Abstract
Background: Systemic lupus erythematosus (SLE) is an inflammatory disease caused by autoimmune dysregulation, which mainly affects young women, usually free from atherosclerosis. Accelerated atherosclerosis is a well established complication of SLE and it cannot be explained by Framingham risk factors alone, and has been attributed to complex interactions between traditional risk factors and factors associated with the disease per se, or its treatment. Arterial stiffness and endothelium function may serve as a valuable measure to be counted in the follow-up of these patients prior to a potential cardiovascular event. The aim of the study was to evaluate atherosclerosis, inflammatory process, immune mediated, using imaging techniques and to identify the role of molecules known to be involved in inflammation, hsCRP, homocysteine, IL-6, ESR and fibrinogen, in the development and perpetuation of atherosclerosis in patients with systemic lupus erythematosus. Methods Our prospective study included 53 patients diagnosed with systemic lupus erythematosus and fulfilled the revised ACR (American College of Rheumatology) criteria for the classification of SLE. Exclusion criteria were <18 years of age, history of CVD, infections, diabetes mellitus, dyslipidemia. Results: We enrolled 53 patients with SLE, 50 (94%) women and 3 (6%) men, with a mean age of 31,92 years (SD 5,55; limits 22-44) with no significant difference between sex (31,65±3,4 years in women and 37,33±4,05 years in men). The measurement of inflammation markers revealed increased values for all the variables: ESR had a mean value of 69,19± 14,18mm, fibrinogen 445,66 ±4,56mg%; IL-6 had a mean value of 11,209 ±1,56pg/ml; homocysteine 17,721±2,5374 µmol/l and for hs CRP the mean value was 3,493±1,12 mg/l. The assesement of arterial stiffness showed a mean value of 23,32% (SD 5,82; 95%CI 21,716 - 24,925) for AIx and 9,1m/s (SD 0,49; 95%CI 8,971 - 9,244) for cfPWV. There was a positive, significant correlation between AIx and hsCRP (r=0,612; 95%CI 0,4104 - 0,7576; p<0,001), (r=0,526; 95% CI 0,2979 to 0,6971; p=0,0001), for AIx and homocysteine (r=0,526; 95%CI 0,2979 to 0,6971; p=0,0001). The correlation coefficient with AIx was similar for ESR and fibrinogen (r=0,63 and 0,60). IL-6 and AIx correlated correlated positively, (r=0,369; 95%CI 0,1097 - 0,5813), statistically significant (p=0,006), but the correlation was not powerful. hsCRP and cfPWV were related (r=0,652; 95%CI 0,4677-0,7862; p<0,001); cfPWV also correlated with IL-6 (r=0,6552; 95%CI 0,4677- 0,7862; p<0,0001), homocysteine (r=0,9174; 95%CI 0,8606- 0,9517; p<0,0001), ESR (r=0,74) and fibrinogen (r=0,64). Conclusions: In summary, our data suggest that arterial stiffness is related to the level of systemic inflammation, and that inflammation is involved in the early alteration of arterial wall. Increase in arterial stiffness can be detected by applanation tonometry, and may serve as an important predictor of future cardiovascular events, since an early diagnosis may have a significant value in preventing the development of major vascular disease.
Keywords: systemic lupus erythematosus, inflammation markers, augmentation index, pulse wave velocity
Background
Systemic lupus erythematosus (SLE) is a complex multisystem inflammatory disease caused by autoimmune dysregulation, which mainly affects young women, usually free from atherosclerosis.
Accelerated atherosclerosis is a well established complication of SLE. In individuals with SLE the prevalence of cardiovascular disease (CVD) ranges from 6% to 10%, and the risk of developing CVD is 4–8 times higher than in normal population [1],[2][3],[4]. Moreover, acute myocardial infarction is reported as a cause of death in 3–25% of SLE patients in different surveys [5],[6],[7].
The high prevalence of atherosclerosis in SLE, however, cannot be explained by Framingham risk factors alone, and has been attributed to complex interactions between traditional risk factors and factors associated with the disease per se, or its treatment [8],[9],[10].
