Abstract
Adenoviral vectors have shown great promise as vaccine carriers and in gene transfer to correct underlying genetic diseases. Traditionally, construction of adenoviral vectors is complex and time consuming. In this paper, we provide an improved method for efficient generation of novel adenoviral vectors by using direct cloning. We introduce a feasible and detailed protocol for the development of chimpanzee adenoviruses (Ads) as molecular clones, as well as for the generation of recombinant virus from the molecular clones. Recombinant viruses are genetically stable and induce potent immune responses in animals. Generation of new Ad molecular clones or new recombinant Ad can be achieved in 2 months or 2 weeks, respectively.
INTRODUCTION
Vectors based on Ads are commonly used for gene transfer because of their broad tropism, high transduction efficiency and relatively low risk in safety. Until now, most vectors are based on human serotype 5 (AdHu5). This virus is endemic in most human populations, and neutralizing antibodies specific to AdHu5 can be detected in up to 40–60% of humans1. Pre-existing neutralizing antibodies dampen gene transfer efficacy and increase vector-mediated toxicity2. Vectors based on rare human Ad serotypes and Ad from other species are being explored to overcome the impact of pre-existing immunity1–6.
Three major methods have traditionally been used to generate recombinant Ad vectors. The most commonly used method is based on homologous recombination in a packaging cell line. In this method, the gene of interest is cloned into a shuttle vector and concomitantly transfected with the Ad genome into HEK 293 cells or other cells that provide E1 in trans. Recombination between the shuttle vector and the Ad genome replaces the E1 domain with the gene of interest. However, recombination is relatively rare and the isolation of recombinant Ad vector using this method is laborious, time consuming and requires multiple rounds of plaque purification7. The second method is based on homologous recombination in Escherichia coli. The commercially available pAdEasy system is based on this method. It is efficient but more complex and requires a three-step transformation, including the use of two different E. coli strains8,9. The third method is based on direct cloning of the Ad genome into a plasmid vector. The genome can then be manipulated in vitro and, following transfection into a packaging cell line, the virus can be rescued. This method is technically challenging because the Ad genome is large (around 36 kb) and contains few useful restriction sites that allow its assembly into a full-length molecular clone8. However, this method is highly desirable, because it is straightforward and it completely eliminates potentially contaminating infectious material from the original Ad genome10 that would not be eliminated by homologous recombination and thus affect the usefulness of Ad vectors for clinical development.
In this paper, we describe a simple strategy that allows the efficient development of Ad molecular clones by direct cloning. Briefly, we took advantage of suitable unique restriction sites within a portion of the genome rather than within the whole genome. Such sites are present in all Ad genomes that we have vectored thus far. Thereafter, we assembled the genome part by part into one plasmid. To clone the foreign gene of interest into the Ad molecular clone, a shuttle vector was used containing two very rare restriction sites, I-CeuI and PI-SceI. The same sites were placed into the molecular clone to allow the insertion of the trans-gene’s expression cassette into the deleted E1 domain. Virus can be rescued by transfection of packaging cell lines with the linearized recombinant molecular clone.
Overview of the protocol
This protocol describes the development of an E1- or E1/E3-deleted Ad molecular clone and the cloning of the gene of interest into the E1/E3-deleted Ad genome, using chimpanzee-derived Ad serotype 6 (AdC6) as an example. The Ad genome contains four segments that encode early gene products (E1–E4), and five segments that encode five late gene products (L1–L5). The E1, E2 and E4 gene products regulate transcription and translation of late genes and are necessary for viral replication. E3 gene products subvert immune responses by altering antigen presentation and cytokine and apoptosis pathways, but are unnecessary for viral replication11. Deletion of the E3 in addition to the E1 domain increases the permitted size of the inserted expression cassette to ~7.5 kb.
To generate the E1-deleted AdC6 molecular clone, the 5′ right-inverted terminal repeat (ITR) was amplified by PCR and cloned into the pNEB193 vector. Using restriction enzyme sites that are unique in assembly but not necessarily unique to the full AdC6 genome, the right half of the AdC6 genome was then cloned piecemeal into the pNEB193 vector. The left ITR was amplified by PCR and cloned into a different pNEB193 vector. Using the same strategy as above, the remainder of the left fragment of the AdC6 genome was assembled into the pNEB193 vector. Approximately 2.6 kb of the E1 region between SnaBI and NdeI sites was omitted and replaced with a linker that contains the rare enzyme sites of I-CeuI and PI-SceI. This step removes the entire E1a and E1b 19-kDa homolog-coding regions and 74% of the E1b 55-kDa homolog-coding region. Finally, using two suitable enzymes, the E1-deleted left fragment of the Ad genome was released from the pNEB193 vector and inserted into the vector containing the right fragment of the genome, effectively generating the E1-deleted infectious molecular clone of AdC6.
To delete the E3 domain, a fragment including this domain was cut from the E1-deleted molecular clone. The ends of the molecular clone were recombined by ligation. The ~4 kb E3 region was removed from the fragment using suitable restriction enzymes, i.e., Eco47III and SwaI. The resulting fragment was then cloned back into the E1-deleted molecular clone to generate the E1/E3-deleted Ad molecular clone.
To generate a recombinant molecular clone, first, the gene of interest was cloned into the commercially available pShuttle vector under the control of CMV promoter; other promoters can also be used (see Box 1). The expression cassette was released by digestion with I-CeuI and PI-SceI, and then cloned into the Ad molecular clone that had been cut with the same enzymes. Recombinant Ad vector was generated by rescue in packaging cells such as HEK 293 cells (see Box 2).
Box 1. ALTERNATIVE REGULAToRY ELEMENTS.
Adenovirus vectors produced as described in this protocol use the cytomegalovirus promoter to regulate transgene product expression. Alternative promoters can be used on modification of the existing pShuttle vector or on construction of a new shuttle vector. Transgene product toxicity can prevent successful viral rescue and expansion. This can be overcome by insertion of inducible silencing elements such as tetracycline operators into the expression cassette36. Others have explored the use of multicistronic cassettes37.
Box 2. PACKAGING CELL LINES.
Chimpanzee-origin adenovirus (Ad) vectors can readily be grown in packaging cell lines that provide E1 of AdHu5 in trans. HEK 293 cells are freely available to academic investigators. Some of the E1-deleted AdHu5 genomes may recombine with the E1 gene of the packaging cell line, resulting in a replication-competent Ad virus. The E1 flanking regions of chimpanzee-origin Ad viruses show limited homology with those of AdHu5, making recombination in HEK 293 cells highly unlikely. Other E1 transcomplementing packaging cell lines have been described such as PERC6 cells38, which are not readily available to academia. Some E1-deleted Ad serotypes cannot be rescued on cell lines that provide E1 of AdHu5 virus in trans. Examples are the chimpanzee-origin AdC1 vector or human serotypes AdHu26 or AdHu35 (refs. 25,39). For such vectors, the open reading frame 6 of E4 has to be replaced with that of AdHu5 virus. Again, such replacement can be made in the viral molecular clone using cloning techniques similar to those described above.
