Abstract
Portable wireless neuro-stimulators have been developed to facilitate long-term cognitive and behavioral studies on the central nervous system in freely moving animals. These stimulators can provide precisely controllable input(s) to the nervous system, without distracting the animal attention with cables connected to its body. In this study, a low power backpack neuro-stimulator was developed for animal brain researches that can provides arbitrary stimulus waveforms for the stimulation, while it is small and light weight to be used for small animals including rats. The system consists of a controller that uses an RF link to program and activate a small and light microprocessor-based stimulator. A Howland current source was implemented to produce precise current controlled arbitrary waveform stimulations. The system was optimized for ultra-low power consumption and small size. The stimulator was first tested for its electrical specifications. Then its performance was evaluated in a rat experiment when electrical stimulation of medial longitudinal fasciculus induced circling behavior. The stimulator is capable of delivering programmed stimulations up to ± 2 mA with adjusting steps of 1 μA, accuracy of 0.7% and compliance of 6 V. The stimulator is 15 mm × 20 mm × 40 mm in size, weights 13.5 g without battery and consumes a total power of only 5.l mW. In the experiment, the rat could easily carry the stimulator and demonstrated the circling behavior for 0.1 ms current pulses of above 400 μA. The developed system has a competitive size and weight, whereas providing a wide range of operation and the flexibility of generating arbitrary stimulation patterns ideal for long-term experiments in the field of cognitive and neuroscience research.
Keywords: Electrical stimulation, neurophysiology, programmable current stimulator, ultra-low power
INTRODUCTION
Electrical stimulation of the nervous system has long been one of the fundamental tools of neuro-physiological and cognitive studies and its applications to treat different neurological disorders and disabilities such as tremor, Parkinson, deafness, blindness, depression and epilepsy are rapidly growing.[1] Computer-controlled or programmable current stimulators are widely used in related animal research studies to deliver a variety of precisely adopted stimulation patterns to the nervous tissue. In experiments on anesthetized animals a stimulator with cable connections is generally adequate. However, in awake animals such connections not only limits the subject's free movements, but also increases the risk of infection, may distract its attention or produce emotional distress and interferes with the natural behavior of the animal.[2] A portable stimulator, that is worn by the animal and is controlled wirelessly by a remote module, significantly reduces these problems. In designing such portable systems, small size, light weight and low power consumption are the major constrains, especially when the system is designed for small animals such as rat and mice. This necessitates the use of a small, low weight and therefore low capacity battery for the system's power supply. Although recent development of high-power density lithium-ion and lithium-polymer batteries has provided the same power in significantly smaller batteries,[3] design for ultra-low power consumption is still critically important in portable neuro-stirnulators; particularly when the stimulator is to be used continuously in a long-term experiment.
Specific design techniques, such as aggressive voltage scaling, dynamic power-performance management, energy efficient signaling and micro watt designed integrated circuits must be employed to decrease power consumption without significant decrease in system's performance.[4]
Another important factor that should be considered is the advantages of using electrical current stimulation instead of voltage stimulation to have stable neural responses and controllability over the tissue and electrode damage.
In recent years, design of portable stimulator/recorder systems for neuro-physiological and cognitive studies has attracted many researchers.
Xu et al.[2] have developed a multichannel portable neuro-stimulator for freely moving rats, which is wirelessly controlled by a base-station connected to a computer. The back-pack system can deliver continuous stimulations for up to 7 h with a 6 V l20 mAh lithium battery, but it can only apply biphasic voltage pulses with constant amplitude to the tissue.
Song et al.[5] in their study have designed a remote control multimode micro-stimulator for brain stimulation of freely moving animals. The system consists of a micro-controller unit and a backpack micro-stimulator which is 18 mm × 28 mm in size and works continuously for up to 20 h with a lithium battery (3.6 V, 650 mAh). Only biphasic rectangular voltage pulses can be generated by this stimulator.
Feng et al.[6] have developed a remote control system to deliver stimulus pulses into the rat brain. The system consists of a PC based program, a Bluetooth module and a backpack stimulator module which is 36 mm × 22 mm × 15 mm in size. The system is powered by two lithium cells (3.7 V, 120 mAh) and works for up to 8 h. Although the system can generate current pulses, the amplitude is limited to 120 μA that is too low for typical electrical stimulations of the nervous system.
Ye et al.[7] have designed a multi-channel telemetry system for brain stimulation and neuronal recording in small freely behaving animals. Their system consists of a head stage, a back-pack and a remote Personal Digital Assistant that wirelessly control and receive information from the back-pack, using a bluetooth transceiver. Their system works only for 2 h with a 2200 mAh lithium-polymer battery that is too low for long term neuro-physiological study.
