Abstract
Background:
Patient-specific implants are used for cranioplastic skull reconstruction when large bone flaps must be replaced or where there are complex or critical contours, especially near the face. These implants have a low complication rate, with poor fit and postoperative infection being the most common complications. We report here a potentially serious hazard that may arise from the use of porous implants.
Case Description:
A 45-year-old woman sustained severe head trauma in a motor vehicle accident that required urgent surgical intervention. Because of progressive resorption of her native bone flap, she underwent replacement of her native flap with a hard tissue replacement/patient-matched implant cranioplasty. Eight years later, she sustained a traumatic laceration over her vertex that necessitated removal of her cranioplastic implant because of persistent local infection. Intraoperatively, the dural flap was ingrowing and firmly adherent to the inside surface of the porous cranioplasty. After several failed attempts to remove the whole implant piecemeal, we attempted to dissect the dural flap from the brain surface to remove it together with the cranioplastic implant but exposure of the extensive cortical adhesions between the brain surface and the dural flap was compromised by the hard overlying cranioplastic implant. Despite our meticulous attempts to cut off these cortical adhesions, a perisylvian blood vessel was avulsed, resulting in intraparenchymal hemorrhage.
Conclusion:
In this case, dural adhesion and ingrowth to the underside of the cranioplasty implant led to disastrous bleeding when the implant needed to be removed years after initial implantation.
Keywords: Adhesion, complication, cranioplasty, dura
BACKGROUND AND IMPORTANCE
Cranial reconstruction after neurosurgical procedures is common practice. Implants are intended for reconstruction and augmentation in craniofacial procedures to fill defects resulting from disease, surgical trauma, or traumatic injury. Numerous alloplastic materials are available for use when native bone cannot be used, including titanium mesh, polymethylmethacrylate (PMMA), silicone rubber, polyethylene, polytetrafluoroethylene, porous hydroxyapatite ceramics, and hard tissue replacement (HTR).[4] Some properties of an ideal bone substitute are resistance to deformation and fracture, compressive strength, ease of application, a hydrophilic surface to promote blood flow, radio-opacity to facilitate postoperative radiographic monitoring, availability, nonresorbability, and ease of removal should reoperation of replacement become necessary.
Cranial reconstruction using computed tomography (CT)-generated patient-matched alloplastic implants has been used for medium and large defects in the upper craniofacial region. The polymer implants consist of either nonporous substance, such as polyetheretherketone (PEEK) or solid titanium metal, or a porous composite material composed of PMMA, polyhydroxyethylmethacrylate (PHEMA), and a calcium hydroxide coating. Porous cranioplasty implants have been applauded for their durability and ease of use as well as the ability to allow for vascular, soft tissue, and bone ingrowth – all of which supplement the acceptance of the foreign object. Additionally, porous cranioplasty implants have been reported in numerous studies to have low rates (0-11%) of complications such as infection and resorption.[1,3,5,6] It has been noted, however, that a potential liability with the use of porous cranioplasties is that the ingrowth of soft tissue may render secondary removal difficult in cases that demand later operation.[1] We present a case in which a patient with a porous plastic HTR/patient-matched implant (HTR PMI) cranioplasty who required delayed cranioplasty removal exhibited dural adhesion and ingrowth into the implant that ultimately led to intraoperative and postoperative bleeding and irreversible brain damage. To the best of our knowledge, this scenario has never been described in the literature before.
Clinical presentation
History
A 45-year-old female patient suffered a severe traumatic brain injury caused by a high-speed rollover motor vehicle accident. Her injuries included comminuted skull fractures, acute right-sided subdural hematoma, subarachnoid and intraparenchymal hemorrhages, and multiple brain contusions. On initial presentation at our facility, she was unconscious and intubated and had unequal pupils. She immediately underwent a large right decompressive hemicraniectomy, evacuation of subdural and right temporal intraparenchymal hematomas, and control of bleeding from lacerated anterior perisylvian arterial vessels. The dura, augmented with an autologous pericranial graft, was loosely closed over the injured brain, and the scalp was closed over a subgaleal drain.