This protocol is direct, efficient and reproducible. Provided the sequence of the Ad genome is known, molecular clone generation from a purified Ad averages 2 months and a recombinant Ad vector can be efficiently generated and purified to high titers within an additional 2 weeks. Ads can be cloned as vectors for vaccine development or gene transfer with the use of this protocol. Data obtained from a molecular clone of chimpanzee-derived Ad serotype 7 (AdC7) are also shown.
Results obtained previously using this protocol
Molecular clones from a number of chimpanzee and human Ads have been derived through the use of this protocol. The resulting Ad vectors have undergone extensive testing in vitro. Vectors were genetically stable and efficient expression of the transgene product was maintained through a minimum of 15 passages. Testing in experimental animals showed that vectors induced potent immune responses to a variety of antigens, including those derived from rabies virus1, 12–14, influenza virus15, human papilloma virus16,17 and human and simian immunodeficiency viruses18–28.
MATERIALS
REAGENTS
EcoRI (New England Biolabs, cat. no. R0101S)
KpnI (New England Biolabs, cat. no. R0142S)
PmeI (New England Biolabs, cat. no. R0560S)
PacI (New England Biolabs, cat. no. R0547S)
XbaI (New England Biolabs, cat. no. R0145S)
SnaBI (New England Biolabs, cat. no. R0130S)
NdeI (New England Biolabs, cat. no. R0111S)
SphI (New England Biolabs, cat. no. R0182S)
HindIII (New England Biolabs, cat. no. R0104S)
AgeI (New England Biolabs, cat. no. R0552S)
MluI (New England Biolabs, cat. no. R0198S)
SbfI (New England Biolabs, cat. no. R0642S)
BstAPI (New England Biolabs, cat. no. V0269S)
SwaI (New England Biolabs, cat. no. R0604S)
I-CeuI (New England Biolabs, cat. no. R0699S)
PI-SceI (New England Biolabs, cat. no. R0696S)
SgfI (Promega, cat. no. R5104)
Eco47III (Promega, cat. no. R6731)
Phusion hot start high-fidelity DNA polymerase (England Biolabs, cat. no. F-540S)
dNTP mix (10 mM each; Invitrogen, cat. no. R72501)
Alkaline phosphatase (AP; Roche, cat. no. 13826120)
T4 DNA ligase (Roche, cat. no. 13827621)
DNA ladders (Invitrogen, cat. nos. 10381-010 and 15628-019)
Agarose (low melting point; Sigma-Aldrich, cat. no. A9414-25G)
UltraPure agarose (Invitrogen, cat. no. 15510-027)
l-broth (LB) capsules (MP Biomedicals, cat. no. 3001-031)
Ampicillin (Mediatech, cat. no. 61-238-RH)
Kanamycin (Roche, cat. no. 106801)
Pronase (Boehringer Mannheim, cat. no. 165921)
Proteinase K (Boehringer Mannheim, cat. no. 745723)
Complete protease inhibitor cocktail tablets (Roche, cat. no. 04693124001)
SDS solution (10% (wt/vol); Ambion, cat. no. 9822)
Tris-HCl (1 M, pH 8.0; Sigma-Aldrich, cat. no. T-2694)
Plasmid DNA mini and midi preparation kit (Qiagen)
pShuttle (Clontech, cat. no. K1650-1)
pNEB193 (New England Biolabs, cat. no. N3051S)
Lipofectamine 2000 transfection reagent (Invitrogen, cat. no. 11668-027)
DMEM (Mediatech, cat. no. 10-017-CV)
Leibovitz’s L-15 medium (L-15; Mediatech, cat. no. 10-045-CV)
FBS (PAA Laboratories, cat. no. A11-034)
Penicillin-streptomycin (Mediatech, cat. no. 30-002-CI)
Trypsin-EDTA (Mediatech, cat. no. 25-053-CI)
Primers and oligo linker (Integrated DNA Technologies)
Codon-optimized HIV gag (GenScript)
DNeasy extraction kit (Qiagen)
Distilled water
Mice
BALB/c mice (ACE Animals)
Cells
MAX efficiency Stbl2 competent cells (Invitrogen, cat. no. 10268-019)
HEK 293 (ATCC, cat. no. CCL-243)
Virus
AdC6 (Simian Ad23/Pan6; ATCC, cat. no. VR-592)
AdC7 (Simian Ad24/Pan7; ATCC, cat no. VR-593)
Monoclonal antibody
HIV-1 gag (183-H12-5C; NIH, cat. no. 1513)
β-Actin (Santa Cruz Biotechnology, cat. no. SC-47778)
Buffers
Potassium chloride (for KCM buffer; see REAGENT SETUP)
Calcium chloride (for KCM buffer; see REAGENT SETUP)
Magnesium chloride (for KCM buffer; see REAGENT SETUP)
Dulbecco’s phosphate-buffered saline (DPBS; Mediatech, cat. no. 21-031-CM)
TAE buffer (Mediatech, cat. no. 46-010-CM)
RIPA buffer (Pierce, cat. no. 89900)
EQUIPMENT
Six-well culture plates
T25 tissue culture flasks
T175 tissue culture flasks
Centrifuges (Eppendorf, cat. nos 5415D and 5810R)
Ultracentrifuge (Beckman Coulter)
SW 32.1 Ti ultracentrifuge rotor (Beckman Coulter)
Ultraclear centrifuge tubes (Beckman, cat. no. 344061)
Thermal cycler (Eppendorf Mastercycler gradient, Eppendorf, cat. no. 5331)
Water bath
Agarose gel chamber
Power supply (Bio-Rad)
Spectrophotometer
Bacteria incubator (37 °C)
Humidified tissue culture incubator (37 °C, 5% CO2)
Orbital shaker (37 °C)
Gel imaging system (Gel logic 200, Kodak)
Microscope
Freezer (−80 °C)
LSRII (Beckman Coulter)
Flowjo software 7.1.1 (Treestar)
REAGENT SETUP
Propagate and purify wild-type Ad
The wild-type AdC6 was purchased from ATCC, propagated in HEK 293 cells and purified by CsCl gradient centrifugation as described previously29. The number of virus particles (vp) per milliliter was determined by measuring UV absorbance at 260 nm (A260) using a spectrophotometer and was calculated using the following equation30:
KCM buffer (5×)
Combine 0.5 M KCl, 0.15 M CaCl2 and 0.25 M MgCl2; buffer can be stored at room temperature (24 °C) for several months.
