) | 0.11 (0.058–0.2) | 0.58 (0.14–2.3) | 0.14 (0.027–0.77)‡ |
| Etoposide | 0.24 (0.17–0.36) | 0.046 (0.013–0.15) | ND | ND |
| Floxuridine | 0.0016 (0.0013–0.002) | 0.0001* | ND | ND |
| Fluorouracil | 0.36 (0.3–0.43) | 0.22 (0.17–0.29) | ND | ND |
| Fluvastatin | 4.5 (1.6–13) | ND | ND | ND |
| Gemcitabine | 0.0021 (0.0018–0.0025) | 0.00032 (0.00012–0.00087) | ND | ND |
| Lovastatin | 4 (3.3–4.8) | 2.1 (0.11–38)‡ | 13 (4.7–38) | ND |
| Methotrexate | 0.0052 (0.0024–0.012) | 0.15 (0.05–0.46) | ND | ND |
| Mitoxanthrone | 0.18 (0.11–0.29) | 0.19 (0.12–0.31) | 0.12 (0.072–0.2)‡ | ND |
| Nocodazole | 0.11 (0.036–0.32) | 0.094 (0.037–0.23)‡ | 0.015 (0.0073–0.03)‡ | ND |
| Pemetrexed | 0.035 (0.027–0.046) | 15 (0.57–410) | ND | ND |
| Pitavastatin | 3.5 (1.5–8.6) | ND | 5 (2.8–8.7) | ND |
| Podofilox | 0.0064 (0.0015–0.028) | 0.0074 (0.0048–0.012)‡ | 0.0039 (0.0025–0.0059)‡ | ND |
| Pyrimethamine | 4.9 (3–8.1) | 0.87 (0.2–3.9) | ND | ND |
| Raltitrexed | 0.003 (0.0024–0.0038) | 0.34 (0.18–0.63) | ND | ND |
| Rosuvastatin | 2.6 (2.3–3) | ND | ND | ND |
| Simvastatin | 3 (2.6–3.5) | ND | 9.4 (6–15) | 5.8 (4.7–7.2) |
| Tenoposide | 0.052 (0.003–0.89) | 0.11 (0.0097–1.2) | 7.7 (1.5–39) | 29 (3.8–230) |
| Thioguanine | 0.44 (0.082–2.4) | 0.8 (0.52–1.2) | 11 (2.3–52) | ND |
| Topotecan | 0.075 (0.05–0.11) | 0.18 (0.06–0.53) | 0.09 (0.026–0.31) | ND |
| Trifluridine | 0.038 (0.034–0.043) | 0.0029 (0.0022–0.0039) | ND | ND |
| Trimetrexate | 0.035 (0.0028–0.45) | 0.0047 (0.0033–0.0066) | ND | ND |
| Vinblastine | 0.012 (0.0028–0.053) | 0.015 (0.011–0.021)‡ | ND | ND |
| Vincristine | 0.0094 (0.0044–0.02) | 0.01 (0.0071–0.014)‡ | ND | ND |
ND = EC50 could not be determined in the concentration range tested.
indicates questionable EC50 due to regression artifacts.
indicates regression curve failed to reach 50% efficacy.
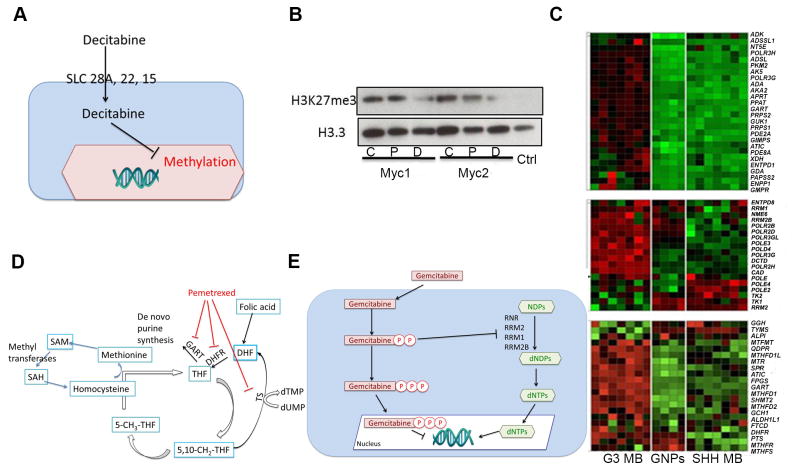
We selected decitabine, pemetrexed, and gemcitabine for further study due to their selectivity and diversity in mechanism of action. As noted earlier, mouse and human G3 MBs are marked by a high level of H3K27me3. Decitabine is an S-adenosyl methionine and cytidine analogue. At low dose and prolonged exposure, decitabine targets DNA and histone methylation, whereas it induces DNA damage at high doses (Figure 2A) (Palii et al., 2008). After 72 hr of treatment, the EC50 for decitabine was 1.3 μM against Myc1 with 100% efficacy (Figure S2A, blue curve, Table 1), whereas efficacy against Trp53-null never exceeded 35% (Figure S2A, red curve, Table 1). Treatment of Myc1 with 0.5 μM decitabine for 72 hr significantly decreased H3K27me3 levels while 40 nM of pemetrexed had no effect (Figure 2B).
Figure 2. Pattern of sensitivity of Trp53-null and G3 neurospheres to the different classes of compounds.
(A)An outline of the decitabine pathway. (B) Detection of H3K27me3 by immunoblotting from cell lysates of Myc1 and Myc 2, untreated (C), or treated with decitabine (D) or pemetrexed (P). (Ctrl) represents recombinant human H3.(C) mRNA levels of enzymes involved in purine (top), pyrimidine (middle) metabolism and the folate pathway (one carbon pool and folate biosynthesis) (bottom) (from KEGG pathway) between mouse G3 MB, SHH MB, and Trp53-null GNPs. (D) Pemetrexed effects on the folate pathway: pemetrexed inhibits phosphoribosylglycinamideformyltransferase(GART), dihydrofolate reductase (DHFR) and thymidylate synthase (TS). (E) Gemcitabine targets:gemcitabine blocks DNA replication and deoxynucleotide triphosphate (dNTP) synthesis. See also Figure S2.
A comparative study of gene expression profiles in mouse G3 and SHH MBs and GNPs suggested that mouse G3 MBs were sensitive to inhibitors of purine (Figure 2C, top panel), pyrimidine (Figure 2C, middle panel) and folate (Figure 2C, bottom panel) metabolism compared to SHH MBs and GNPs. In agreement with this gene expression pattern, pemetrexed and gemcitabine targeted these pathways. Pemetrexed targets three enzymes in the folate pathway; phosphoribosylglycinamideformyltransferase(GART), dihydrofolate reductase (DHFR), and thymidylate synthase(TS) (Figure 2D) (Chattopadhyay et al., 2007). After 72 hr of pemetrexed treatment, the EC50 for Myc1 was 35 nM (Figure S2A, blue curve, Table 1) compared to 15 μM in Trp53-null (Figure S2A, red curve, Table 1). Gemcitabine disrupts DNA synthesis via incorporation in DNA or inhibition of the ribonucleotide reductase (RNR) (Figure 2E) (van Moorsel et al., 2000). When incorporated into DNA, gemcitabine causes single strand breaks that lead to apoptosis (Ewald et al., 2007). After 72 hr treatment, the Myc1 EC50 for gemcitabine was 2.1 nM with 100% efficacy whereas efficacy against Trp53-null never exceeded 63% (Figure S2A, Table 1).