Sharma et al.[1] have designed a multichannel bidirectional telemetry controller for stimulation and recording. The back-pack controller consumes 420 mW and operates for 8 h without the need to recharge the battery. However the size of their system is 13 cm × 6 cm × 3 cm which is too large to be used on small animals.
Forni et al.[8] in their study have reported the development of a single channel portable micro-stimulation system for chronic deep brain stimulation (DBS) in freely moving animals. The system is 28 mm × 17 mm × 5 mm and is supplied by two lithium polymer batteries (3 V, 36 mAh). The system can deliver current pulses with amplitudes up to 120 μA, frequencies up to 130 Hz and pulse widths up to 80 μs. Although these ranges for the stimulation parameters are the same as for commercial DBS systems, it is believed that the system's ranges of operation may be limited if it is to be used in research experiments.
Although the rectangular current pulses are still the most used stimulation waveforms in research studies, as well as in clinical use, increasing studies have demonstrated that the stimulus waveform can have a significant impact on the performance and efficiency of the stimulations.[9] Stimulus waveforms have been suggested to decrease the threshold of the stimulation or selectively activate the target neural elements.
In this paper, design of an ultra-low power backpack neuro-stimulator system is presented with features which make it very convenient for different animal neuroscience and neural engineering applications. The system includes a miniature stimulator with the flexibility of delivering arbitrary waveform current pulses and a remote controller module, which programs and controls the stimulator via an RF link. The system is designed for ultra-low power consumption and therefore is suitable for long term electrical stimulation on freely moving animals. The performance of the system was evaluated both electronically and by conducting a neurophysiological experiment.
MATERIALS AND METHODS
System Overview
A neuro-stimulator system was developed for long-term neural stimulation of the central nervous system (CNS) in small freely moving animals. The system was designed for small size so that it can be carried by small animals and low power consumption for long-term use without the need for changing the battery. In addition, the system should have the flexibility of producing various stimulation patterns necessary for different neuro-physiological applications.
The block diagram of the system is demonstrated in Figure 1, which consists of two separate modules, a remote controller and a back-pack stimulator. The remote controller provides a simple user interface to adjust the stimulation parameters, programs the back-pack stimulator wirelessly through an RF link and activates/deactivates the stimulator module for delivering the stimulations. The back-pack module works based on instructions received from the controller module. It is specifically designed to produce arbitrary controlled current stimulus waveforms for precise and reproducible neural stimulation.
Figure 1.
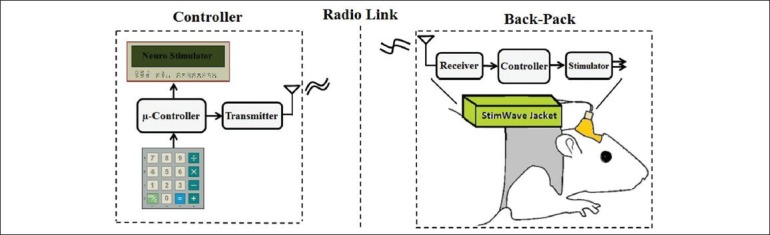
The block diagram of the designed neuro-stimulator system. The controller module (left) provides a user interface to set the stimulation parameters and controls the stimulator module (right) via an on-off keying (OOK) RF link. The stimulator module creates the stimulation pattern using a l2 bit Digital-to-Analog Converter and deliver the proportional current pulses using the Howland circuit
The Controller Module
The detailed design of the remote controller module is demonstrated in Figure 2. The core of the controller module is a PIC18F452 microcontroller (Microchip Tech), which provides a simple user interface based on a 4 × 4 keyboard and a 16 × 2 LCD. The user can set the parameters of the stimulation pattern and then send them via ATX-433-IA RF module (Abacom Technology) to the stimulator. The parameters are checked to be in specified ranges before sending, in order to avoid unwanted irreparable effects. After the stimulator is programmed, the controller module can send an activation code to the stimulator to start applying the programmed train of stimulations. To eliminate the effects of the errors in the output current, a calibration feature was implemented in the controller module to adjust the offset and gain of the system. This significantly improves the accuracy of the currents generated. The baud rate for the RF link is 1200 bits/s which is enough to send the parameters of the stimulation pattern to the stimulator. This allowed for using energy efficient on-off keying (OOK) modulation for sending the data. The ARX-433-ULC (Abacom Technology) receiver module consumes only 70 μA at 3 V which was versatile for our ultra-low power design of the stimulator module. However, OOK modulation is vulnerable to noise and special considerations were needed for reliable transmission of data. Each data packet is preceded by two specified start bytes which must be received correctly for approving the data packet. To ensure good performance despite changes in the strength of the received signal, the OOK receiver module uses the averaged value of the received signal as the threshold to determine the value of the received bits. A drawback of this technique is that if for an adequate period of time, the number of transmitted 1 s does not compensate the number of 0 s, the average value of the signal tends towards either its minimum (identified as zero) or maximum (identified as one), which results in noisy output signal. To avoid this, Manchester coding was used that keeps the average value of the transmitted signal on the midline. In addition, each data packet was preceded by three dummy bytes of 10101010. These bytes are discarded in the receiver. However, they move the receiver threshold to the midline and therefore the receiver can detect the consecutive data correctly. The data packet for sending each parameter includes an extra byte that indicates the number of ‘1’ bits in the binary code of that parameter. This byte is used in the stimulator module to ensure that each parameter is received correctly. The stimulator does not confirm the reception of the parameters unless all the parameters are received correctly. This increases the reliability of the system to an appropriate level so that unwanted stimulation patterns are never generated.