LB Broth
Mix 25 l-broth capsules with 1 liter of distilled water, and then autoclave.
PROCEDURE
Isolation of genomic DNA from wild-type Ad  TIMING ~6 h
TIMING ~6 h
1∣ To 100 μl of purified virus (1 × 1013 vp ml−1), add 15 μl of 10% SDS, 15 μl of 100 mg ml−1 pronase, and 3 μl of 20 mg ml−1 proteinase K; then add sterile water to reach a final volume of 150 μl. Incubate for 3 h at 37 °C.
2∣ Use a commercial kit (e.g., DNeasy) to extract genomic DNA of wild-type Ad according to the manufacturer’s instructions.
Construction of E1-deleted Adc6 molecular clone  TIMING 5 weeks
TIMING 5 weeks
3∣ Cloning EK (EcoRI to KpnI) fragment of AdC6 into pNEB193 plasmid: digest 0.5 μg of pNEB193 plasmid DNA with KpnI and EcoRI for 2 h at 37 °C. Conduct digestion in a total volume of 30 μl.
4∣ Inactivate the digest for 20 min at 65 °C. Thereafter, add 1 μl of AP for 30 min at 37 °C.
- 5∣ Aliquot 50 ng of genomic DNA of wild-type AdC6 as template. PCR-amplify the EK fragment using the 5′ and 3′ primers 5′-CACCTCCCGGTACCACATCAC-3′ and 5′-CGGAATTCCGGCGATCGCGCATCATCAATAATATACCTCAAAC-3′. Conduct PCR in a total volume of 50 μl as follows:
Phusion HF buffer (5×) 10 μl (1× Final concentration) MgCl2 (50 mM) 1 μl (2.5 mM Total final concentration) dNTP mix (10 mM) 1 μl (200 μM Each final concentration) Forward primer (20 μM) 1 μl (400 nM Final concentration) Reverse primer (20 μM) 1 μl (400 nM Final concentration) Genomic DNA (50 ng μl−1) 1 μl (1 ng μl−1 Final concentration) Phusion polymerase (2 U μl−1) 0.5 μl (0.02 U μl−1 Final concentration) Distilled water 34.5 μl Total volume 50.0 μl Per reaction
Mix the reaction by vortexing and spin down. Run the PCR for 32 cycles at 94 °C for 1 min, 58 °C for 1 min and at 72 °C for 2.5 min.
6∣ Digest 12 μl of the PCR amplicon with KpnI and EcoRI for 2 h at 37 °C. Conduct digestion in a total volume of 20 μl.
-
7∣ Run the digestion products on 1% (wt/vol) low-melting agarose gel in TAE buffer. Cut out the bands and place gel slices into Eppendorf tubes. Incubate for 5 min at 68 °C. Cool for 1 min at 37 °C. Set up an in-gel ligation reaction in a total volume of 20 μl, i.e., use 4 μl of vector in liquefied gel, 12 μl of insert in liquefied gel and mix both with 1 μl of 5 U of T4 DNA ligase. Ligate at 16 °C overnight.
 CRITICAL STEP Heating the gel slices containing DNA for 5 min at 68 °C is enough to melt the agarose. Try not to exceed 70 °C to avoid denaturing the DNA.
CRITICAL STEP Heating the gel slices containing DNA for 5 min at 68 °C is enough to melt the agarose. Try not to exceed 70 °C to avoid denaturing the DNA. -
8∣ Melt the ligation products for 5 min at 65 °C. Thereafter, dilute in 180 μl of 1× KCM buffer. Transform 25 μl of diluted ligation product into 50 μl of MAX efficiency Stbl2 competent cells and spread the transformation mix onto an ***amplicillin-containing LB plate (100 μg ml−1 amplicillin). Incubate plates for 14 h at 30 °C.
 TROUBLESHOOTING
TROUBLESHOOTING -
9∣ Select five to ten colonies and grow in LB medium for 12–14 h in a shaker at 30 °C and 180 r.p.m. shaking speed. Isolate plasmid DNA by minipreparation. Digest plasmids with the same enzymes used in cloning to check the size of the insert.
 CRITICAL STEP Large genomes transformed into MAX efficiency Stb12 competent cells are often unstable. To minimize damage to the DNA, the temperature for bacterial growth in the shaker should not exceed 30 °C, and shaking speed and time should not exceed 200 r.p.m. and 14 h, respectively.
CRITICAL STEP Large genomes transformed into MAX efficiency Stb12 competent cells are often unstable. To minimize damage to the DNA, the temperature for bacterial growth in the shaker should not exceed 30 °C, and shaking speed and time should not exceed 200 r.p.m. and 14 h, respectively.
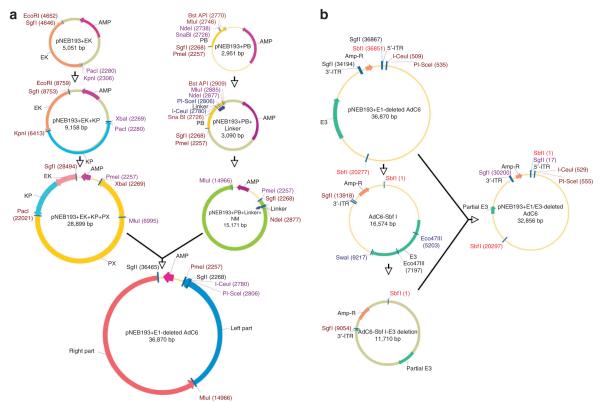
For this and all subsequent cloning steps, confirmation that the desired clone was obtained is made by restriction enzyme digestion, and a fragment of DNA is sequenced to ensure correct ligation. In our example, the new construct was termed PNEB193 + EK (Fig. 1). SgfI and EcoRI are sites added to the end of the right ITR of AdC6 by PCR amplification. Both EcoRI and KpnI sites are unique in this part of the genome. A schematic overview of the cloning procedure is shown in Figure 1a.
10∣ Cloning of the KP (KpnI to PacI) fragment of AdC6 into the PNEB193 + EK plasmid: digest 0.5 μg of PNEB193 + EK plasmid DNA with PacI and KpnI. Conduct the reaction in a total volume of 30 μl and incubate for 3 h at 37 °C.
11∣ Repeat Step 4.