Decitabine, pemetrexed and gemcitabine were further tested on four additional, mouse G3 MB lines derived from independently-derived tumors and infections; all displayed comparable potency and efficacy compared to Myc1 (Figure S2B). By contrast, we found a SHH MB-derived line was as sensitive to pemetrexed as Myc1 but was greater than 5–fold less sensitive to gemcitabine compared to Myc1 (Figure S2C).
Pharmacological assessment of decitabine, pemetrexed and gemcitabineon mouse G3 MBs
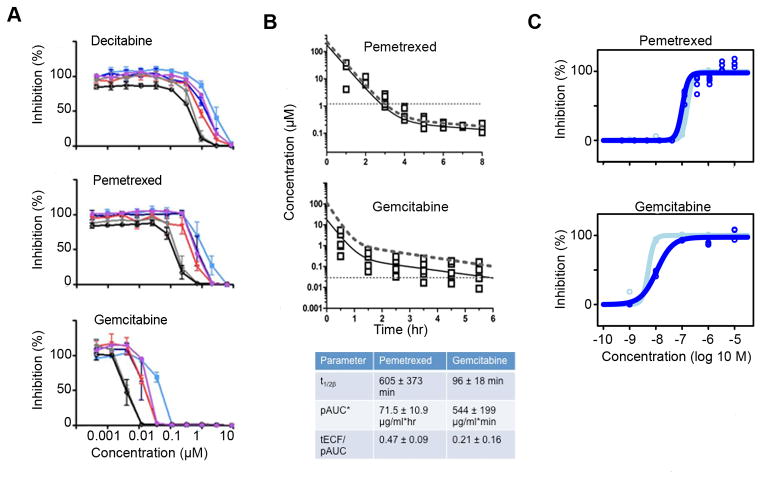
To determine the concentration-time threshold required to inhibit proliferation of neurospheres in vitro, we performed “wash-out” experiments with each of the three compounds. TheEC50 values for decitabine were approximately 2.4 μM, 920 nM, and 500 nM after 1, 10, and 24 hr drug exposure, respectively (Figure 3A, top panel). While theEC50 for pemetrexed was 1.2 μM after 1hr exposure, it decreased to 500 nM after 10 hr, and to 180 nM after 24 hr (Figure 3A, middle panel). With gemcitabine, the EC50 values were 44 nM, 13 nM, and 3.8 nM after 1, 10, and 24 hr exposure, respectively (Figure 3A, bottom panel).
Figure 3. Effects of pemetrexed and gemcitabine on mouse and human G3 neurospheres in vitro and pharmacokinetics in G3 MB-bearing mice.
(A) In vitro “washout” of decitabine (top panel), pemetrexed (middle panel), and gemcitabine (bottom panel) on Myc1. Cells incubated with drugs for indicated times(1hr, light blue line; 3hr, red line; 6hr, dark blue line; 10hr, brown line; 24hr, grey line; 72hr, black line) after which the medium was replaced by fresh medium and plates were read 72 hr after. (B) Concentration-time plot for pemetrexed (top panel) and gemcitabine (middle panel). Observed tumor extracellular fluid concentrations (tECF; open squares), population simulation of tECF concentration-time data (solid black line), and population simulation of total plasma concentration-time data (dotted line) included in both plots. The horizontal dashed line represents the 1hr EC50 derived from Myc1. PK parameters are described in the bottom panel (t1/2=half-life, pAUC=area under the plasma concentration-time curve; tECF/AUC=tumor extracellular fluid/AUC ratio). Values in the bottom panel represent averages +/− standard deviations. (C) Neurospheres from two G3 MB PDXs, TB-12-5950 (light blue curves) and OA-2012-1 (dark blue curves), treated with concentrations from 1 nM to 10 μM of pemetrexed and gemcitabine, and read 72 hr later; top panel, pemetrexed EC50=160 nM (light blue line) or 100 nM (dark blue line); bottom panel, gemcitabine EC50=5.1 nM (light blue line) or 11 nM (dark blue line). See also Figure S3.
Although decitabine affected the viability of Myc1 and decreased H3K27me3 in vitro (Figure 2B), a review of the published preclinical and clinical pharmacokinetic data (Chabot et al., 1983; George et al., 2010) strongly suggested that the maximally achievable decitabine brain concentrations in humans would be well below those required to induce significant inhibition of proliferation. Therefore, decitabine was not considered for further in vivo studies.
For gemcitabine and pemetrexed, total plasma and tumor extracellular fluid (tECF) drug concentrations were assessed in separate groups of mice bearing Myc1-induced G3 MB after single intravenous (IV) injections of pemetrexed (200 mg/kg) and gemcitabine (60 mg/kg). A three-compartment pharmacokinetic model adequately described the plasma and tECF concentration-time data for each drug. The murine plasma pharmacokinetics for each agent differed only modestly from previously published reports, with the clearance of gemcitabine and pemetrexed being around 2-fold higher in our studies (Rocchetti et al., 2007; Wang et al., 2004; Woodland et al., 1997). The plasma exposure of each drug, quantified by the area under the plasma concentration-time curve (pAUC), was similar to that achieved in humans at clinically relevant dosages (Figure 3B, upper and middle panels) (Malempati et al., 2007; Reid et al., 2004). We found that the tECF concentrations of pemetrexed and gemcitabine exceeded the in vitroEC50 vs. time threshold suggesting that these compounds should have in vivo efficacy in G3 MB (Figure 3B, bottom panel).
To address how much drug crosses the normal blood brain barrier (BBB), studies of pemetrexed and gemcitabine were conducted in six non-tumor bearing mice. Pemetrexed (200 mg/kg IV) or gemcitabine (60 mg/kg IV) was administered, the brain harvested and the drug concentration was measured in both the brain parenchyma and plasma. The brain to plasma ratio of pemetrexed and gemcitabine in these samples was 7.3% and 45%, respectively, indicating that both drugs cross a normal BBB in a mouse model.
Pemetrexed and gemcitabine inhibit human G3 MB proliferation in vitro
To assess whether pemetrexed and gemcitabine inhibited proliferation of human G3 MB in vitro, we generated patient-derived xenografts (PDXs) from primary G3 MBs overexpressing the MYC protein with or without MYC amplification and derived neurospheres for two of them. Gene expression profiling of Icb-1572 (Zhao et al., 2012) and TB-12-5950 (SJCRH) confirmed clustering with previously published human G3 MB (Robinson et al., 2012), and demonstrated that a similar profile was maintained through several passages in mice (Figure S3A). Fluorescence in situ hybridization (FISH) analysis revealed that MYC was not amplified in Icb-1572 (Figure S3B, left panel)(Shu et al., 2008) but was amplified in TB-12-5950 that also displayed leptomeningeal dissemination (Figure S3B, right panel). PDX OA-2012-1 overexpressed the MYC protein without amplification, as measured by a CGH (Ayrault, unpublished results), whereas PDX Med-511-FH was confirmed to have MYC amplification by nanostring analysis (Olson, unpublished results).
TB-12-5950, OA-2012-1 and human neural stem cells H9 formed neurospheres in vitro allowing us to test the effects of pemetrexed and gemcitabine invitro. Neurospheres were treated for 72 hr at doses ranging from 1 nM to 10 μM. Cell viability was measured. For TB-12-5950 and OA-2012, EC50 for pemetrexed were 160 nM and 100 nM (Figure 3C, top panel), while those for gemcitabine were 5.1 nM and 11 nM, respectively (Figure 3C, bottom panel). These EC50 values were similar to those found for Myc1 (within 2–5 fold) (Table 1). For H9 cells, the EC50 for pemetrexed was 0.29 μM and for gemcitabine 0.0015 μM (Figure S3C), which corresponded to the response seen in mouse control Trp53-null (Figure S2A, Table 1).