Figure 2.
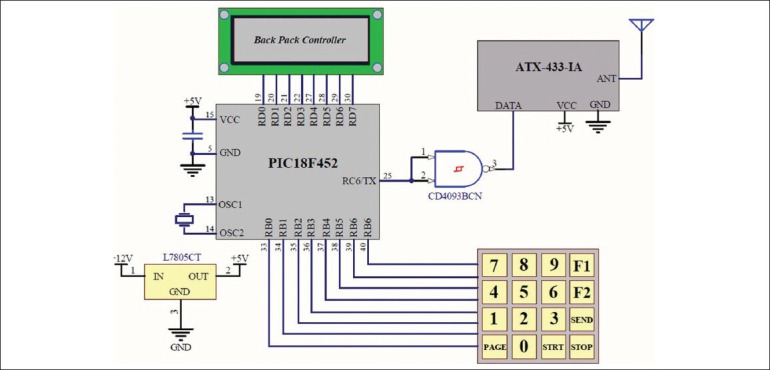
Schematics of the controller module. It provides a simple interface for programming and control of the stimulator module. The core of the module is a PIC micro-controller which receives and displays the stimulation parameters and programs the stimulator module via a on-off keying RF module, ATX-433-IA (Abacom Technology)
The Back-pack Stimulator Module
Figure 3 shows the detailed design of the ultra-low power stimulator module. A PIC16F88 microcontroller (Microchip Tech) was used for its small size and micro power consumption.
Figure 3.
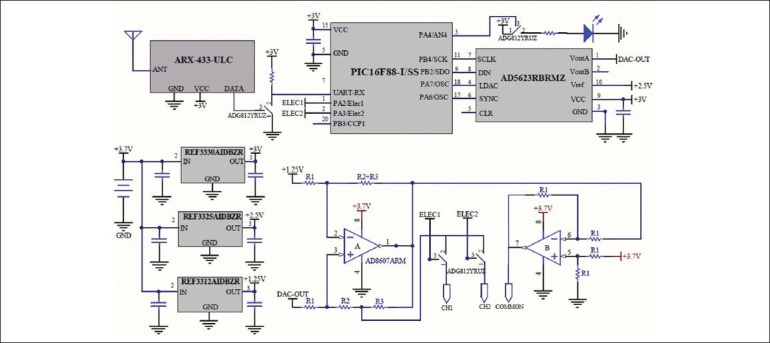
Schematics of the backpack stimulator module. The stimulation waveform is generated by a micro controller and a Digital-to-Analog converter, making the generation of arbitrary stimulus waveforms possible. The Howland circuit converts the waveform into current for more stable neural stimulation. R1 = 220 kΩ, R2 = 16.5 kΩ and R3 = 50 Ω
The microcontroller receives the stimulation parameters and commands from the receiver module via its UART (Universal Asynchronous Receiver Transmitter) module. Currently, it is programmed to generate trains of biphasic rectangular pulses, however the stimulator hardware was designed for the flexibility of generating arbitrary stimulus waveforms. As soon as receiving the activation command, the microcontroller starts generating the programmed stimulation pattern using a 12 bit digital to analog converter (AD5623 of Analog Devices). Using the internal 1.25 V voltage reference, the Digital-to-Analog Converter provides voltages in the range of 0-2.5 V. The voltage is then converted to a controlled current stimulus by a voltage-controlled current pump [Figure 4]. The controlled current output ensures more stable neural response regardless of the electrode and tissue impedance. The current pump is a modified Howland circuit.[10,11] which provides very good linearity and high output impedance. An extra operational amplifier (AD8607 of Analog Devices) is used in this circuit as an inverting amplifier which doubles the compliance of the current pump for a float load.
Figure 4.