12∣ Digest 1 μg of genomic DNA of wild-type AdC6 with PacI and KpnI to release the KP fragment from AdC6. Conduct the reaction in a total volume of 20 μl and incubate the mixture for 3 h at 37 °C.
13∣ Repeat Steps 7–9. Both KpnI and PacI sites are unique in this part of the genome. The new construct was termed PNEB193 + EK + KP.
14∣ Cloning of the PX (PacI to XbaI) fragment of AdC6 into the PNEB193 + EK + KP plasmid: digest 0.5 μg of PNEB193 + EK + KP plasmid DNA with XbaI and PacI. Conduct the reaction in a total volume of 30 μl and incubate for 3 h at 37 °C.
15∣ Repeat Step 4.
16∣ Digest 1 μg of genomic DNA of wild-type AdC6 with XbaI and PacI to release the XP fragment. Conduct the reaction in a total volume of 20 μl and incubate for 3 h at 37 °C.
17∣ Repeat Steps 7–9. Both PacI and XbaI sites are unique in this part of the genome. The new construct was termed PNEB193 + EK + KP + PX.
18∣ Cloning the PB (PmeI to BstAPI) fragment of AdC6 into the PNEB193 plasmid: digest 0.5 μg of pNEB293 plasmid DNA with PmeI for 3 h at 37 °C, then add BstAPI at 1.5 μl for 3 h at 60 °C. Conduct digestion in a total volume of 30 μl.
19∣ Inactivate the digest for 20 min at 80 °C; add 1 μl of AP for 30 min at 37 °C.
-
20∣ Use 50 ng of genomic DNA of wild-type AdC6 as template, amplify PB fragment by PCR for introduction of the PmeI and SgfI sites to the end of the left ITR of AdC6 and introduce NdeI, MluI and BstAPI at the right end of this fragment. The following 5′ and 3′ primers were used: 5′-GGGTTTAAACCCGCGATCGCGCATCATCAATAATATACCTCAAAC-3′ and 5′-AAGGGCACCATCTGCACGCGTCGCCATATGGAATACGTAAAAACACCGGACTTTG-3′. Conduct the PCR reaction in a total volume of 50 μl as follows.
Phusion HF buffer (5×) 10 μl (1× Final concentration) MgCl2 (50 mM) 1 μl (2.5 mM Total final concentration) dNTP mix (10 mM) 1 μl (200 μM Each final concentration) Forward primer (20 μM) 1 μl (400 nM Final concentration) Reverse primer (20 μM) 1 μl (400 nM Final concentration) Genomic DNA (50 ng μl−1) 1 μl (1 ng μl−1 Final concentration) Phusion polymerase (2 U μl−1) 0.5 μl (0.02 U μl−1 Final concentration) Distilled water 34.5 μl Total volume 50.0 μl Per reaction Vortex and spin down all the fluids to obtain a pellet. Run PCR for 32 cycles at 94 °C for 1 min, 62 °C for 50 s and 72 °C for 40 s.
21∣ Digest 12 μl of PCR amplicon with PmeI for 3 h at 37 °C; thereafter, add 1.5 μl of BstAPI and incubate for 3 h at 60 °C. Conduct digestion in a total volume of 30 μl.
22∣ Repeat Steps 7–9. Both PmeI and BstAPI sites are unique in this part of the genome. The new construct was termed PNEB193 + PB.
23∣ Cloning of the linker into the PNEB193 + PB plasmid: digest 0.5 μg of PNEB193 + PB plasmid DNA with SnaBI and NdeI. These two enzyme sites are unique in this plasmid. Conduct the reaction in a total volume of 20 μl and incubate for 3 h at 37 °C.
24∣ Repeat Step 4.
25∣ A linker with a length of 165 bp containing the extremely rare enzymes I-CeuI and PI-SceI was synthesized by Integrated DNA Technologies. The sequence of the linker is as follows: 5′-cgctacgtacgatatcatttccccgaaagtgccacctgaccgtaactataacggtcctaaggtagcgaagcatctatgtcgggtgcggagaaagaggtaatgaaatggcattatgggtattatgggtctgcattaatgaatcggtcagatatcgacatatgctgc-3′.
Figure 1.
Flowchart of cloning E1/E3-deleted AdC6 molecular clone. (a) Sequence of cloning E1-deleted AdC6 molecular clone. Details are represented in Steps 3–34. Clones of the right and left halves of wild-type AdC6 were inserted sequentially into two pNEB193 plasmids; PmeI and MluI are attached to the left fragment of AdC6 and to the right fragment of AdC6 in pNEB193 vector, respectively. (b) Sequential illustration of cloning E1/E3-deleted AdC6 molecular clone. The procedure is detailed in Steps 35–44. In brief, one fragment including E3 domain was cut from E1-deleted molecular clone, E3 region was then removed from the fragment by digestion of Eco47III and SwaI. Finally, the resulting fragment was cloned back into E1-deleted molecular clone.
Digest 0.3 μg of the linker with SnaBI and NdeI. Conduct the reaction in a total volume of 20 μl and incubate for 3 h at 37 °C.
26∣ Repeat Steps 7–9. The new construct was termed PNEB193 + PB + Linker.
27∣ Cloning NM (NdeI to MluI) fragment into PNEB193 + PB + Linker plasmid: digest 0.5 μg of the PNEB193 + PB + Linker plasmid with NdeI and MluI. Conduct the reaction in a total volume of 30 μl and incubate for 3 h at 37 °C.
28∣ Repeat Step 4.
29∣ Digest 1 μg of genomic DNA of wild-type AdC6 with NdeI and MluI to release the NM fragment. Conduct the reaction in a total volume of 20 μl and incubate for 3 h at 37 °C.
30∣ Repeat Steps 7–9. Both NdeI and MluI sites are unique in this fragment of the genome. This new construct was termed PNEB193 + PB + Linker + NM.
31∣ Construct the full-length E1-deleted AdC6 molecular clone: digest 0.5 μg of PNEB193 + EK + KP + PX plasmid with PmeI and MluI. Conduct the reaction in a total volume of 30 μl. Incubate for 3 h at 37 °C.
32∣ Repeat Step 4.
33∣ Digest 0.5 μg of PNEB193 + PB + Linker + NM with PmeI and MluI to release the fragment of PB + Linker + NM.
-
34∣ Repeat Steps 7–9. Both PmeI and MluI sites are unique in the genome of AdC6. The new construct represents an infectious clone of the E1-deleted AdC6 within a PNEB193 vector. It was termed AdC6–000.