Pemetrexed and gemcitabine activity in mouse G3 MBs in vivo
To determine how efficacious pemetrexed and gemcitabine were in suppressing proliferation of mouse G3 MB in vivo, we stereotactically transplanted 1×105 purified tumor cells, retrovirally transduced with luciferase, in the cortices of CD1 mice. We previously determined that this cell number induces MBs that kill the animals within 15 days post-transplant, and recapitulate the primary tumors (Kawauchi et al., 2012). Bioluminescence detection of tumor progression correlated with tumor volume measured by Magnetic Resonance Imaging (MRI) (Figure S4A, B and C). Hematoxylin and Eosin (H&E) staining of tumor sections performed three days post-transplant confirmed the presence of an organized tumor mass that was vascularized and surrounded by blood vessels (Figure S4D).
The schedule and dosage of drug delivery was calculated based upon modeling and simulation of data from our pharmacokinetic studies, and related to pAUC values tolerable in pediatric clinical trials (Malempati et al., 2007; Reid et al., 2004). Mice transplanted with Myc1 were treated by tail vein injection with 200 mg/kg pemetrexed (Figure 4A), 60 mg/kg gemcitabine (Figure 4B), or the combination of both drugs (Figure 4C). When combined, pemetrexed and gemcitabine were either given together in the same injection (Figure 4C, top panels) or split by a 2-day interval (first gemcitabine, 2 days later pemetrexed) (Figure 4C, bottom panels) to reduce stress on the mice.
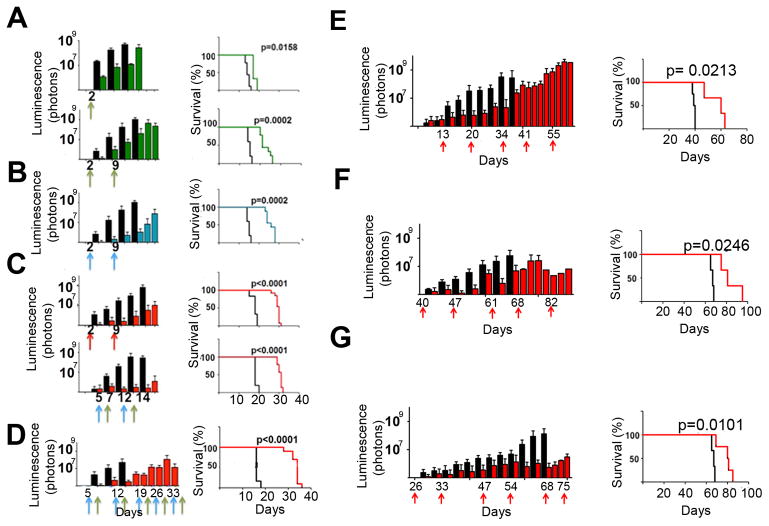
Figure 4. In vivo efficacy of pemetrexed and gemcitabine against G3 MBs.
Mice injected with luciferase marked 1×105 Myc1 (A–D) or 1×106 G3 MB PDX cells (E–G). Treatment was initiated when luciferase signal reached 5.105 photons/sec. Luciferase measurements (left panels), survival (right panels). (A) Top panels: mice treated at day 2 (green arrow) with pemetrexed (green bars and line) or saline (black bars and line); Bottom panels: mice treated at days 2 and 9 with pemetrexed (green arrows). n=10 drug treatment, n=5 saline. (B) Mice treated at days 2 and 9 (blue arrows) with gemcitabine (treated group, blue bars and line) or saline (control group, black bars and line). n=10 drug treatment, n=5 saline. (C) Top panels: mice treated at days 2 and 9 (red arrows) with pemetrexed and gemcitabine administered together (treated group, red bars and line) or saline (control group, black bars and line); Bottom panels: mice treated with gemcitabine at days 5 and 12 (blue arrows) and pemetrexed at days 7 and 14 (green arrows) (treated group, red bars and line) or saline (control group, black bars and line). n=10 treated group, n=5 control group. (D) Long-term treatment of Myc1-bearing mice with vehicle (black bars and line), or treated (red bars and line) with pemetrexed (green arrows) and gemcitabine (blue arrows). (E) Mice bearing G3 MB PDX Icb-1572 (passage 8) treated at day 13 after implant and days, 20, 34 and 41 (red arrows) with pemetrexed and gemcitabine (n=5, red bars and red line) or saline (n=5, black bars and black line). (F) Mice bearing G3 MB PDX TB-12-5950, treated after implant at days 40, 47, 61 and 68 (red arrows) with pemetrexed and gemcitabine (n=5, red bars and line) or saline (n=5, black bars and line). (G) Mice bearing G3 MB PDX Med-511-FH treated at days 26, 33, 47 and 54 (red arrows) with pemetrexed and gemcitabine (n=5, red bars and line) or saline (n=5, black bars and line). See also Figure S4.
Five mice treated with vehicle survived up to 14 to 20 days after transplant, as expected (Figure 4A–C, black bars). One dose of pemetrexed at day 3 increased median survival by 3 days, (Figure 4A, top panels). Mice treated with two doses of pemetrexed at days 3 and 10 had an increased 7 days median survival (Figure 4A, bottom panels). Two doses of gemcitabine at 60 mg/kg, at days 2 and 9 after tumor implant, had an 11 days increased median survival by compared to mice treated with vehicle (Figure 4B). Treatment of mice with the two drugs given at the same time on days 2 and 9 increased their median survival by 13 days compared to vehicle treated animals (Figure 4C, top panels). Similarly, mice treated with gemcitabine at days 5 and 12 and with pemetrexed at days 7 and 14 (Figure 4C, bottom panels) had a 12 days median survival (from 18 to 30 days). Therefore, mice receiving both drugs together or separately had a similar increased median survival compared to vehicle treated animals.
We repeated the efficacy studies with Myc2 and obtained similar results (Figure S4E). The survival of animals treated with vehicle was 11 days, whereas treatment with pemetrexed at days 4 and 11 increased the median survival up to 28 days. Mice treated with gemcitabine at days 4 and 11 had a median survival of 26 days whereas co-treatment with pemetrexed and gemcitabine given together increased the median survival by 33 days (Figure S4E).
Because the treatment of mice with the two drugs administered singly or together was well tolerated, based on weekly observations of signs of morbidity (loss of motion, head dome, lethargy) and white blood cell count, and because the tumors came back despite treatment, we assessed the effects of a longer treatment course on tumor growth. Ten Myc1-induced G3 MB-bearing mice were either treated with vehicle or once a week with gemcitabine from days 4 to 32 and pemetrexed from days 6 to 34, after tumor implant. Long-term treatment increased mouse median survival by 18 days (Figure 4D). Similar results were obtained when mice were transplanted with Myc2, and treated long-term with the two drugs administered together (Figure S4F).
Pemetrexed and gemcitabine inhibit human G3 MB proliferation in vivo
The PDX Icb-1572, passage 8, was marked with luciferase and implanted in the cortex of CD1 mice that were treated 13 days after tumor implant with pemetrexed and gemcitabine at days 13, 20, 34, and 41 (Figure 4E). When added together, the two drugs increased mouse median survival by 21 days. We confirmed these results in two other G3 MB PDXs, with MYC amplification, TB-12-5950, passaged once in NSG and three times in CD1 mice and Med-511-FH, passaged once in NSG animals (Olson, unpublished) (Figure 4F, 4G). Since each PDX had a different proliferation rate, treatment was initiated at different times after implant when the luminescence signal reached 5×105 photons/sec [TB-12-5950, 40 days (Figure 4F) and Med-511-FH, 26 days (Figure 4G)] (Figure S4G). In both cases, all mice treated with vehicle died of tumor burden with a median survival of 67 days after implant whereas those treated with gemcitabine and pemetrexed survived longer up to 81 days. Thus, for each PDX, the median survival was significantly longer in the treatment group than the control.