Over the next 18 days, the patient made a good recovery and was discharged to inpatient rehabilitation. One month later, she underwent bone flap replacement using her native bone pieces, which had been sterilely preserved at −20°C. She was able to return home functionally independent, although she did not resume work as a school bus driver.
Three years later, because of progressive resorption of her cranial flap, she underwent replacement of her native bone with a porous plastic patient-specific implant (HTR PMI, W. Lorenz Surgical, Jacksonville, FL). At this surgery, her dura was noted to be completely healed and intact, and it was easily separated from the old bone pieces without being breached or having unusual bleeding. The patient recovered from this surgery uneventfully.
Examination and operation
Eight years later, the patient presented after sustaining a small traumatic laceration over her cranial implant at the vertex, which exposed a small portion of the plate. After 6 weeks, the wound had not healed completely despite her use of antibiotics and topical measures. On examination, there was exposed hardware but no erythema or signs of generalized infection. A CT scan of her head at that time showed no obvious radiographic complications [Figure 1].
Figure 1.
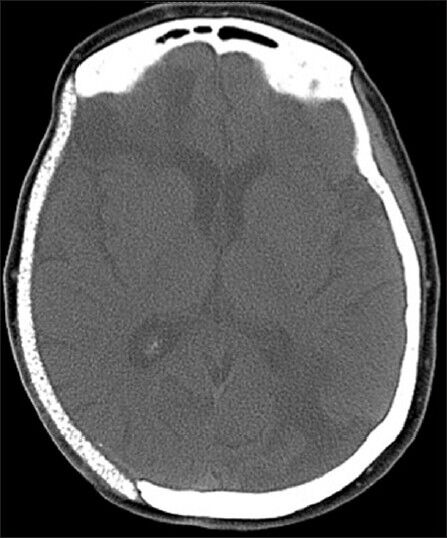
Noncontrast CT scan of the head showing an intact cranioplasty site without any obvious signs of infection or thickening below the cranioplasty to suggest dural adhesion
Because there was clear contamination of the implant and the wound was not healing, we undertook removal of the implant, with a plan for delayed reconstruction. At surgery, the patient's original scalp incision was reopened, and the scalp flap was elevated without difficulty. After the cranial fixation hardware was removed, the skull implant was noted to be firmly adhered to the underlying dura. We began removing it piecemeal with Leksell rongeurs, beginning in the occipital–parietal area. Below the posterior edge of the implant it became clear that the dura had grown into and firmly adhered to the inferior 1 mm surface of the porous implant over the covered defect [Figure 2].
Figure 2.
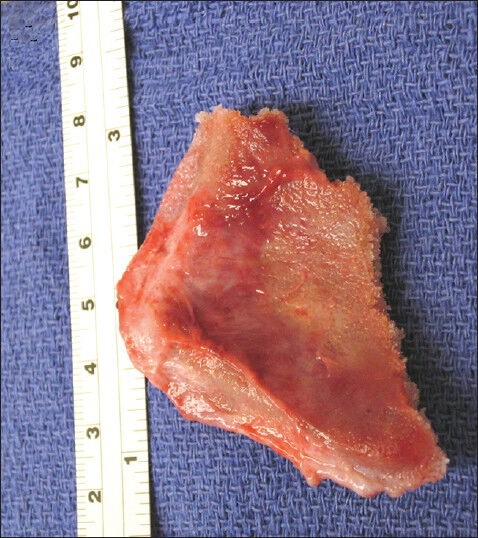
Intraoperative picture showing the inferior surface of part of the cranioplastic porous implant after it has been removed in a piecemeal fashion. The picture demonstrates complete ingrowth of the dura into the inferior surface of the porous implant
The adhesion could not be separated with Penfield instruments or curettes; hence, we resorted to a strategy of trying to separate the dura from the underlying brain to remove it piecemeal with the implant. Because of the previous trauma, there were numerous areas of dural adhesion to the brain, especially in the perisylvian frontotemporal region. Exposure of these cortical adhesions to the dura was further compromised by the hard, nonretractable nature of the overlying plastic implant. Despite our best efforts to clear these adhesions, an M3-M4 middle cerebral artery branch in the vicinity of her previous contusion and intraparenchymal hematoma was avulsed partway into the depths of the sylvian fissure. An operating microscope was used to explore this area, and hemostasis was obtained. Once all of the implant was removed, the resected dura was replaced with a large sheet of resorbable dural substitute (Durepair, Medtronic Corporation, Minneapolis, MN), and the scalp was closed.