 TROUBLESHOOTING
TROUBLESHOOTING
Generate E1/E3-deleted Adc6 molecular clone  TIMING ~10 d
TIMING ~10 d
35∣ Digest 0.5 μg of AdC6-000 plasmid with SbfI. Conduct the reaction in a total volume of 20 μl and incubate for 3 h at 37 °C.
36∣ Run the digest on 1% (wt/vol) low-melting agarose gel in TAE buffer; cut out a band containing a fragment of ~19 kb and place it into an Eppendorf tube. Incubate for 5 min at 68 °C. Cool for 1 min at 37 °C. Assemble an in-gel ligation reaction as described in Step 7.
37∣ Repeat Steps 8 and 9. The ligation results in the new construct AdC6-Sbf1.
38∣ Digest 0.5 μg of AdC6-Sbf I plasmid DNA with Eco47III and SwaI. Both of these enzyme sites are unique in this plasmid and both enzymes produce blunt ends. Conduct the reaction in a total volume of 20 μl and incubate for 3 h at 37 °C.
39∣ Run all digests on 1% (wt/vol) low-melting agarose gels in TAE buffer; cut out a band containing a fragment of ~15 kb and place it into an Eppendorf tube. Heat for 5 min at 68 °C. Cool down for 1 min at 37 °C. Conduct an in-gel ligation as described in Step 7.
40∣ Repeat Steps 8 and 9. The ligation results in the new construct AdC6-Sbf1-E3 deletion.
41∣ Digest 0.5 μg of AdC6-000 plasmid with SbfI. Conduct the reaction in a total volume of 20 μl and incubate for 3 h at 37 °C.
42∣ Inactivate the digest for 20 min at 65 °C. Thereafter, add 1 μl of AP for 30 min at 37 °C.
43∣ Digest AdC6-Sbf1-E3 deletion with SbfI. Perform the reaction in a total volume of 20 μl and incubate for 3 h at 37 °C.
44∣ Repeat Steps 7–9. The new construct is the E1/E3-deleted AdC6 molecular clone, and was termed AdC6 010. A schematic overview of the cloning procedure is shown in Figure 1b.
Clone foreign gene into the Adc6 010 vector  TIMING ~1 week
TIMING ~1 week
45∣ Cloning of the HIV gag gene into pShuttle: digest 0.5 μg of pShuttle plasmid DNA with KpnI and XbaI for 3 h at 37 °C. Conduct digestion in a total volume of 20 μl.
46∣ Repeat Step 4.
47∣ Codon-optimized HIV gag was synthesized by GenScript and provided in pUC57 vector flanked by XbaI and KpnI sites. Digest 0.5 μg of pUC57-HIV gag with KpnI and XbaI. Conduct digestion in a total volume of 20 μl and incubate for 3 h at 37 °C.
-
48∣ Repeat Steps 7–9 to generate pShuttle-HIV gag.
 CRITICAL STEP pShuttle contains kanamycin resistance gene. The transformation mixture should be spread onto a kanamycin-containing LB plate (50 μg ml−1 of kanamycin).
CRITICAL STEP pShuttle contains kanamycin resistance gene. The transformation mixture should be spread onto a kanamycin-containing LB plate (50 μg ml−1 of kanamycin). 49∣ Cloning of the HIV gag gene into the AdC6 010 vector: digest 0.5 μg of the AdC6 010 plasmid with I-CeuI and PI-SceI. Conduct the reaction in a total volume of 20 μl and incubate for 3 h at 37 °C.
50∣ Repeat Step 4.
51∣ Digest 0.5 μg of pShuttle-HIV gag plasmid with I-CeuI and PI-SceI. Conduct the reaction in a total volume of 20 μl and incubate for 3 h at 37 °C.
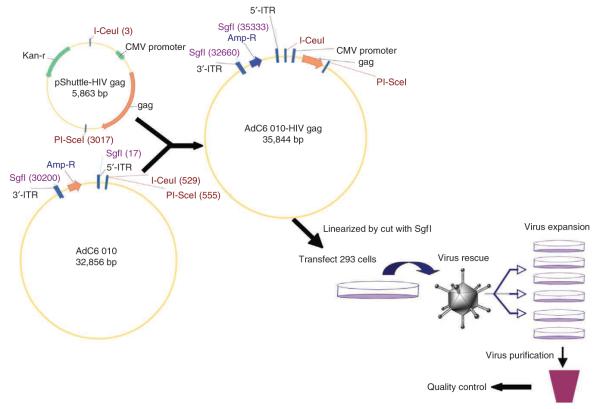
52∣ Repeat Steps 7–9. The new construct was termed AdC6 010-HIV gag. A schematic overview of the cloning procedure is shown in Figure 2.
Figure 2.
Schematic representation of the cloning of the gene of interest into E1/E3-deleted AdC6 and virus production. Foreign gene of interest was cloned into pShuttle and then into E1/E3-deleted AdC6 molecular clone (for detailed procedures refer to Steps 45–52). Recombinant molecular clone was then transfected into HEK 293 cells after linearization. Virus was expanded and purified after rescue (refer to Steps 53–61).
Transfection of HEK 293 cells with the linearized Adc6 010-HIV gag construct and rescue of virus  TIMING 6–8 d
TIMING 6–8 d
53∣ Digest 5 μg of AdC6 010-HIV gag with SgfI to linearize the plasmid. Conduct the reaction in a total volume of 50 μl and incubate for 3 h at 37 °C.
54∣ Inactivate the digestion mixture for 20 min at 65 °C.
55∣ Combine the inactivated digestion mixture with Lipofectamine 2000 transfection reagent; transfect one T25 flask of HEK293 cells at a confluency of 80–90% following the manufacturer’s instruction.
-
56∣ At 4 h after transfection, substitute the medium with DMEM contain ing 5% FBS and incubate at 37 °C. Virus plaques become visible 6–8 d later. Substitute the medium if necesssary.
 TROUBLESHOOTING
TROUBLESHOOTING
Virus amplification in HEK 293 cells  TIMING ~10 d
TIMING ~10 d
57∣ Harvest transfected HEK 293 cells once virus plaques are readily detectable, and resuspend in 1 ml of L15 medium. Freeze and thaw cells three times. Spin the lysate for 8 min at 4,000 r.p.m. at 4 °C.
58∣ Use half of the resulting supernatant to infect one T175 flask of HEK 293 cells at a confluence of 90%. Reserve the remainder of the supernatant at −80 °C. After 24–48 h, once viral plaques become visible, harvest infected cells and resuspend in 1 ml of L15 medium. Repeat the above freeze-and-thaw procedure and save the supernatant.