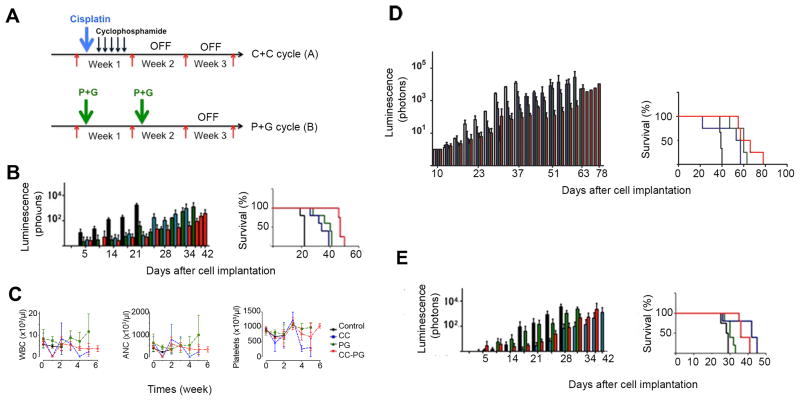
Effects of the treatment of mouse G3 and SHH MBs with pemetrexed and gemcitabine combined to cisplatin and cyclophosphamide in vivo
In an attempt to compare the pemetrexed and gemcitabine combination to agents already in clinical use, we treated mice bearing mouse G3 MB with cisplatin and cyclophosphamide (cycle A) followed with pemetrexed and gemcitabine (cycle B) (Figure 5A)(White and Sterling-Levis, 2008). Vincristine, the third drug used in the clinic could not be used due to intolerable toxicity in mice (data not shown). Mice treated with vehicle had to be euthanized 21 days after implant (Figure 5B, black bars and lines). Mice treated for two cycles with pemetrexed and gemcitabine combined (cycle B) had an 18 days increased median survival compared to vehicle treated mice (Figure 5B, green bars and line, p=0.035). Mice treated IV with cisplatin, 5 mg/kg at day 1 and intraperitoneally(IP) with cyclophosphamide, 130 mg/kg for 5 consecutive days, days 2–6, had a median survival of 12 days longer than the vehicle treated mice (Figure 5B, blue bars and line, p=0.035); however, two of the five mice died from acute drug-induced toxicity. Remarkably, mice treated with cisplatin and cyclophosphamide alternating with pemetrexed and gemcitabine survived 25 days longer than the vehicle-treated animals but still succumbed to tumors (Figure 5B, red bars and line, p=0.0069). Therefore the combination of cisplatin and cyclophosphamide with pemetrexed and gemcitabine resulted in a longer median survival than either treatment alone.
Figure 5. Treatment of mouse G3 and SHH MBs with pemetrexed and gemcitabine combined with cyclophosphamide and cisplatin.
(A)Treatment schedule with cisplatin(C, bold blue arrow) at day 1 and cyclophosphamide (C, dark blue arrows) from days 2 to 6 every 3 weeks (C+C, cycle A, top panel) or pemetrexed and gemcitabine together twice every 3 weeks (P+G, cycle B, bottom panel). (B) Mice bearing mouse G3 MB treated with saline (black bars and line), C+C at each course, (blue bars and line), P+G at each course (green bars and line), or with C+C for the first course and P+G for the second course (red bars and line). (C) White blood cells (WBC), neutrophils (ANC) and platelets counts for mice treated with saline (black curves), C+C at each course (blue curves lines), P+G at each course (green curves) or alternative cycle of C+C first followed by P+G (red curves). (D) Mice bearing G3 MB PDX Icb-1572 treated with saline (black bars and line) (median survival 39 days), with C+C at each course (blue bars and curve) (median survival 55 days, p=0.0724), with P+G at each course (green bars and curve), (median survival 60 days, p=0.0084), or with C+C for the first course and P+G for the second course (red bars and curve) (median survival 62 days, p=0.0044). (E)Mice bearing mouse SHH tumors treated with saline (black bars and line) (median survival 28 days), with C+C at each course (blue bars and curve) (median survival 42 days, p=0.045), with P+G at each course (green bars and curve), (median survival 30 days, p=0.1042), or with C+C first course and P+G second course (red bars and curve) (median survival 36 days, p=0.040).
We evaluated the potential toxicity of each therapeutic regimen by analyzing blood chemistries, including while blood cells, absolute neutrophil and platelets count, once a week from cohorts of animals bearing mouse G3 MB treated with cisplatin and cyclophosphamide (Figure 5C, blue lines), pemetrexed and gemcitabine (Figure 5C, green lines), versus the combination of both cycles (Figure 5C, red lines). One cycle of cisplatin and cyclophosphamide dramatically decreased the number of white blood cells, neutrophils and platelets, but this was reversible since the values recovered to normal once treatment was stopped (Figure 5C).
To assess whether the combination of pemetrexed, gemcitabine and/or cisplatin and cyclophosphamide could also suppress proliferation of human G3 MB and mouse SHH MB, tumor cells purified from Icb-1572 and from a mouse SHH MB marked with luciferase were implanted in the cortex of recipient CD1 mice. Animals were treated with the same regimen as for mouse G3 MB. Mice bearing the Icb-1572 tumor died at 39 days post-implant when treated with vehicle (Figure 5D, black line). When treated with two rounds of cycle A, they died at 55 days (Figure 5D, blue line), but survive up to 60 days with two rounds of cycle B (Figure 5D, green line). Mice treated with cycle A and B died 63 days after transplant (Figure 5D, red line). Mice with mouse SHH MB, treated with vehicle had to be sacrificed 28 days after transplant (Figure 5E). Mice treated for two rounds of cycle B had a 2 day increased median survival compared to vehicle treated mice (Figure 5E, green bars and line) but when treated with two rounds of cycle A survived 14 days longer than vehicle treated mice (Figure 5E, panels, blue bars and line). Finally, mice treated with alternating cycle A and B survived 8 days longer than the vehicle-treated animals but succumbed to tumors (Figure 5E, red bars and line). This demonstrated that treatment with pemetrexed and gemcitabine had little effect on the survival of mice bearing mouse SHH MB. However, SHH MBs responded to cisplatin and cyclophosphamide, as expected from the clinical experience.
Resistance to pemetrexed and gemcitabine
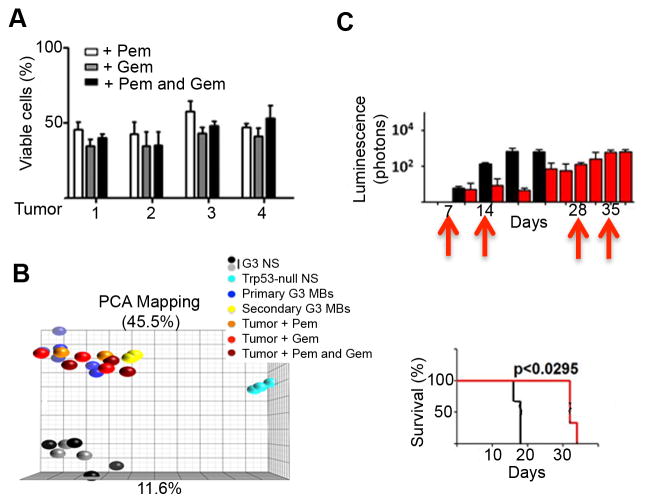
In all cases, tumors relentlessly regrew and eventually killed the animals. To test whether tumor regrowth could potentially be due to intrinsic drug resistance, tumor cells were purified from four G3 MBs, twofrom untreated animals (tumors 1 and 2) and two from mice treated with pemetrexed and gemcitabine (tumors 3 and 4) (Figure 4D), and grown as neurospheres in presence or absence of drugs (Figure 6A). Regardless of whether the tumor cells came from untreated or treated animals, they had similar sensitivity to pemetrexed and gemcitabine, suggesting that tumor regrowth was not due to intrinsic resistance to either drug (Figure 6A). Moreover, gene expression analysis of tumors harvested at the time of sacrifice from treated or control mice showed no significant difference in their global transcriptome; principal component analysis (PCA) of either primary or secondary G3 MBs from untreated animals or from G3 MBs treated with pemetrexed, gemcitabine or both, clustered together (Figure 6B).