Arterial stiffness and endothelium function may serve as a valuable measure to be counted in the follow-up of these patients, prior to a potential cardiovascular event [11]. Two major estimates of vascular function, in particular augmentation index (AIx), indicating systemic arterial stiffness, and carotid-femoral pulse wave velocity (PWV), the parameter of regional arterial stiffness, have intensively been explored during last decade in different ways in rheumatic patients [12],[13].
Study objective: The aim of the study was to evaluate atherosclerosis, inflammatory process, immune mediated, using imaging techniques and to identify the role of molecules known to be involved in inflammation, hsCRP, homocysteine, IL-6, ESR and fibrinogen, in the development and perpetuation of atherosclerosis in patients with systemic lupus erythematosus.
Methods
Our prospective study included 53 patients diagnosed with systemic lupus erythematosus between december 2007 until may 2009, in Rheumatology Department, Emergency County Hospital Craiova. At study enrolment, all patients signed the informed consent and fulfilled the revised ACR (American College of Rheumatology) criteria for the classification of SLE [14].
Exclusion criteria were <18 years of age, history of CVD, infections, diabetes mellitus, dyslipidemia. Routine hematology and chemistry (fasting blood glucose and lipid profile), Westergren ESR, high sensitive C-reactive protein-hsCRP, fibrinogen, C3, C4, CH50, lupus anticoagulant, and IgG antibodies to dsDNA and IgG/ IgM anticardiolipin antibodies, chest X-ray and electrocardiography were done. In order to evaluate the inflammatory status we also determined homocysteine and IL-6.
Arterial stiffness, was assessed measuring carotid to femoral pulse wave velocity (cfPWV) and augmentation index (AIx). Carotid and femoral pulse waves were obtained non-invasively by applanation tonometry (SphygmoCor device, AtCor Medical, Sydney, Australia). Pulse waves recorded consecutively from the carotid and femoral arteries were referenced to a simultaneously recorded ECG, and transit time was computed from the time difference between the carotid and femoral waveforms. The distance between the carotid and femoral arteries was measured, and PWV was calculated, as the ratio between distance and time. AIx was calculated from carotid pulse wave, using the integrated software. The systolic part of the central arterial waveform is characterized by two pressure peaks. The first peak is caused by left ventricular ejection, whereas the second peak is a result of pulse wave reflection. The difference between both pressure peaks reflects the degree to which central arterial pressure is augmented by wave reflection. AIx, a measure of systemic arterial stiffness, is calculated as the difference between the second and the first systolic peak expressed as a percentage of pulse pressure. For carotid-femoral PWV, a threshold more than 12 m/s has been related to higher risk of coronary artery disease in hypertensive patients. So-called normal values of AIx are assessed individually for particular patient adjusting for age, blood pressure, height, weight and gender and the individual values of AIx can variate from around −20% in young athletes to about 40% in elderly hypertensive patients [11].
Results
We enrolled 53 patients with SLE, 50 (94%) women and 3 (6%) men, with a mean age of 31,92 years (SD 5,55; limits 22-44) with no significant difference between sex (31,65±3,4 years in women and 37,33±4,05 years in men).
The measurement of inflammation markers revealed increased values for all the variables: ESR had a mean value of 69,19 mm (DS=14,18; CI95% 65,279 - 73,098), fibrinogen 445,66 mg% (DS 74,56; 95%CI 425,108-466,213mg%); for hs CRP the mean value was 3,493 mg/l, (SD 1,12; 95%CI 3,181-3,804), 31 de patients (58%) had values that show an increased cardiovascular risk (>3mg/l). Homocysteine had a mean value of 17,721 µmol/l (SD 2,5374; 95%CI 17,021 - 18,420), most of the patients (51) having values over the limits. IL-6 had a mean value of 11,209 pg/ml (SD 1,56; 95% CI 10,778-11,640).
The assesement of arterial stiffness showed a mean value of 23,32% ( SD 5,82; 95%CI 21,716 - 24,925) for AIx and 9,1m/s (SD 0,49; 95%CI 8,971 - 9,244) for cfPWV.