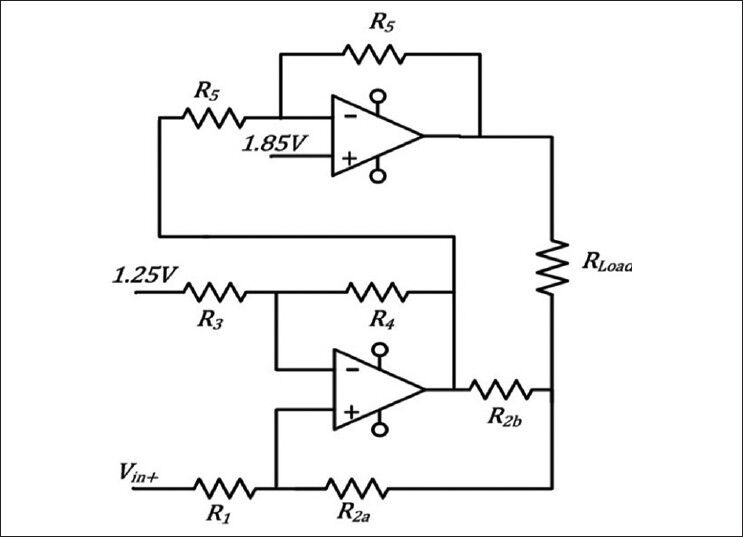
The Howland current pump (Lower opamp) provides the controlled current stimulus to the tissue (Rload in Figure 4). The circuit works with a single supply of 3.7 V. Therefore, an inverter amplifier (upper opamp) was used for the other electrode, so that bidirectional stimulation can be applied to the tissue with a high compliance
Equation 1 shows the input output relationship of the modified Howland current source:

The Howland circuit is directly powered by the 3.7 V battery, to have the maximum possible compliance. To make the current pump both sink and source the current while powered by a single supply, the negative input of the pump (Vin-) was set to 1.25 V. The main output of the current pump may connect to two electrodes via separate parallel analog switches (ADG812 of Analog Devices). This ensures that actually no current is injected to the tissue when the stimulator is idle and forms two, not simultaneously active, channels of stimulation.
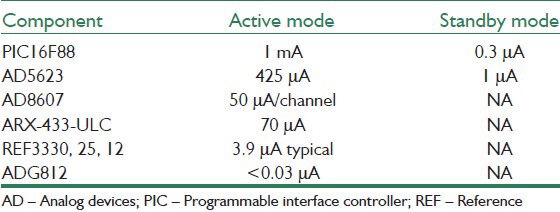
Low power consumption of the chips [listed in Table 1] allowed to use a precision micro-power low dropout voltage reference (REF3330 of Texas Instruments) to fix the power supply of all the chips (except the Howland circuit) on 3 V from the slowly decreasing voltage of a namely 3.7 V battery until the voltage of the battery decreases to 3.2 V. A small LED was used as an indicator of the systems state. It blinks a few times when the data packet for programming the stimulus pattern is received correctly, as well as when the stimulation starts and ends, to ensure proper operation of the system.
Table 1.
Power consumption of the components of the backpack stimulator for 3 V power supply
The Stimulation Pattern
One of the advantages and the novelty of the presented design is its ability to generate arbitrary stimulus waveforms. The use of waveforms other than the traditional rectangular pulses is being more studied to improve the performance and efficiency of the stimulation in many applications. However, to evaluate the implemented system, the micro controller was programmed to just generate asymmetric biphasic rectangular pulse trains that are generally needed in neurophysiologic experiments. The form of the possible stimulus pattern is shown in Figure 5.
Figure 5.
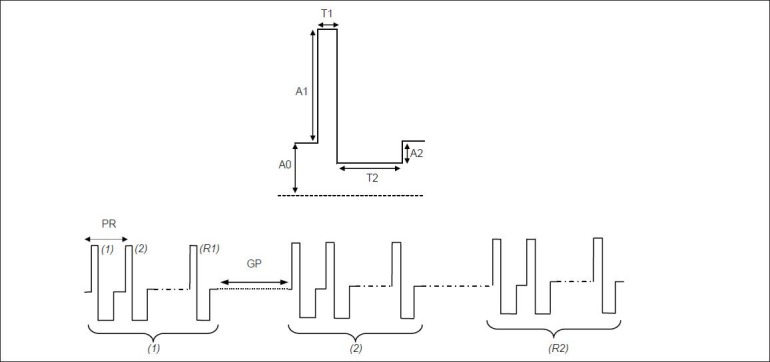
The general form of the stimulation pattern which was implemented for the stimulator provides repeating trains of biphasic stimulation with programmable parameters of the baseline and positive and negative pulse amplitudes, the duration of the two pulses as well as the stimulation frequency, the number of biphasic pulses in each stimulus train, the gap between trains and the total number of the stimulus trains
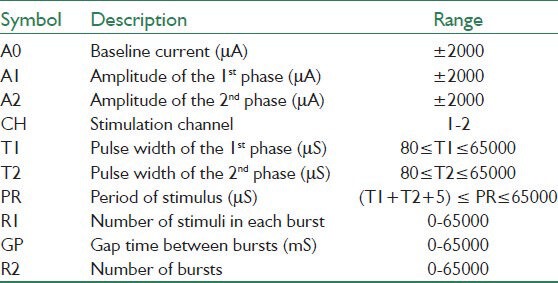
The achievable ranges for the stimulation parameters are presented in Table 2.