Figure 6. Resistance of mouse G3 MBs to gemcitabine and pemetrexed treatment.
(A) Viability of tumor cells purified from 4 individual tumors (1–4) arisen from animals treated in Figure S4F grown in vitro and treated with 40 nM pemetrexed (pem, white bars), 5 nM gemcitabine (gem, grey bars) or both drugs together (black bars). Cell viability was measured after 72 hr treatment by trypan blue normalized to cells treated with DMSO. (B) Principal component analysis (PCA) for G3 neurospheres (G3 NS, black and grey dots, n=7), Trp53-null neurospheres (NS, clear blue dots, n=3), primary G3 MBs (dark blue dots, n=3), secondary G3 MBs (yellow dots, n=3), or G3 MBs (tumors) from animal treated with pem(orange dots, n=3), gem (red dots, n=3) or both drugs (dark red dots, n=3). (C) Mice implanted with tumors harvested from mice treated in (A) were treated with vehicle (black bars, top; black line, bottom) or with pemand gem combined (red bars, top; red line, bottom) at days 7, 14, 28 and 35 (red arrows). See also Figure S5.
To gage whether mouse G3 MBs harvested from animals treated long-term with pemetrexed and gemcitabine (Figure S4F) remained sensitive to the two drugs in vivo, tumor cells were purified from G3 MBs from treated mice at time of euthanasia and re-implanted into the cortices of naïve recipients. Mice bearing MB were either left untreated (Figure 6C, black bars and line) or treated with the two drugs added together, once a week from days 6 to 34 (Figure 6C, red bars and line). Whether the tumors were from animals treated or not, mouse median survival was similar (compare Figure 6C to Figure S4F). To assess the role of pharmacokinetics in drug resistance, mice bearing G3 MB were treated with pemetrexed and gemcitabine at days 3, 10, and 24. Plasma and brain samples were collected 1, 3, and 6 hours after the last day 24 treatment. Plasma exposure of both pemetrexed and gemcitabine were equivalent to that observed after single-dose treatment, and brain concentrations remained above the respective EC50 concentrations for both drugs. These results suggest that neither intrinsic acquired drug resistance of tumor cells nor altered drug delivery accounted for the observed persistent tumor growth after therapy.
Others have shown that the spatial distribution of chemotherapeutics in solid tumors is highly dependent on the presence of blood vessels (Minchinton and Tannock, 2006; Saggar et al., 2013). Two independently derived mouse G3 MBs were immunostained with an antibody to caspase 3 after treatment with pemetrexed and gemcitabine. Caspase 3 staining was not detected in the vehicle-treated tumors (Figure S5A, control) but was found in tumors from treated mice. Even though tumors were well vascularized, areas of apoptosis were detected (Figure S5A). Moreover, we found that pemetrexed and gemcitabine induced cell death in tumor cells in vitro by Annexin V staining (Figure S5B) and that gemcitabine induced histone 2AX (H2AX) foci after 1 hr treatment at 5 nM (Figure S5C, bottom panels). Proliferation measured by bromo-deoxy-uridine (BrdU) incorporation was similar in treated and untreated MBs (Figure S5D). These data suggest that host factors and/or vascularization of the tumors may limit the efficacy of pemetrexed and gemcitabine.
Purine metabolism is directly linked to the folate pathway through the action of GART. We found that increasing the concentration of folate in the medium (up to 10 μM) significantly decreased the sensitivity of mouse G3 MB neurospheres to pemetrexed highlighting the importance of the folate pathway in these tumors (Figure S5E). We also noticed that when G3 MB-bearing animals were treated with cisplatin/cyclophosphamide or pemetrexed/gemcitabine for one cycle, tumor cells harvested from these mice responded to the four drugs similarly in culture with no significant differences in EC50 (Figure S5F).
DISCUSSION
G3 MB, characterized by high levels of MYC expression due to gene amplification or overexpression (Northcott et al., 2011; 2012), is a particularly aggressive tumor for which current therapy is inadequate. The generation of a MYC-driven murine model of G3 MB and its propagation as robust neurospheres enabled the testing and identification of compounds from a ‘bioactive’ library including 830 FDA-approved drugs, to select and prioritize agents for clinical development. The integration of this in vitro drug screen with in vivo pharmacokinetic and pharmacodynamic studies identified two FDA-approved drugs, pemetrexed and gemcitabine as therapy for this deadly disease.
Overall the screening process identified all expected drugs known to have clinical effect including vincristine, vinblastine, and etoposide (cisplatin and cyclophosphamide were not tested due to incompatibility with the high throughput screen (HTS) as well as agents not previously known to be active. Among the latter category, decitabine was a very interesting “hit” since its use in vitro resulted in a decrease of H3K27me3, an epigenetic mark characteristic of G3 and G4 MBs (Dubuc et al., 2013; Robinson et al., 2012). Unfortunately, it was unsuitable for further in vivo studies due to predicted inadequate CNS exposure in humans. Nonetheless, the in vitro efficacy of decitabine suggested that similar hypomethylating drugs with adequate brain penetration should be considered for the treatment of G3 MBs. On the other hand, pemetrexed and gemcitabine showed high anti-proliferative potency against mouse and PDX G3 MBs in vitro and in vivo. When used as single agents, each inhibited mouse and human G3 MB neurospheres proliferation. When administered in combination to mice bearing murine or PDXs G3 MB, survival time doubled compared to vehicle treated animals. In contrast, mouse SHH MBs did not respond to the combination of pemetrexed and gemcitabine suggesting that the combination of the two drugs should specifically target G3 MBs. It would be informative to know whether this combination chemotherapy has a similar efficacy in G4 MBs. However, if the response to the drugs is MYC driven, then average lower levels of MYC expression in G4 MB (Northcott et al., 2011), would suggest that it will not be an effective therapeutic approach. Furthermore the large number and heterogeneity of G4 MB, without the availability of several adequate mouse models, preclude comprehensive pre-clinical studies similar to those that cover the spectrum of G3 MB with MYC overexpression.
Previous reports suggested that the combination of gemcitabine and pemetrexed was synergistic(Adjei, 2002; Tonkinson et al., 1999) which prompted their use in the treatment of non-small cell lung cancer (NSCLC), pancreatic cancer and advanced breast cancer(Monnerat and Le Chevalier, 2006). Clinical trials in adults have shown this combination of drugs to be well tolerated for multiple cycles at doses of gemcitabine ranging between 1250–1500 mg/m2 on day 1 and 8 and of pemetrexed at 500 mg/m2 on day 8. Response rates have been promising in the range of 10–44% in NSCLC. Furthermore, a number of clinical trials have tested different delivery schedules of pemetrexed and gemcitabine, alternating the administration sequence and intervals between administration (Ma et al., 2005; Monnerat and Le Chevalier, 2006).