Correlations between inflammation markers and arterial stiffness
The measurement of hsCRP, identified 31 patients with values higher than 3mg/l, indicating a high cardiovascular risk. In these patients the mean AIx was 26,06% (SD 5,57; 95%CI 24,022-28,107). For 41% (22 patients), hsCRP was between 1-3mg/l, values that show a medium cardiovascular risk, with a mean AIx of 19,45% (SD 3,58%; 95%CI 17,866 - 21,043). The difference between the two groups was statistically significant (p<0,001). There was a positive, significant correlation between AIx and hsCRP (r=0,612; CI 95% 0,4104 - 0,7576; p<0,001), linear regresing showing the same result (r2=0,375; F ratio=30,65; p<0,001) (Fig.1).
Fig.1.
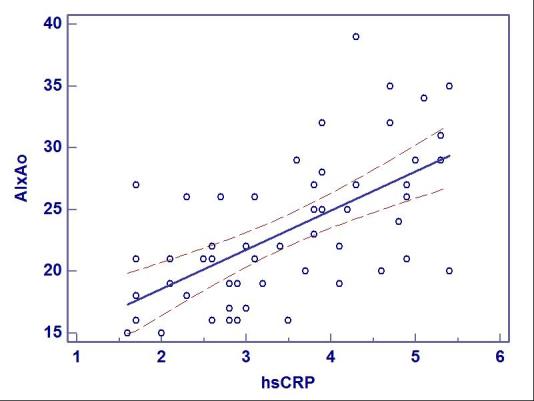
Linear regression and correlation coefficient for AIx and hsCRP
However, the correlation between AIx and hsCRP was different regarding the cardiovascular risk: there was a significant positive correlation in patients with hsCRP>3mg/l (r=0,41; 95%CI 0,0654-0,0667) and a inverse correlation in patients with values between 1 and 3mg/l (r=-0,017; 95%CI 0,435-0,407; p=0,94).
AIx was higher (24,06%; SD 5,85%; 95%CI 22,289-25,847) in patients with values of IL-6 over the limits (>9,7pg/ml), compared with those with normal values (19,6%; SD 4,24; 95%CI 16,405 - 22,928 ) (p=0,037). IL-6 and AIx correlated positively, (r=0,369; 95%CI 0,1097 - 0,5813), statistically significant (p=0,006), but the correlation was not powerful. Similar results were established using linear regression (r2=0,136; F ratio=8,04; p=0,007). Homocysteine significantly correlated with AIx (r=0,526; 95%CI 0,2979- 0,6971) (p=0,0001). The correlation coefficient with AIx was similar for ESR and fibrinogen (r=0,63 and 0,60).
Using multiple regression we tried to establish the dependence between AIx and inflammatory markers: the multiple correlation coefficient was 0,6281 using Enter method and 0,6127 using Stepwise method (Table 1).
Table 1.
Multiple regression for AIx and inflammation markers
| Method | Enter | Stepwise |
| Coefficient of determination R2 | 0,3946 | 0,3754 |
| R2-adjusted | 0,3575 | 0,3632 |
| Multiple correlation coefficient | 0,6281 | 0,6127 |
| Residual standard deviation | 4,6654 | 4,6446 |
| F-Ratio | 10,6441 | 30,6563 |
| Significance level | p<0,001 | p<0,001 |
cfPWV was 9,342 m/s (DS 0,364; 95%CI 9,208 – 9,475) in patients with hsCRP over 3mg/l, significantly higher than the ones with values between 1 and 3mg/l (8,777m/s; DS 0,471; 95% CI 8,568-8,986) (p <0,001). hsCRP and cfPWV were related (r=0,652; CI 95% 0,4677-0,7862; p<0,001), similar results being assessed using linear regression (r2=0,4293; F-ratio=38,3572; p<0,001) (Fig.1). cfPWV also correlated with IL-6 (r=0,6552; 95%CI 0,4677- 0,7862; p<0,0001), homocysteine (r=0,9174; 95%CI 0,8606- 0,9517; p<0,0001), ESR (r=0,74) and fibrinogen (r=0,64). The multiple correlation coefficient between cfPWV and inflammatory markers was 0,91 using both methods (Enter and Stepwise) (Table 2).