59∣ Use three-fourths of the supernatant to infect four T175 flasks of HEK 293 cells at a confluence of 90%. After 24 h, harvest infected cells, and resuspend in 1 ml of L15 medium. Repeat the above freeze-and-thaw procedure and save the supernatant.
60∣ Use three-fourths of the supernatant, infect 40 T175 flasks of HEK 293 cells at a confluence of 90%. After 24 h, harvest infected cells, and resuspend in 30 ml of 10 mM Tris-HCl buffer. Repeat the above freeze-and-thaw procedure and save the supernatant.
61∣ Purify the virus from the supernatant by CsCl gradient centrifugation, determine the number of vps by spectrophotometry and determine virus infectivity by titration on HEK 293 cells31.
Testing genetic stability of the vector  TIMING 3–4 weeks
TIMING 3–4 weeks
62∣ Repeat Step 58 to passage the virus serially 15 times (p15). Thereafter, repeat Steps 59–61 to expand and purify the p15 virus.
63∣ Repeat Steps 1 and 2 to isolate the genomic DNA of the p15 virus. Digest 1.5 μg of p15 genomic DNA with SphI, HindIII and AgeI. Conduct the reactions in a total volume of 20 μl and incubate for 3 h at 37 °C. Use identical digestions for viral DNA from the first passage virus.
64∣ Run all digests in 1% (wt/vol) agarose gel to determine whether they have the correct fragments (shown in Fig. 3a).
Figure 3.
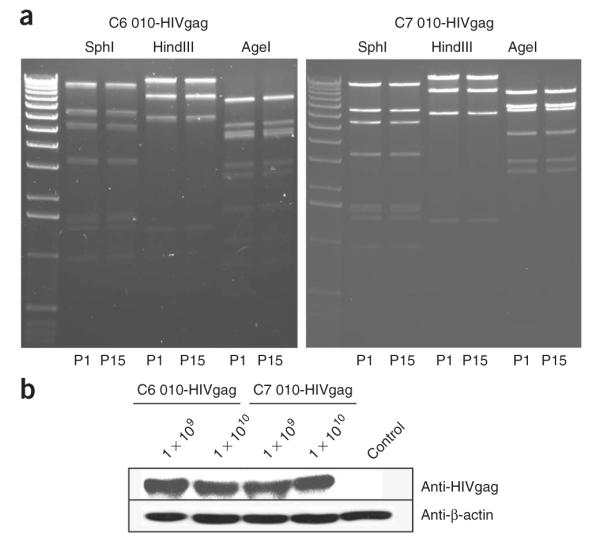
Genetic stability test and the expression of adenoviral vectors. (a) Genomic DNAs from passage 15 of the recombinant adenovirus were purified and then digested with SphI, HindIII and AgeI. The same digestions for the virus DNA from the first passage were set in parallel. Digests were run on 1% (wt/vol) agarose gel. (b) HEK 293 cells were infected with 1 × 109 and 1 × 1010 vps of AdC6 010-HIV gag or AdC7 010-HIV gag. After 24 h, cells were harvested and lysed. Total protein of each sample was run on 10% SDS–polyacrylamide gel electrophoresis gel. The expression of HIV gag was detected by blotting with anti-HIV Gag antibody; antibody to β-actin was included.
Expression of the transgene product  TIMING 4 d
TIMING 4 d
65∣ Seed HEK 293 cells in a six-well plate at a density that results in 90% confluency within 24 h.
66∣ The next day, infect the cells in separate wells with 1 × 109 or 1 × 1010 vps of recombinant Ad vector.
67∣ After 24 h, harvest cells and resuspend in 100 μl of RIPA buffer with protease inhibitors.
68∣ Run the western blot to detect the expression of HIV gag by blotting with an anti–HIV Gag antibody. β-Actin is included as a loading control (Fig. 3b).
Testing the immune responses induced by Ad vectors in mice  TIMING 2–3 weeks
TIMING 2–3 weeks
69∣ Immunize female BALB/c mice (6–8 weeks old) with either AdC6 010-HIV gag or AdC7 010-HIV gag dosed at 1 × 108, 1 × 109 or 1 × 1010 vps.
70∣ After 2 weeks, obtain blood samples from the mice and determine frequencies of HIV gag–specific CD8+ T cells by staining peripheral blood mononuclear cells with fluorochrome-labeled antibodies to CD8 and a MHC class I tetramer specific for the HIV Gag immunodominant epitope (AMQMLKETI) as described32.
71∣ Analyze stained cells on an LSRII (Beckman Coulter) and conduct postacquisition analysis with Flowjo software 7.1.1 (Treestar).
 TROUBLESHOOTING
TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 8 | No development of bacterial colonies |
Ligation was not properly conducted |
Determine ratio of backbone and insert; cool the gel to 37 °C before adding T4 DNA ligase |
| 34 | No large construct; partial construct |
DNA shearing; broken constructs |
Avoid high vortexing speed or excessive centrifugation when isolating large plasmid DNA |
| 56 | No formation of viral plaques | Transfection was not efficient; molecular clone is flawed |
Determine quality of plasmid DNA and optimal ratio of DNA in transfection mix; sequence key regions of the molecular clone |
 TIMING
TIMING
Steps 1 and 2, Isolate genomic DNA of wild-type Ad: 6 h
Steps 3–34, Generate E1-deleted AdC6 molecular clone: ~5 weeks
Steps 35–44, Generate E1/E3-deleted AdC6 molecular clone: ~10 d
Steps 45–52, Clone gene of interest into E1/E3-deleted AdC6 molecular clone: ~1 week
Steps 53–56, Rescue virus from packing cell line: 6–8 d
Steps 57–61, Expand and purify virus: ~10 d
Steps 62–64, Test genetic stability: ~3–4 weeks
Steps 65–68, Test transgene expression: 4 d
Steps 69–71, Test immune responses in mice: 2–3 weeks
ANTICIPATED RESULTS
The virus yields for AdC6 010-HIV gag and AdC7 010-HIV gag preparation from cultures of adherent HEK 293 cells (40 T175 flasks) should be greater than 2 × 1013 vps, with a vp–infectious unit ratio below 200 (see Box 3). Alternative methods based on amplification of Ad vectors in suspension cultures have been described previously33. Viruses should remain stable on serial passages. After immunization, vectors should elicit readily detectable transgene product-specific CD8+ T-cell responses (Fig. 4).
Box 3. TITRATION OF ADENOVIRUS (Ad) VECTORS.