Both pemetrexed and gemcitabine have been used to treat pediatric cancers with each agent exhibiting an acceptable toxicity profile. A Phase I pediatric clinical trial conducted in children with refractory solid tumors evaluated a range of pemetrexed dosages from 400 to 2480 mg/m2, and identified a maximum tolerated dose (MTD) in children of 1910 mg/m2 IV given once every 21 days with folate and B 12 supplementation (Malempati et al., 2007). The results of a pediatric Phase 1 study with gemcitabine as single agent showed that the MTD was 1200 mg/m2 when given as a 30-min infusion once weekly for 3 consecutive weeks of a 4-week cycle. The MTD for a 2-week schedule has not been established; however the dose escalation concluded at 2100 mg/m2 given on day 1 and 8 without reaching a defined MTD, indicating that this regimen was well tolerated (Reid et al., 2004).
Our studies in mice bearing G3 MB clearly showed that both gemcitabine and pemetrexed exceeded their respective EC50 values in the tumor extracellular fluid and studies in mice not bearing tumor showed that both drugs penetrated into the brain. This finding is consistent with published reports (Kerr et al., 2001; Stapleton et al., 2007; Kumthekar et al., 2013) in other animal models and humans that showed modest, but clinically relevant brain penetration of both gemcitabine and pemetrexed.
Our preclinical studies suggest that both pemetrexed and gemcitabine are efficacious on mouse G3 MB when given 7 days apart, and we observed no difference in efficacy when gemcitabine preceded pemetrexed or when administered together. The plasma AUC values for pemetrexed (200 mg/kg/dose) and gemcitabine (60 mg/kg/dose) in mice were similar to that reported in humans at dosages in the 400–670 mg/m2 ranges for pemetrexed and 1250 mg/m2 for gemcitabine (Malempati et al., 2007; Reid et al., 2004). This suggested that the combination of pemetrexed and gemcitabine was clinically feasible at dosages well below the already established MTD in children.
The comparison of pemetrexed and gemcitabine in combination to the more clinically analogous combination of cisplatin and cyclophosphamide led to the unexpected finding that the integration of these four drugs produced a heightened tumor response. We assessed the effect of cisplatin/cyclophosphamide alone, pemetrexed and gemcitabine alone, or a combination of those drugs. Whereas treatment with pemetrexed and gemcitabine or cyclophosphamide and cisplatin increased the median survival of mice bearing murine G3 MB by 18 and 12 days, respectively, versus vehicle treated animals, when given as alternating cycles, median survival was increased by 25 days. Moreover, no additional toxicity was detected. When transplanted with the PDX Icb-1572, the combination of the two cycles led to an increased median survival of 23 days. In contrast, treatment of mice bearing mouse SHH MB with cycles of pemetrexed, gemcitabine and cisplatin, cyclophosphamide did not improve mouse survival compared to treatment with cisplatin and cyclophosphamide alone. These results provide stronger rationale for adding pemetrexed and gemcitabine to the current therapeutic regimen for the treatment of human G3 MBs.
Folates are essential for purine and pyrimidine synthesis and consequently rapid cellular division and proliferation of cancer cells. GSEA confirmed that both the folate and purine metabolic pathways were significantly enriched in mouse G3 MBs compared with GNPs and SHH MBs. Although the anti-folate agents methotrexate and 5-fluorouracil have been widely used for decades in the treatment of malignant tumors, pemetrexed is a relatively new therapeutic agent currently approved as a first line treatment for mesothelioma (Dowell et al., 2012), NSCLC in association with platinum-based chemotherapy (Mubarak et al., 2012), and in newly diagnosed brain metastasis (Bailon et al., 2012). It also showed clinical activity in other adult tumors such as breast, colorectal, bladder, cervical, gastric and pancreatic cancers (Adjei, 2004; Chattopadhyay et al., 2007; Warwick et al., 2013). Genes regulating pyrimidine metabolism were also enriched in mouse G3 MBs compared with mouse GNPs and SHH MBs, in agreement with the anti-proliferation effects of gemcitabine, a pyrimidine cytidine analogue.
In summary, our studies identified gemcitabine and pemetrexed, two FDA-approved drugs as efficacious in increasing survival of mice bearing PDX G3 MBs in which MYC is overexpressed. In combination with two chemotherapeutic drugs in current clinical use, cyclophosphamide and cisplatin, we found increased prolonged survival of mice bearing either mouse or human G3 MB for as long as 40 days after tumor implant compared to untreated animals. Pemetrexed and gemcitabine have both been evaluated in single agent phase 1 studies in childhood CNS and solid tumors, and are recommended in phase 2 clinical trials, although there is very limited data specific to MB. Due to the specificity of this combination therapy in G3 over SHH MBs that harbor high MYC levels and have a worse prognosis, it seems prudent to evaluate this therapy inhuman G3 MBs. G3, MYC amplification or overexpression, presence of metastatic disease and large cell/anaplastic MBs are all overlapping poor prognostic features of MB that represent a huge challenge to the clinical management of the disease since as a group these have about a 60% 5 year overall survival (OS) in comparison to an 80% 5 year OS in patients who do not harbor these characteristics. What’s more the majority of these patients currently get maximal “high risk” therapy-which includes high dose craniospinal radiation and adjuvant post-radiation chemotherapy. Current and future protocols are risk stratifying patients based on the presence or absence of these and other molecular characteristics. However without having any additional effective agents to add to high risk therapy there will be little change to these patients’ overall survival.
The data presented in this article is exciting from a clinical perspective because it suggests that pemetrexed and gemcitabine can be added to currently used chemotherapy with an enhanced effect and little additional myelosuppressive toxicity. Therefore these medications, which have already been used clinically in combination with other chemotherapy regimens in other cancers, could be incorporated into high risk MB therapy for patients with G3 MBs. We do not know whether the combination of pemetrexed and gemcitabine will be effective in G3 MBs that do not overexpress or amplify MYC. However, since approximately 17% of G3 MB amplify the MYC gene (Northcott et al 2012), and > 75% of G3 MB exhibit high MYC expression (Northcott et al., 2011), this supports our recommendation to trial this therapy in G3 MB. These agents must be introduced in stringently designed clinical trials that can strictly monitor for expected and unexpected toxicities of these agents as well as measure whether the suggested enhanced effect of this therapy translates to the human disease.
EXPERIMENTAL PROCEDURES
Development of mouse G3 and SHH MB and G3 MB PDXs
Mouse G3 MBs were generated by orthotopic transplantation of GNPs from cerebella of P7 Trp53−/−; Cdkn2c−/− mice infected with MYC encoding retroviruses (Kawauchi et al., 2012). Mouse SHH MBs spontaneously occur in Ptch1+/−; Trp53−/− animals (Wetmore et al., 2001). G3 MB PDXs were developed from primary tumor samples, from previously untreated patients, implanted into the cortex or cerebella of NSG mice. The generation of PDXs is provided in detail in Supplemental Information. In all cases, primary human brain tumor specimens were obtained under written informed consent approved by the Institutional Review Boards of SJCRH, the Necker Sick Children’s Hospital, Paris, France, the Baylor College of Medicine in Houston, Texas, and the Seattle Children’s Hospital. All animal studies were conducted according to the NIH guidelines and regulation and de-identified specimens were used to make patient-derived xenograft mice in accordance with the SJCRH, Fred Hutchinson Cancer Center and Baylor College of Medicine’s IACUC-approved protocols. The care and use of animal studies in Orsay, France were performed by strictly applying European and National Regulation in force for the Protection of Vertebrate Animals used of experimental and other scientific purposes (directive 86/609). The protocol also complied with internationally established 3R principles, in accordance with UKCCCR guidelines.