Table 2.
The stimulation parameters and their valid range of values
The stimulator can deliver specified number of repetitive asymmetric biphasic current pulses, providing good flexibility for using the system in different experiments. The generated train of pulses may be followed by a silence period and repeated for a certain number of times. Since the stimulus waveform is built in the stimulator module, the only data that is transmitted through the RF link is the stimulation parameters. This, substantially reduces the load on the RF link and allows implementing an ultra-low power receiver in stimulator module. The amplitude and duration of each pulse, the period of stimulation, the number of pulses in each train, the silence period and the number of trains can be adjusted for a specific application. This necessitates considering the baseline amplitude A0 at the voltage-controlled current source (VCCS) input to produce zero output current.
Experimental Protocol
Male Wistar rat (321 g) was obtained from Isfahan University of medical sciences, Iran. Rat was kept in animal house and provided with food and water ad libitum and experienced a 12:12-h light-dark cycle (07:00-19:00) in a temperature controlled environment (22 ± 2°C). Rat was allowed to adapt to the laboratory conditions for at least 1 week before surgery. The Animal Research Ethic Committee at Isfahan University of medical sciences approved the study and all experiments were conducted in accordance with the international guidance principles for biomedical research involving animals, revised in 1985.
Rat was anesthetized with chloral hydrate (450 mg/kg, i.p) then was placed in a stereotaxic apparatus (Stoelting, USA) and two holes of one millimeter in diameter was drilled in the skull, one for inserting the stimulation electrode in the medial longitudinal fasciculus AP = −8.2 or −7.5, ML = 0.4 mm, DV = 7.1 mm from skull surface[12] and the other for placing the reference electrode on the brain (the same coordinate on the left). The stimulation electrode was Teflon-coated stainless steel with 1 mm exposed tip. Dental acrylic was used to fix the electrodes. 7 days of post-operative recovery were allowed. A jacket was designed to put the system on the back of the rat. The rat was placed in a large cage to more freely.
Rectangular biphasic current pulses of 0.1 ms width and different amplitudes were applied with the frequency of 83 Hz and the resulted behavior of the rat was monitored.
RESULTS AND DISCUSSION
A prototype portable wireless stimulator system was designed and characterized for neuro-physiological studies. The controller module could reliably send parameters and control the stimulator module wirelessly in a large room and the stimulator module could deliver precise trains of asymmetric biphasic current pulses to the electrodes. The quiescent power consumption of the system was measured only 5.1 mW.
The compliance of the stimulator was measured as 6 V. The electrode tissue impedance in DBS studies is reported to vary between 0.5 and 1.5 kΩ.[13] Therefore the output compliance of the system is large enough for the stimulation of CNS. The system could deliver stimulus pulses up to ± 2 mA to a load of 1.5 kΩ. Obviously, the load resistance can be higher when lower stimulus amplitudes are used.
A sample stimulation train of asymmetric bipolar pulses produced by the system is demonstrated in Figure 6. The time constant of the edges of the pulses was measured to be 12 μs.
Figure 6.
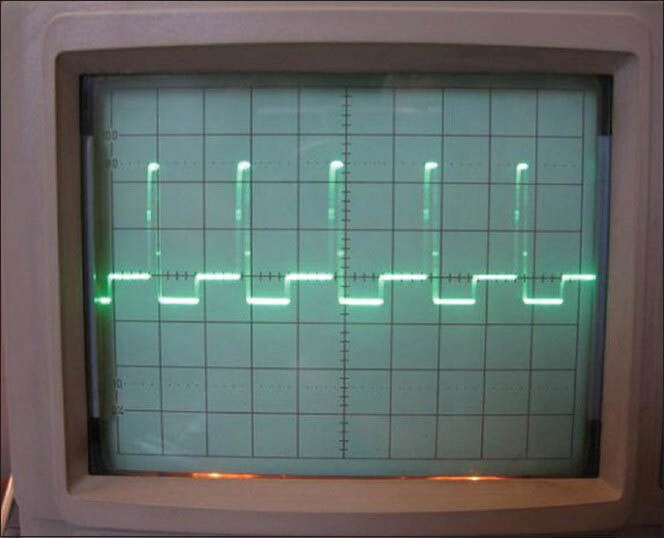
Rectangular biphasic train of the stimulus pulses produced by the developed system. The minimum programmable pulse width was 80 μs, while the edge of the pulses has a time constant of 12 μs
Figure 7 shows the achieved output currents for the loads of 0-1.5 kΩ when an output current of 100 μA is programmed. The VCCS demonstrated a high output impedance of 100 kΩ, which resulted in only 0.7% error in output current amplitude for 0.5-1.5 kΩ range of the tissue impedance.[13]
Figure 7.