Postoperative care
The patient sustained a hemorrhagic infarct secondary to her adhesion-related vascular injury. She had an extended course in the neurosurgical critical care unit, with a left hemiparesis, pneumonia, deep vein thrombosis, and somnolence. She was discharged to a skilled nursing facility and, 6 weeks after her initial surgery, was readmitted for cranioplasty using a custom-made solid titanium implant (Synthes, West Chester, PA). Six months later, her wound was healed without further breakdown or infection. She had returned to her home but had significant residual hemiparesis and need for daily assistance.
DISCUSSION
During the past 15 years, neurosurgeons have used HTR PMIs for cranial reconstruction surgery. HTR PMI is characterized by the marked porosity of its surface, which allows for soft tissue and bone ingrowth. We have found no literature to date on the possible adverse affects of ingrowth.
We report the first case of dural adhesion and ingrowth to a porous HTR cranioplasty noted upon removal 8 years after implantation. This case demonstrates one potential concern when using porous cranioplasty material—dural adhesion to an implant requiring removal in a delayed fashion. Not only can this potentially damage the underlying brain parenchyma, but, as demonstrated by our case, can result in other catastrophic injuries, such as arterial avulsion when attempting to remove the cranioplasty material from the adhered dura and soft tissue below. In a review of the literature, we could identify no other cases requiring removal of porous cranioplasty in such a delayed fashion after initial implantation. Other than our case, the next longest duration to cranioplasty explantation was 37.6 months.[7]
Couldwell et al.[1] used a porous polyethylene implant in a series of 25 patients and noted no complications in 6-15 months of follow-up. No patient in that series had infection or required removal of the porous polyethylene implant. Liu et al.[6] reported a large series of 611 surgical procedures in which a porous polyethylene implant was used in predominantly skull base surgeries requiring cranioplasty or reconstruction, and no patient experienced infection or required further surgery other than the initial operation. The mean follow-up period in that study was 4 years (range 1-8 years).
In 1997, Roberson and Rosenberg[9] were among the first to report the use of HTR PMI cranioplasties in traumatic cranial defects. They noted no complications in their two patients (one with 6-month follow-up and another at 2-year follow-up). Eppley et al.[3] described a series of 14 patients who required HTR PMI cranioplasties after trauma, brain tumor, and cerebrovascular accidents, and all patients noted good aesthetic results with no implants requiring removal for infection. Eppley[2] reported another series of seven patients with bony tumors of the anterior cranial vault and orbit who received HTR PMI implants and were monitored for at least one year (average 2.6 years). The implants for these patients were constructed preoperatively from a three-dimensional CT scan, and an anatomical model was used to predict the amount of bony excision to be performed. There were no complications in any of the patients in this series.
Petersen and Hollins[8] retrospectively evaluated 17 patients who underwent 19 HTR PMI cranioplasties for trauma or postoperative defects. Two of the 17 patients (12%) had complications, including one case of gradual implant exposure necessitating bone graft modification and one case of infection requiring explantation of the graft, but no dural adhesion was noted.
Nassiri et al.[7] reported a significantly higher rate of complications (23.8%) in their retrospective review of 21 patients who underwent HTR PMI reconstruction for large cranial defects. Five patients were noted to have complications, which were described as exposure (one patient), infection of the implant (three patients), and/or soft tissue infection (one patient). The four patients that had implant exposure or infection had their implants removed with no adhesion (time to complication: 3.6-37.6 months). The one patient with a soft-tissue infection was treated with intravenous antibiotics for 4 weeks and did not require graft removal (time to complication: 1.4 months). Although the indication for HTR PMI cranial reconstruction in this series was bone flap loss secondary to infection in more than half (13/21) of the patients, interestingly these patients were the least likely to have a complication after HTR PMI implant. Only one patient with a previously infected bone flap required removal of an infected HTR PMI cranioplasty at 3.6 months after implantation. The authors also looked at whether a history of diabetes or smoking was a significant risk factor for HTR PMI failure, but found no correlation. They did, however, note that two of the HTR PMI infections were in cases where the craniotomy bone flap was never replaced because of traumatic intracerebral hematoma and elevated intracranial pressure in patients with multiple comorbidities. Their findings suggested that decompressive craniectomies in trauma patients may be risky indications for the use of HTR PMI.