Cell culture
Mouse and human G3 MBs, mouse SHH MBs and Trp53-null GNPs were grown as neurospheres in supplemented neurobasal medium, as described previously (Kawauchi et al., 2012). HepG2 and TERT− BJ lines were purchased from ATCC© (#77400, CRL-4001) and human neural stem cells (H9) from Invitrogen.
High-Throughput Screen
The SJCRH “bioactive library consisted of 7389 compounds (6568 unique) that were screened on mouse G3 MB and Trp53-null neurospheres. The high throughput primary creen (HTS) was performed at one 10 μM concentration and the luminescent signal from the plates were read 72 hr after treatment using CellTiter-Glo©, as described previously (Atkinson et al., 2011). Dose-response experiments identified 35 FDA-approved drugs from which three, decitabine, pemetrxed and gemcitabine, were chosen for further analysis. Additional details concerning the chemical library screened, the HTS assay protocol, the hits validation and data analysis, and “wash out” experiments were reported in Supplemental Information.
Pharmacological Studies
Murine plasma and tumor extracellular fluid (tECF) samples were analyzed for pemetrexed and gemcitabine using separate validated LC-MS/MS assays. To assay gemcitabine samples, tetrahydrouridine (THU) was added during sample collection to prevent deamination of gemcitabine to dFdU by cytidinedeaminase. For pemetrexed and gemcitabine plasma pharmacokinetic (PK) studies, a serial sacrifice plasma-only PK study (single sample per mouse) was performed in tumor-bearing mice to obtain initial plasma PK parameter estimates. These estimates were used to inform a D-optimal, limited sampling model (LSM) for microdialysis experiments, minimizing blood withdrawal and maximizing information content. Using a previously published microdialysis technique (Zhuang et al., 2006), we assessed pemetrexed and gemcitabine tumor penetration separately in orthotopically implanted mouse G3 MBs. Prior to the in vivo microdialysis study, microdialysis probe recovery was assessed for each compound using an in-vitro recovery technique. More details on pemetrexed and gemcitabine LC-MS/MS bioanalytical assays, plasma pharmacokinetic studies (PK) and cerebral microdialysis studies were described in Supplemental Information.
Statistical Analysis
Statistical analyses were performed in the GraphPadPrism software version 5.0. Kaplan-Meyer (Log rank test) was used for testing significant mouse survival.
Gene expression profiling
Total RNA was extracted using Trizol, as previously published (Kawauchi et al, 2012). RNA was subjected to Affymetrix Gene Chip analysis (HT430PM, Affymetrix). Data were analyzed with Spot fire(Kawauchi et al., 2012) and for gene set enrichment analysis (GSEA) (Broad Institute). Gene expression of xenografts TB-12-5950 and Icb1572 were compared to gene expression profiles from 72 primary human medullo blastoma samples (Robinson et al., 2012). Total RNA was extracted from the snap frozen human MBs and xenograft samples using STAT-60. mRNA profiles were generated using U133 Plus 2.0 microarray (Affymetrix, Santa Clara, CA). The data was imported into Spotfire Decision Site (Palo Alto CA, USA) and for each probe set and subject z-scores were calculated by computing the mean and standard deviation across subjects within each probe set.
Immunoblotting, in vivo BrdU incorporation, caspase 3 and BrdU immunostaining, Gamma-H2AX and fluorescence in situ hybridization analysis
All procedures and reagents were described in details in Supplemental Information.
Supplementary Material
HIGHLIGHTS.
Group3 medulloblastoma (G3 MB) neurospheres were screened with FDA-approved drugs.
Pemetrexed and gemcitabine inhibited mouse and human G3 and mouse SHH MBs in vitro.
Pemetrexed and gemcitabine increased survival of mice bearing mouse and human G3 MB.
Pemetrexed and gemcitabine did not affect survival of mice bearing mouse SHH MB.
SIGNIFICANCE.
Despite the recent identification of four molecular groups of human MB, patients are currently treated with similar chemotherapies independent of classification. Patients with G3 MB are characterized by high incidence of metastasis and poor prognosis. Thus, more effective therapeutic approaches for these patients are desperately needed. The development of a mouse model of G3 MB enabled production of cultured neurospheres that provided an ideal platform to identify additional chemotherapies. We found two FDA-approved drugs that significantly inhibited mouse and human G3 MBs’ neurosphere cultures, mouse allografts and xenografts from G3 MB primary patient samples, but not mouse SHH MBs. These findings provide a strong rationale for combination therapy with pemetrexed and gemcitabine to treat patients with G3 MB.
Acknowledgments
We thank Charles J. Sherr, Richard Gilbertson and all members of the Brain Tumor Program for helpful discussions and insights, and members of the Roussel and Sherr laboratories for helpful comments. We are indebted to Dana Farmer, Shelly Wilkerson, Jose Grenet and Coralie Lefevre for excellent technical expertise, the Small Imaging Core for mouse surgery, MRI, luciferase imaging, and assistance with PK studies, the diagnostic laboratory core for blood analysis, the flow cytometry core for Annexin V and FACS analysis, Marc Valentine for FISH analysis, John Gray for lentiviruses, Brian Murphy for IP, Daisuke Kawauchi for neurospheres, Cynthia Wetmore, Michael Dyer and Richard Rahija for establishment of PDXs and Chuck Rock for the folate rescue experiment. This work was supported in part by NIH grants CA-096832 (MFR), CA-155360 & CA-114567 (JMO), Cancer Center Core Grant CA-021765 (MFR, AG), Institut National du Cancer (AVENIR INSERM team, INCaR10067LS; OA, LB), CNRS (OA, LB) and Institut Curie (OA, LB), a V foundation translational award (MFR, AG), and the American Lebanese-Syrian Associated Charities (ALSAC) of St. Jude Children’s Research Hospital (SJCRH).
Footnotes
All authors declare having no financial conflicts of interest.