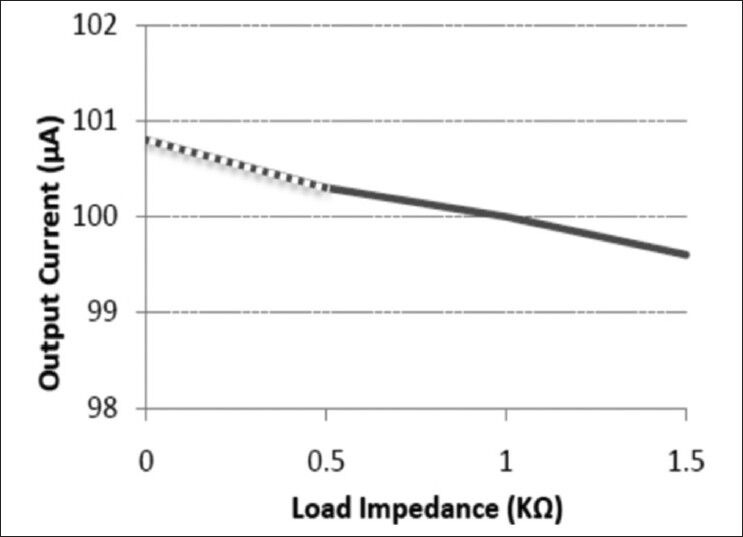
The measured output current versus load impedance. The output current is almost constant with the load impedance
Figure 8 shows the measured output current of the stimulator versus the programmed current for an example load of 1.5 kΩ. The input-output characteristics function of the whole system is acceptably linear in the range of its operation.
Figure 8.
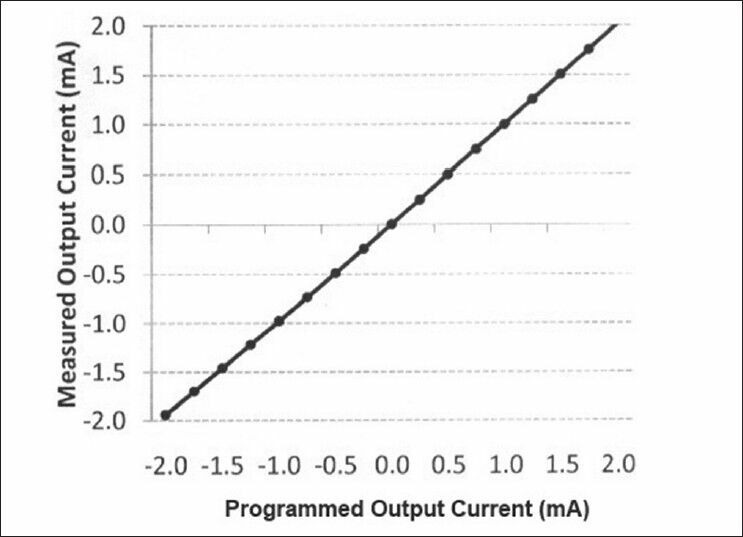
The measured output current of the system versus the programmed current demonstrates the almost linear transfer function of the system, even in the limits
The performance of the system was evaluated in a rat experiment. Figure 9 shows the backpack system placed on the back of the rat, with the electrodes on the head. The rat could carry the stimulator module without any problem.
Figure 9.