Table 2.
2 Multiple regression for cfPWV and inflammation markers
| Method | Enter | Stepwise |
| Coefficient of determination R2 | 0,8443 | 0,8417 |
| R2-adjusted | 0,8277 | 0,8386 |
| Multiple correlation coefficient | 0,9188 | 0,9174 |
| Residual standard deviation | 0,2054 | 0,1988 |
| F-Ratio | 50,9551 | 271,1915 |
| Significance level | p<0,001 | p<0,001 |
Discussion
Our study confirmed the inflammatory status of SLE patients and showed that the inflammatory response is an important factor contributing to atherosclerotic damage. The main findings from the present study are that vascular injury is an early event in patients with SLE, showing an organ damage, and that the level of systemic inflammation is an important mediator of this process, increasing the arterial stiffness. AIx, correlated with inflammation mediators, the strongest correlation being assessed for hsCRP. cfPWV, considered as the “gold-standard” measurement of arterial stiffness [11], positively correlated with all inflammation markers, and the multiple correlation coefficient showed a strong dependence of the two variables (r=0,91).
Our data are in harmony with the results from the studies focusing on SLE and arterial wall functioning: Brodszki, et al., Roman, et al., Selzer, et al. [15],[16],[17]. These studies demonstrated an increased arterial stiffness and signs of premature vascular ageing in SLE patients without manifest cardiovascular disease and without significant atherosclerotic lesions. The authors conclude that other mechanisms beside atherosclerosis might be involved in the pathogenesis of arterial stiffening in SLE patients. The association of arterial stiffening with circulating levels of C-reactive protein and IL-6, the citokine that stimulates its synthesis, implicates chronic inflammation as important mediator of this process [15]. Serum levels of CRP predict cardiovascular risk [18],[19], and inflammation is believed to be a key process in atheroma formation. CRP is deposited in the arterial intima in early atherosclerotic lesions [20], induces an inflammatory and atherogenic phenotype in endothelial cells, interferes with endothelial progenitor cell survival and function, and stimulates cell migration and proliferation [21].
SLE itself remains an independent risk factor for early atherosclerosis, suggesting a link between chronic inflammation and accelerated vascular damage [22].
Given the cardiovascular risk associated with SLE and other connective tissue diseases, it would appear to be fundamental to identify potentially modifiable risk factors and establish evidence based guidelines to help clinicians in planning strategies able to reduce long-term cardiovascular morbidity and, consequently, mortality.
Conclusions
In summary, our data suggest that arterial stiffness is related to the level of systemic inflammation, and that inflammation plays a role in the process of arterial stiffening.
Increase in arterial stiffness can be detected early, by applanation tonometry, and may serve as an important predictor of future cardiovascular events, directly linked to acceleration of atherosclerotic arterial wall damage. The assessment of augmentation index, the parameter of systemic arterial stiffness, and carotid-femoral pulse wave velocity, the parameter of regional muscular vessel flexibility, may have a beneficial diagnostic, prognostic and therapeutic relevance and may serve for designing algorithms of diagnosis and treatment.