ACCESSION NUMBER
The NCBI Gene Expression Omnibus accession number for the microarray results reported in this paper is GSE46406.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjei AA. Preclinical and clinical studies with combinations of pemetrexed and gemcitabine. Semin Oncol. 2002;29:30–34. doi: 10.1053/sonc.2002.37468. [DOI] [PubMed] [Google Scholar]
- Adjei AA. Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent. Clin Cancer Res. 2004;10:4276s–4280s. doi: 10.1158/1078-0432.CCR-040010. [DOI] [PubMed] [Google Scholar]
- Atkinson JM, Shelat AA, Carcaboso AM, et al. An integrated in vitro and in vivo high-throughput screen identifies treatment leads for ependymoma. Cancer Cell. 2011;20:384–399. doi: 10.1016/j.ccr.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailon O, Chouahnia K, Augier A, et al. Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma. Neuro-Oncol. 2012;14:491–495. doi: 10.1093/neuonc/nos004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot GG, Rivard GE, Momparler RL. Plasma and cerebrospinal fluid pharmacokinetics of 5-Aza-2′-deoxycytidine in rabbits and dogs. Cancer Res. 1983;43:592–597. [PubMed] [Google Scholar]
- Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–417. doi: 10.1158/1535-7163.MCT-06-0343. [DOI] [PubMed] [Google Scholar]
- Dowell JE, Dunphy FR, Taub RN, et al. A multicenter phase II study of cisplatin, pemetrexed, and bevacizumab in patients with advanced malignant mesothelioma. Lung Cancer. 2012;77:567–571. doi: 10.1016/j.lungcan.2012.05.111. [DOI] [PubMed] [Google Scholar]
- Dubuc AM, Remke M, Korshunov A, et al. Aberrant patterns of H3K4 and H3K27 histone lysine methylation occur across subgroups in medulloblastoma. Acta Neuropath. 2013;125:373–384. doi: 10.1007/s00401-012-1070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald B, Sampath D, Plunkett W. H2AX phosphorylation marks gemcitabine-induced stalled replication forks and their collapse upon S-phase checkpoint abrogation. Mol Cancer Ther. 2007;6:1239–1248. doi: 10.1158/1535-7163.MCT-06-0633. [DOI] [PubMed] [Google Scholar]
- George RE, Lahti JM, Adamson PC, et al. Phase I study of decitabine with doxorubicin and cyclophosphamide in children with neuroblastoma and other solid tumors: a Children’s Oncology Group study. Ped Blood & Cancer. 2010;55:629–638. doi: 10.1002/pbc.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi D, Robinson G, Uziel T, et al. A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell. 2012;21:168–180. doi: 10.1016/j.ccr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JZ, Berg SL, Dauser R, et al. Plasma and cerebrospinal fluid pharmacokinetics of gemcitabine after intravenous administration in non-human primates. Cancer Chemotherap Pharmacol. 2001;47:411–414. doi: 10.1007/s002800000253. [DOI] [PubMed] [Google Scholar]
- Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumthekar P, Grimm SA, Avram MJ, et al. Pharmacokinetics and efficacy of pemetrexed in patients with brain or leptomeningeal metastases. J Neuronc. 2013;112:247–255. doi: 10.1007/s11060-013-1055-0. [DOI] [PubMed] [Google Scholar]
- Ma CX, Nair S, Thomas S, et al. Randomized phase II trial of three schedules of pemetrexed and gemcitabine as front-line therapy for advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5929–5937. doi: 10.1200/JCO.2005.13.953. [DOI] [PubMed] [Google Scholar]
- Malempati S, Nicholson HS, Reid JM, et al. Phase I trial and pharmacokinetic study of pemetrexed in children with refractory solid tumors: the Children’s Oncology Group. J Clinical Oncol. 2007;25:1505–1511. doi: 10.1200/JCO.2006.09.1694. [DOI] [PubMed] [Google Scholar]
- Massimino M, Cefalo G, Riva D, et al. Long-term results of combined preradiation chemotherapy and age-tailored radiotherapy doses for childhood medulloblastoma. J Neuro-Oncol. 2012;108:163–171. doi: 10.1007/s11060-012-0822-7. [DOI] [PubMed] [Google Scholar]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nature Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- Monnerat C, Le Chevalier T. Review of the pemetrexed and gemcitabine combination in patients with advanced-stage non-small cell lung cancer. Ann Oncol. 2006;17(Suppl 5):v86–90. doi: 10.1093/annonc/mdj958. [DOI] [PubMed] [Google Scholar]
- Mubarak N, Gaafar R, Shehata S, et al. A randomized, phase 2 study comparing pemetrexed plus best supportive care versus best supportive care as maintenance therapy after first-line treatment with pemetrexed and cisplatin for advanced, non-squamous, non-small cell lung cancer. BMC Cancer. 2012;12:423. doi: 10.1186/1471-2407-12-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott PA, Korshunov A, Pfister SM, Taylor MD. The clinical implications of medulloblastoma subgroups. Nat Rev Neurol. 2012;8:340–351. doi: 10.1038/nrneurol.2012.78. [DOI] [PubMed] [Google Scholar]
- Palii SS, Van Emburgh BO, Sankpal UT, et al. DNA methylation inhibitor 5-Aza-2′-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol. 2008;28:752–771. doi: 10.1128/MCB.01799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DW, Li M, Zhang X, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331:435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh TJ, Weeraratne SD, Archer TC, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch T, Jones DT, Zapatka M, et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell. 2012;148:59–71. doi: 10.1016/j.cell.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JM, Qu W, Safgren SL, et al. Phase I trial and pharmacokinetics of gemcitabine in children with advanced solid tumors. J Clin Oncol. 2004;22:2445–2451. doi: 10.1200/JCO.2004.10.142. [DOI] [PubMed] [Google Scholar]
- Robinson G, Parker M, Kranenburg TA, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchetti M, Simeoni M, Pesenti E, et al. Predicting the active doses in humans from animal studies: a novel approach in oncology. Eur J Cancer. 2007;43:1862–1868. doi: 10.1016/j.ejca.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Saggar JK, Fung AS, Patel KJ, Tannock IF. Use of molecular biomarkers to quantify the spatial distribution of effects of anticancer drugs in solid tumors. Mol Cancer Ther. 2013;12:542–552. doi: 10.1158/1535-7163.MCT-12-0967. [DOI] [PubMed] [Google Scholar]
- Shu Q, Wong KK, Su JM, et al. Direct orthotopic transplantation of fresh surgical specimen preserves CD133+ tumor cells in clinically relevant mouse models of medulloblastoma and glioma. Stem Cells. 2008;26:1414–1424. doi: 10.1634/stemcells.2007-1009. [DOI] [PubMed] [Google Scholar]
- Stapleton SL, Reid JM, Thompson PA, et al. Plasma and cerebrospinal fluid pharmacokinetics of pemetrexed after intravenous administration in non-human primates. Cancer Chemotherap Pharmacol. 2007;59:461–466. doi: 10.1007/s00280-006-0285-7. [DOI] [PubMed] [Google Scholar]
- Tonkinson JL, Worzalla JF, Teng CH, Mendelsohn LG. Cell cycle modulation by a multitargeted antifolate, LY231514, increases the cytotoxicity and antitumor activity of gemcitabine in HT29 colon carcinoma. Cancer Res. 1999;59:3671–3676. [PubMed] [Google Scholar]
- Uziel T, Zindy F, Xie S, et al. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes &Dev. 2005;19:2656–2667. doi: 10.1101/gad.1368605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Moorsel CJ, Bergman AM, Veerman G, et al. Differential effects of gemcitabine on ribonucleotide pools of twenty-one solid tumour and leukaemia cell lines. Biochim Biophys Acta. 2000;1474:5–12. doi: 10.1016/s0304-4165(99)00209-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Li M, Rinehart JJ, Zhang R. Pretreatment with dexamethasone increases antitumor activity of carboplatin and gemcitabine in mice bearing human cancer xenografts: in vivo activity, pharmacokinetics, and clinical implications for cancer chemotherapy. Clin Cancer Res. 2004;10:1633–1644. doi: 10.1158/1078-0432.ccr-0829-3. [DOI] [PubMed] [Google Scholar]
- Warwick AB, Malempati S, Krailo, et al. Phase 2 trial of pemetrexed in children and adolescents with refractory solid tumors: a Children’s Oncology Group study. Ped Blood &Cancer. 2013;60:237–241. doi: 10.1002/pbc.24244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61:513–516. [PubMed] [Google Scholar]
- White L, Sterling-Levis K. Multiagent chemotherapy studied in a xenograft model of medulloblastoma/primitive neuroectodermal tumour: analysis of the VETOPEC regimen. J Clin Neurosci. 2008;15:49–54. doi: 10.1016/j.jocn.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Woodland JM, Barnett CJ, Dorman DE, et al. Metabolism and disposition of the antifolate LY231514 in mice and dogs. Drug Metabol & Dispo. 1997;25:693–700. [PubMed] [Google Scholar]
- Zhao X, Liu Z, Yu L, et al. Global gene expression profiling confirms the molecular fidelity of primary tumor-based orthotopic xenograft mouse models of medulloblastoma. NeuroOncol. 2012;14:574–583. doi: 10.1093/neuonc/nos061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Fraga CH, Hubbard KE, et al. Topotecan central nervous system penetration is altered by a tyrosine kinase inhibitor. Cancer Res. 2006;66:11305–11313. doi: 10.1158/0008-5472.CAN-06-0929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.