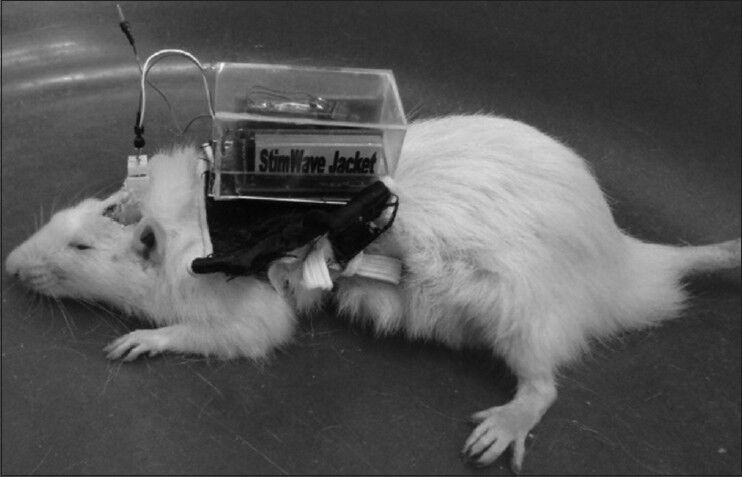
The backpack stimulator on the back of the rat. The rat could easily carry the stimulator module
Upon starting the stimulations, the circling behavior was started. For small amplitude stimulations, the front paws moved to the right, increasing the stimulation amplitude resulted in moving the head to the right and for amplitudes higher than 400 μA, the rat started to circle. The circling was performed around the cylindrical cage that the rat was in, when the stimulation amplitude was just above the threshold. For higher stimulation amplitudes, the circling was more intensive, as the rat seemingly followed her tail. The response was stable in several trials performed including those performed 7 days later. When the stimulation was stopped, the normal sniff and search behavior of the rat came back immediately.
CONCLUSIONS
A portable ultra-low power neuro-stimulator was designed, to deliver arbitrarily stimulus waveforms to the CNS. The stimulator is programmed using a wireless remote controller and is quite flexible in generating various desired stimulation patterns. This makes the developed system a suitable candidate for a large variety of neuro-physiological experiments. The implemented system demonstrates reliable performance with very low power consumption, ideal especially for experiments in which hours of continuous stimulation is required. The system can work continuously for 6 days while continuously delivers stimulus pulses of ± 2 mA without the need to change its battery. Ultra low power consumption of the stimulator could impose constrains on the performance of the system, including the time response of the output pulses and the minimum achievable pulse widths. However, the system was designed to obtain acceptable performance in these parameters. The design was based on commercial off-the-shelf components and provides a solid framework for implementing similar systems by other interested users.
The system worked reliably in a rat experiment to induce the circling behavior. The experiment demonstrated the simplicity of working with the system both for the experimenter and the rat itself.
The performance of the system is compared with some previously developed systems with the similar functionality [Table 3].
Table 3.
Specifications of the developed system, compared with some previously reported systems with the similar functionality
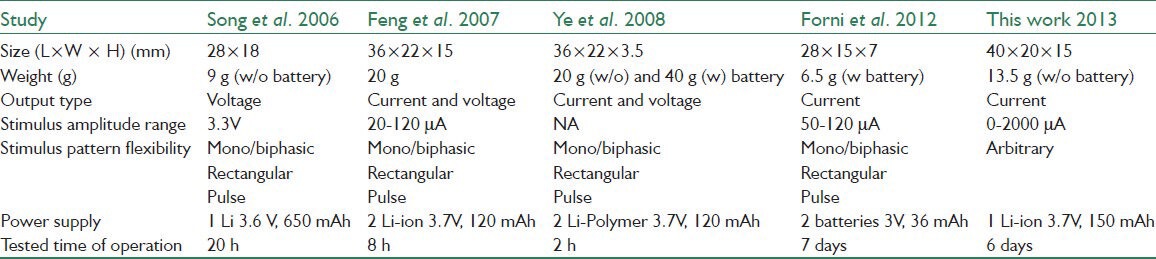
The advantage of the developed system is that it has competitive light weight, small size and power consumption, whereas providing a large range of operation (stimulus amplitudes and pulse widths) and the capability of generating arbitrary stimulus waveforms with controlled current. The system can currently generate trains of rectangular biphasic stimulus pulses, which are the most common stimulation protocol in neuro-physiological experiments. However, generation of arbitrary stimulus waveforms is straight forward with modifications in the programs of the microcontrollers on the control and stimulation modules.
Feng et al.[6] has reported a maximum of 12% error in the stimulus amplitude for their system when the current mode is used. In the present system however the precise current source that is implemented demonstrated only 0.7% error when the load changes from 0.5 to 1.5 kΩ.
Based on the 0.5-1.5 K range of tissue impedance reported in[13] the 6 V compliance of the system is adequate in most of brain stimulation applications, however increasing the compliance of the system can increase the applicability of the developed system in neuroscience and cognitive research specially when small electrodes should be used.
BIOGRAPHIES

Mohsen Mosayebi Samani received the B.Sc. degree in Communication Engineering from Shahid Bahonar University of Kerman, Iran, in 2009. From 2009 to 2010, he was a research scholar in the Biomedical Precision Instrument Laboratory at the university of Isfahan. During 2010.2012, he worked in a biomedical instrument company as a research and development engineer. Mr Mosayebi Samani is currently M.Sc. student of Biomedical Engineering at Tarbiat Modarres University, Tehran, Iran. His research interests include neural engineering and biomedical instrument design and development.