References
- 1.Ippolito A., Petri M. An update on mortality in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2008;26(Suppl. 51):S72–S79. [PubMed] [Google Scholar]
- 2.Urowitz M.B., Gladman D.D., Tom B.D., Ibanez D., Farewell V.T. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J. Rheumatol. 2008;35:2152–2158. doi: 10.3899/jrheum.080214. [DOI] [PubMed] [Google Scholar]
- 3.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr, Jansen-McWilliams L, D’Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 4.Symmons D.P.M., Gabriel S.E. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nature Reviews Rheumatology. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 5.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejia JC, Aydintug AO, Chwalinska-Sadowska H, de Ramon E, Fernandez-Nebro A, Galeazzi M, Valen M, Mathieu A, Houssiau F, Caro N, Alba P, Ramos-Casals M, Ingelmo M, Hughes GR. European Working Party on Systemic Lupus Erythematosus. Morbidity and mortality in systemic lupus erythematosus during a 5 year period: a multicenter prospective study of 1000 patients. Medicine. 1999;78:167–175. doi: 10.1097/00005792-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Jonsson H, Nived O, Surfelt G. Outcome in systemic lupus erythematosus: a prospective study of patients from a defined population. Medicine. 68:141–150. [PubMed] [Google Scholar]
- 7.Hak A.E., Karlson E.W., Feskanich D., Stampfer M.J., Costenbader K. H. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheum. 2009;61:1396–1402. doi: 10.1002/art.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoenfeld Y, Gerli R, Doria A, Matsuura E, Matucci Cerinic M, Ronda Nicoletta, Jara L.J., Abu-Shakra M, Pier Luigi Meroni PL, Sherer Y. Accelerated Atherosclerosis in Autoimmune Rheumatic Diseases. Circulation. 2005;112:3337–3347. doi: 10.1161/CIRCULATIONAHA.104.507996. [DOI] [PubMed] [Google Scholar]
- 9.Urowitz M. B., et al. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care Res. (Hoboken) 2010;62:881–887. doi: 10.1002/acr.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VonFeldt J. M. Premature atherosclerotic cardiovascular disease and systemic lupus erythematosus from bedside to bench. Bull. NYU Hosp. Jt Dis. 2008;66:184–187. [PubMed] [Google Scholar]
- 11.Laurent S, Cockcroft J, VanBortel L, Boutouyrie1 P, Giannattasio Cristina, Hayoz D, Pannier B, Vlachopoulo C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. European Heart Journal. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 12.Bjarnegård N, Bengtsson C, Brodszki J, Sturfelt G, Nived O, Länne T. Increased aortic pulse wave velocity in middle aged women with systemic lupus erythematosus. Lupus. 2006;15:644–650. doi: 10.1177/0961203306071402. [DOI] [PubMed] [Google Scholar]
- 13.Piper MK, Raza K, Nuttall SL, et al. Impaired endothelial function insystemic lupus erythematosus. Lupus. 2007;16:84–88. doi: 10.1177/0961203306074842. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725–undefined. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 15.Roman MJ, Devereux RB, Schwartz JE, et al. Arterial stiffness in chronic inflammatory diseases. Hypertension. 2005;46:194–199. doi: 10.1161/01.HYP.0000168055.89955.db. [DOI] [PubMed] [Google Scholar]
- 16.Brodszki J, Bengtsson C, Länne T, Nived O, Sturfelt G, Marsál K. Abnormal mechanical properties of larger arteries in postmenopausal women with systemic lupus erythematosus. Lupus. 2004;13:917–923. doi: 10.1191/0961203304lu2033oa. [DOI] [PubMed] [Google Scholar]
- 17.Selzer F, Sutton-Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension. 2001;37:1075–1082. doi: 10.1161/01.hyp.37.4.1075. [DOI] [PubMed] [Google Scholar]
- 18.Williams RC Jr, Harmon ME, Burlingame R, Du Clos TW. Studies of serum C-reactive protein in systemic lupus erythematosus. J Rheumatol. 2005;32:454–461. [PubMed] [Google Scholar]
- 19.Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–1221. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 20.Torzewski M, et al. C-reactive protein in the arterial intima. Role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2094–2099. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 21.Verma S, et al. C-reactive protein attenuates endithelial progenitor cell survival, differentiation, and function. Further evidence of a mechanistic link between C-recative protein and cardiovascular disese. Circulation. 2004;109:2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 22.Zardi E.M., Afeltra Antonella. Endothelial dysfunction and vascular stiffness in systemic lupus erythematosus: Are they early markers of subclinical atherosclerosis? Autoimmunity Reviwes. 2010:684–686. doi: 10.1016/j.autrev.2010.05.018. [DOI] [PubMed] [Google Scholar]