CONCLUSIONS
Although HTR PMI cranioplasties have had relatively good outcomes over short follow-up periods, the reported incidence of complications may be underestimated, as complications have previously been reported as late as 37.6 months (Nassiri et al.[7]) and have now been described 8 years after implant in our case. The surgeon must also be wary about using porous implants because the properties of soft-tissue and bony ingrowth may be detrimental in cases of delayed removal. Solid or nonporous constructs such as PMMA, PEEK, or titanium metal may thus be superior to porous implants, especially those that are engineered to promote soft tissue ingrowth when they are used in close contact with dura. With the increasing use of decompressive craniectomies for trauma and stroke, the need for cranioplastic reconstructions may be expected to rise. Porous cranioplasties may not be the best option in this subset population. Further long-term prospective studies to assess the long-term safety and sustainability of porous cranioplastic constructs are necessary.
ACKNOWLEDGMENTS
The authors thank Kristin Kraus, M.Sc., for editorial assistance in preparing this paper.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/19/127377
Contributor Information
Christina M. Sayama, Email: tinamieko@gmail.com.
Mohammad Sorour, Email: mohammad.sorour@hsc.utah.edu.
Richard H. Schmidt, Email: richard.schmidt@hsc.utah.edu.
REFERENCES
- 1.Couldwell WT, Chen TC, Weiss MH, Fukushima T, Dougherty W. Cranioplasty with the Medpor porous polyethylene flexblock implant. Technical note. J Neurosurg. 1994;81:483–6. doi: 10.3171/jns.1994.81.3.0483. [DOI] [PubMed] [Google Scholar]
- 2.Eppley BL. Craniofacial reconstruction with computer-generated HTR patient-matched implants: Use in primary bony tumor excision. J Craniofac Surg. 2002;13:650–7. doi: 10.1097/00001665-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Eppley BL, Kilgo M, Coleman JJ., 3rd Cranial reconstruction with computer-generated hard-tissue replacement patient-matched implants: Indications, surgical technique, and long-term follow-up. Plast Reconstr Surg. 2002;109:864–71. doi: 10.1097/00006534-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Eppley BL. Alloplastic cranioplasty. Oper Tech Plast Reconstr Surg. 2003;9:16–22. [Google Scholar]
- 5.Kahraman S, Kayhall H, Kafadar A, Akboru M, Timurkaynak E. Clinical experience in cranioplasty with porous polyethylene implant. Turk Neurosurg. 2003;13:89–93. [Google Scholar]
- 6.Liu JK, Gottfried ON, Cole CD, Dougherty WR, Couldwell WT. Porous polyethylene implant for cranioplasty and skull base reconstruction. Neurosurg Focus. 2004;16(5):ECP1. doi: 10.3171/foc.2004.16.3.14. [DOI] [PubMed] [Google Scholar]
- 7.Nassiri N, Cleary DR, Ueeck BA. Is cranial reconstruction with a hard-tissue replacement patient-matched implant as safe as previously reported? A 3-year experience and review of the literature. J Oral Maxillofac Surg. 2009;67:323–7. doi: 10.1016/j.joms.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Petersen T, Hollins R. Cranial reconstruction with computer-generated hard-tissue replacement patient-matched implants: Indications, surgical technique, and long-term follow-up. Arch Facial Plast Surg. 2003;5:533–4. doi: 10.1001/archfaci.5.6.533-a. [DOI] [PubMed] [Google Scholar]
- 9.Roberson JB, Rosenberg WS. Traumatic cranial defects reconstructed with the HTR-PMI cranioplastic implant. J Craniomaxillofac Trauma. 1997;3:8–13. [PubMed] [Google Scholar]