E-mail: mosayebi@modares.ac.ir

Amin Mahnam received his B.Sc. degree in Electrical Engineering from Sharif University of Technology in 2000, and M. Sc. and Ph.D. degrees in Biomedical Engineering (BioElectrics) from Amirkabir University of Technology, in 2002 and 2008, respectively. As a research scholar at Duke University, North Carolina, he spent a year working in the Neural Prosthesis Lab of Prof. Warren M. Grill during 2006.2007. He has also been a researcher in the school of cognitive sciences at the institute for research in fundamental sciences (IPM) during 2010.2011. Since 2007, Dr. Mahnam is assistant professor of Biomedical Engineering at the University of Isfahan. His major research interests are neural engineering, and medical instrumentation. He has conducted projects to study.
E-mail: mahnam@eng.ui.ac.ir

Nasrin Hosseini received the B.Sc. degree in Nursing from Iran University of Medical Sciences, Tehran in 1995 and M.Sc. degree in Human physiology from Ahwaz University of Medical Sciences, Ahwaz in 2003.
She received her Ph.D. degree in Human physiology from Isfahan University of Medical Sciences, Isfahan in 2012, and is currently Ph.D student of cognitive neuroscience at the Institute of cognitive neuroscience, Tehran. Her research interests include different aspects of cognition, learning and memory.
E-mail: hosseini.n58@gmail.com
ACKNOWLEDGMENTS
The authors would like to thank Mr. Kargari for generously providing the electrodes.
Footnotes
Source of Support: This study was supported by a grant from the Medical Image and Signal Processing research center at Isfahan University of Medical Sciences
Conflict of Interest: None declared
REFERENCES
- 1.Sharma V, McCreery DB, Han M, Pikov V. Bidirectional telemetry controller for neuroprosthetic devices. IEEE Trans Neural Syst Rehabil Eng. 2010;18:67–74. doi: 10.1109/TNSRE.2009.2036849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu S, Talwar SK, Hawley ES, Li L, Chapin JK. A multi-channel telemetry system for brain microstimulation in freely roaming animals. J Neurosci Methods. 2004;133:57–63. doi: 10.1016/j.jneumeth.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Holmes CF. The role of lithium batteries in modern health care. J. Power Source. 2001;97-98:739–41. [Google Scholar]
- 4.Chandrakasan AP, Verma N, Daly DC. Ultralow-power electronics for biomedical applications. Annu Rev Biomed Eng. 2008;10:247–74. doi: 10.1146/annurev.bioeng.10.061807.160547. [DOI] [PubMed] [Google Scholar]
- 5.Song WG, Chai J, Han TZ, Yuan K. A remote controlled multimode micro-stimulator for freely moving animals. Sheng Li Xue Bao. 2006;58:183–8. [PubMed] [Google Scholar]
- 6.Feng ZY, Chen WD, Ye XS. A remote control training system for rat navigation in complicated environment. J Zhejiang Univ Sci A. 2007;8:323–30. [Google Scholar]
- 7.Ye X, Wang P, Liu J, Zhang S, Jiang J, Wang Q, et al. A portable telemetry system for brain stimulation and neuronal activity recording in freely behaving small animals. J Neurosci Methods. 2008;174:186–93. doi: 10.1016/j.jneumeth.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Forni C, Mainard O, Melon C, Goguenheim D, Kerkerian-Le Goff L, Salin P. Portable microstimulator for chronic deep brain stimulation in freely moving rats. J Neurosci Methods. 2012;209:50–7. doi: 10.1016/j.jneumeth.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Wongsarnpigoon A, Woock JP, Grill WM. Efficiency analysis of waveform shape for electrical excitation of nerve fibers. IEEE Trans Neural Syst Rehabil Eng. 2010;18:319–28. doi: 10.1109/TNSRE.2010.2047610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen DX, Deng X, Yang WQ. Comparison of three current sources for single-electrode capacitance measurement. Rev Sci Instrum. 2010;81:034704. doi: 10.1063/1.3367879. [DOI] [PubMed] [Google Scholar]
- 11.Pouliquen P, Vogelstein, Etienne-Cummings R. Baltimore, MD: Proc. BioCAS. IEEE; 2008. Practical considerations for the use of a Howland current source for neurostimulation; pp. 33–6. [Google Scholar]
- 12.Paxinos G, Watson C. Sandiego: Elsevier; 2007. The Rat Brain in Stereotaxic Coordinates. [DOI] [PubMed] [Google Scholar]
- 13.Volkmann J, Herzog J, Kopper F, Deuschl G. Introduction to the programming of deep brain stimulators. Mov Disord. 2002;17(Suppl 3):S181–7. doi: 10.1002/mds.10162. [DOI] [PubMed] [Google Scholar]