Content of virus particles is determined by spectrophotometry at 260 nm and 280 nm, with the latter determining the purity of the preparation as described above for wild-type Ad virus. The Food and Drug Administration (FDA) requires dosing of Ad vectors according to virus particles (vp), as they determine the toxicity of the vectors. Immunogenicity of the vectors on the other hand depends on the number of virus particles that are able to infect cells and transcribe the transgene product. In general, numbers of infectious virus particles or infectious units (IU) are measured by plaque assays using an agarose overlay or by an end point dilution assay, which determines cytopathic effects. Both assays are performed on a cell line that provides E1 in trans. Plaque formation is dependent on a number of viral factors and may not reliably reflect the interactions between the cell line and different serotypes of E1-deleted Ad vectors. An alternative assay was developed to determine IU contents of AdC6 and other chimpanzee-origin Ad vector batches, and the assay was validated against the standard plaque assay. In this assay, IU content is determined by nested reverse-transcription PCR with transgene or Ad (hexon)-specific primers on RNA isolated from HEK 293 cells infected for 5–7 d with serial dilutions of vector. Eight replicas are used for each Ad vector dilution. A titrated Ad vector from the same serotype is included as a standard to control the assay. It should be noted that AdC6 vectors and vectors based on other chimpanzee serotypes commonly have higher vp-to-IU ratios than those based on AdHu5 virus. Nevertheless, vp-to-IU ratios are typically below 200:1 and should not exceed 400:1. Ad vectors undergoing immunogenicity testing should be dosed according to vp, although the transgene produced only by infectious vector drives the required immune responses. To directly compare two different vectors, vp-to-IU ratios of the vectors should be similar.
Figure 4.
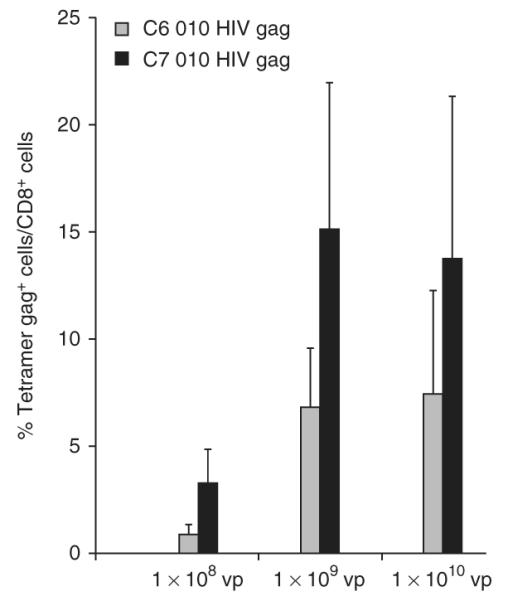
Induction of CD8+ T cells by recombinant adenoviral vectors. Groups of BALB/c mice were immunized i.m. with 1 × 108, 1 × 109 and 1 × 1010 vps of AdC6 010-HIV gag or AdC7 010-HIV gag. Peripheral blood mononuclear cells of individual mice were analyzed 14 d later by tetramer staining and for cell surface-expressed CD8 and tetramer HIV gag. Graph shows mean percentages of CD8+ Gag tetramer cells/CD8+ cells from individual mice ± s.d.
Depending on the transgene product, other methods may have to be used to test for immune responses. Transgene products designed to induce B-cell responses should be tested for induction of antibodies from serum or plasma by suitable assays such as neutralization assays for viral glycoproteins or enzyme-linked immunosorbent assays for antigens that fail to elicit neutralizing antibodies. B-cell responses can be enumerated by ELISpot assays for antibody-secreting cells or memory B cells34. Induction of antibody responses can be assessed in inbred or outbred strains of mice. Induction of T cells can be tested for by a variety of assays if T-cell epitopes or their restricting elements are not defined; the definition of both is a prerequisite for the development of tetramers. Alternative T cell assays include intracellular cytokine staining upon a short in vitro stimulation of lymphocytes with their cognate antigen; ELISpot assays, which do not distinguish between CD8+ and CD4+ T-cell subsets, unless one of these subsets is depleted from the lymphocyte preparation before analyses; and others such as 51Cr-release or cytokine-release assays, which assess function rather than magnitude of a T-cell response. It should be noted that Ad vectors induce preferentially potent CD8+ T-cell responses, whereas induction of CD4+ T-cell responses is less prominent. Attention should be paid to the presence of a suitable T-cell epitope within the transgene product when selecting a mouse strain for immunogenicity testing of Ad vectors. In cases in which the transgene product lacks an epitope that can be recognized by T cells of any of the available inbred mouse strains, testing may have to be conducted in another species or in HLA transgenic mice35.
The timeline described above is based on our experience with AdC6. A number of problems can be encountered during the procedures, and this can significantly expand the time line. Problems we have encountered in the past included contamination of wild-type Ad virus with variants, which required repeated cloning of the virus by plaque purification (add ~4 weeks); inaccurate sequence information, which did not identify all of the sites for restriction enzymes we had planned to use (add anywhere from 1 to 8 weeks if the viral genome has to be resequenced or if a new cloning strategy has to be developed and implemented); problems with ligations (add ~3–6 d); and, most worrisome, problems with viral rescue. We typically try at first to rescue a molecular clone that does not carry a transgene. If virus cannot be rescued, it commonly means that the molecular clone is flawed, which can best be detected by sequencing of key regions (ITRs and regions that could have been modified during cloning).
ACKNOWLEDGMENTS
This work was funded by grants from NIAID and the Commonwealth of Pennsylvania. We thank C. Cole for her help in preparing the paper.
Footnotes
AUTHOR CONTRIBUTIONS D.Z. designed and performed experiments, analyzed data and wrote the paper. X.Z. designed and performed experiments and analyzed data. A.B., H.L., H.C., J.C.S., Y.L., W.G.-D. and Z.X. performed experiments and analyzed data. H.C.J.E. supervised all experimental procedures and edited the paper.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Xiang Z, et al. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 2002;76:2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peruzzi D, et al. A novel chimpanzee serotype-based adenoviral vector as delivery tool for cancer vaccines. Vaccine. 2009;27:1293–1300. doi: 10.1016/j.vaccine.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;7225:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy S, et al. Characterization of a family of chimpanzee adenoviruses and development of molecular clones for gene transfer vectors. Hum. Gene Ther. 2004;15:519–530. doi: 10.1089/10430340460745838. [DOI] [PubMed] [Google Scholar]
- 5.Seshidhar RP, et al. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 6.Roy S, et al. Generation of an adenoviral vaccine vector based on simian adenovirus 21. J. Gen. Virol. 2006;87:2477–2485. doi: 10.1099/vir.0.81989-0. [DOI] [PubMed] [Google Scholar]
- 7.Davis AR, Wivel NA, Palladino JL, Tao L, Wilson JM. Construction of adenoviral vectors. Methods Mol. Biol. 2000;135:515–523. doi: 10.1385/1-59259-685-1:515. [DOI] [PubMed] [Google Scholar]
- 8.Mizuguchi H, Kay MA. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum. Gene Ther. 1998;9:2577–2583. doi: 10.1089/hum.1998.9.17-2577. [DOI] [PubMed] [Google Scholar]
- 9.Danthinne X, Werth E. New tools for the generation of E1-and/or E3-substituted adenoviral vectors. Gene Ther. 2000;7:80–87. doi: 10.1038/sj.gt.3301047. [DOI] [PubMed] [Google Scholar]
- 10.Gao G, et al. High throughput creation of recombinant adenovirus vectors by direct cloning, green-white selection and I-Sce I-mediated rescue of circular adenovirus plasmids in 293 cells. Gene Ther. 2003;22:1926–1930. doi: 10.1038/sj.gt.3302088. [DOI] [PubMed] [Google Scholar]
- 11.Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol. Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 2003;77:10780–10789. doi: 10.1128/JVI.77.20.10780-10789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang Z, Li Y, Gao G, Wilson JM, Ertl HC. Mucosally delivered E1-deleted adenoviral vaccine carriers induce transgene product-specific antibody responses in neonatal mice. J. Immunol. 2003;171:4287–4293. doi: 10.4049/jimmunol.171.8.4287. [DOI] [PubMed] [Google Scholar]
- 14.Zhou D, Cun A, Li Y, Xiang Z, Ertl HC. A chimpanzee-origin adenovirus vector expressing the rabies virus glycoprotein as an oral vaccine against inhalation infection with rabies virus. Mol. Ther. 2006;14:662–672. doi: 10.1016/j.ymthe.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 15.DiMenna LJ, et al. Augmentation of primary influenza A virus specific CD8+ T cell responses in aged mice through blockade of an immunoinhibitory pathway. J. Immunol. 2010;184:5475–5484. doi: 10.4049/jimmunol.0903808. [DOI] [PubMed] [Google Scholar]
- 16.Lasaro MO, et al. Targeting of antigen to the herpesvirus entry mediator augments primary adaptive immune responses. Nat. Med. 2008;14:205–212. doi: 10.1038/nm1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Z, et al. Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology. 2000;270:146–161. doi: 10.1006/viro.2000.0271. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald JC, et al. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 2003;170:1416–1422. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- 19.Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM, Ertl HC. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J. Immunol. 2003;171:6774–6779. doi: 10.4049/jimmunol.171.12.6774. [DOI] [PubMed] [Google Scholar]
- 20.Pinto AR, Fitzgerald JC, Gao GP, Wilson JM, Ertl HC. Induction of CD8+ T cells to an HIV-1 antigen upon oral immunization of mice with a simian E1-deleted adenoviral vector. Vaccine. 2004;22:697–703. doi: 10.1016/j.vaccine.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Reyes-Sandoval A, et al. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J. Virol. 2004;78:7392–739. doi: 10.1128/JVI.78.14.7392-7399.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatsis N, et al. Chimpanzee-origin adenovirus vectors as vaccine carriers. Gene Ther. 2006;3:421–429. doi: 10.1038/sj.gt.3302675. [DOI] [PubMed] [Google Scholar]
- 23.Tatsis N, et al. A CD46-binding chimpanzee adenovirus vector as a vaccine carrier. Mol. Ther. 2007;15:608–617. doi: 10.1038/sj.mt.6300078. [DOI] [PubMed] [Google Scholar]
- 24.Lin SW, Cun AS, Harris-McCoy K, Ertl HC. Intramuscular rather than oral administration of replication-defective adenoviral vaccine vector induces specific CD8+ T cell responses in the gut. Vaccine. 2007;25:2187–2193. doi: 10.1016/j.vaccine.2006.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCoy K, et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 2007;81:6594–6604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tatsis N, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatsis N, Lin SW, Harris-McCoy K, Garber DA, Feinberg MB, Ertl HC. Multiple immunizations with adenovirus and MVA vectors improve CD8+ T cell functionality and mucosal homing. Virology. 2007;367:156–167. doi: 10.1016/j.virol.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tatsis N, et al. Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens. J. Immunol. 2009;182:6587–6599. doi: 10.4049/jimmunol.0900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green AP, et al. A new scalable method for the purification of recombinant adenovirus vectors. Hum. Gene Ther. 2002;13:1921–1934. doi: 10.1089/10430340260355338. [DOI] [PubMed] [Google Scholar]
- 30.Bastaki M, Braiterman LT, Johns DC, Chen YH, Hubbard AL. Absence of direct delivery for single transmembrane apical proteins or their ‘secretory’ forms in polarized hepatic cells. Mol. Biol. Cell. 2002;13:225–237. doi: 10.1091/mbc.01-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varnavski AN, Calcedo R, Bove M, Gao G, Wilson JM. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005;12:427–436. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- 32.Lin SW, Hensley SE, Tatsis N, Lasaro MO, Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J. Clin. Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joo HM, He Y, Sangster MY. Broad dispersion and lung localization of virus-specific memory B cells induced by influenza pneumonia. Proc. Natl. Acad. Sci. USA. 2008;105:3485–3490. doi: 10.1073/pnas.0800003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taneja V, David CS. HLA transgenic mice as humanized mouse models of disease and immunity. J. Clin. Invest. 1998;101:921–926. doi: 10.1172/JCI2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortin V, Thibault J, Jacob D, Garnier A. High-titer adenovirus vector production in 293S cell perfusion culture. Biotechnol. Prog. 2004;20:858–863. doi: 10.1021/bp034237l. [DOI] [PubMed] [Google Scholar]
- 36.Stanton RJ, McSharry BP, Armstrong M, Tomasec P, Wilkinson GW. Re-engineering adenovirus vector systems to enable high-throughput analyses of gene function. Biotechniques. 2008;45:659–662. 664–668. doi: 10.2144/000112993. [DOI] [PubMed] [Google Scholar]
- 37.Sakhuja K, Reddy PS, Ganesh S, Cantaniag F, Pattison S, Limbach P, et al. Optimization of the generation and propagation of gutless adenoviral vectors. Hum. Gene Ther. 2003;14:243–54. doi: 10.1089/10430340360535797. [DOI] [PubMed] [Google Scholar]
- 38.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limbach KL, Richie TL. Viral vectors in malaria vaccine development. Parasite Immunol. 2009;31:501–509. doi: 10.1111/j.1365-3024.2009.01141.x. [DOI] [PubMed] [Google Scholar]