Abstract
Background & objectives:
There is a paucity of data from India on response to treatment of tuberculosis (TB) in patients with human immunodeficiency virus (HIV)-TB co-infection. This study was done to assess the frequency and pattern of TB, outcome of anti-tuberculosis treatment, and the factors related to poor outcome of TB treatment in adult patients with HIV infection.
Methods:
Retrospective review of case records of HIV-TB co-infected patients attending the antiretroviral therapy (ART) clinic in a tertiary care centre in north India was done.
Results:
Of the 1754 patients included in the study, 583 (33.2%) were diagnosed with active TB and 466 (79.9%) of them had CD4 count less than 200/μl at diagnosis. Extrapulmonary TB was diagnosed in 372 (63.8%) patients [76 (20.4%) had disseminated TB], and pulmonary TB in 211 (36.2%) patients. Favourable outcome (cure and completed treatment) was observed in 332 (77%) patients. Unfavourable outcome included default (8.1%), treatment failure (1.6%), and death (13.2%). At 1-year post-treatment follow up, 12 (3.6%) patients had disease relapse. CD4 count of less than 200/μl at diagnosis [OR-2.32, CI (1.06-5.09)], and retreatment cases [OR-2.91, CI (1.22-6.89)] were independent predictors of unfavourable outcome.
Interpretation & conclusions:
There is an urgent need to strengthen the information, education, communication activities and expand the ART services to meet the requirement of early testing and treatment initiation in patients co-infected with HIV-TB. The findings highlight the need for performing drug susceptibility testing (DST) for patients starting retreatment regimen to improve treatment outcome.
Keywords: Acquired immune deficiency syndrome, clinical profile, human immunodeficiency virus, immunosuppression, tuberculosis
The dual epidemics of tuberculosis (TB) and human immunodeficiency virus (HIV) infection is a major public health problem, particularly in resource-limited settings such as India. TB is the most common opportunistic infection in HIV infected patients, particularly in developing countries1,2. Patients with HIV-TB co-infection frequently have advanced HIV disease and are at an increased risk of death and new opportunistic infections3. The HIV-TB co-infection has been aptly described as the “cursed duet”4.
The World Health Organization (WHO) estimated 8.8 million incident cases of TB globally in 2010; with 12-14 per cent of cases among people with HIV5. India accounted for maximum number of incident cases of TB (2-2.5 million) worldwide, with an estimated 5.0 per cent (3.3-7.1%) having HIV co-infection5. Despite the high burden of disease, there is a paucity of data from India on response to anti-tuberculosis treatment (ATT) in patients with HIV-TB co-infection6,7,8. Information on the pattern of TB, the outcome of ATT and the associated factors will help in planning interventions to improve outcomes in these patients.
The present study was carried out to assess the frequency and pattern of TB, outcome of ATT, and the factors related to poor outcome of TB treatment in HIV-infected patients with TB, attending a tertiary care health facility in north India.
Material & Methods
The case records of HIV/AIDS patients attending the All India Institute of Medical Sciences (AIIMS) hospital, a tertiary care centre in New Delhi, India, over a period of six years between May 2005 and April 2011 were retrospectively reviewed. Patients with HIV/AIDS attending various departments/facilities in the hospital were referred to the Antiretroviral Therapy (ART) clinic for further evaluation and treatment. The ART clinic works under the aegis of the National AIDS Control Organization (NACO), an initiative of the Ministry of Health and Family Welfare of the Government of India. The hospital provides tertiary care to the population of Delhi and neighbouring States and most of the patients come from the lower socio-economic strata. The study included HIV infected adult patients registered at the ART clinic between May 2005 and April 2011 who were diagnosed with active TB. Patients referred from other hospitals with incomplete baseline data or transferred out before complete baseline evaluation were excluded from the study. All patients were followed up till end of the study (i.e. April 2011). Hence, those who were registered earlier had longer follow up than those who were registered later.
HIV infection was documented by commercially available third generation enzyme-linked immunosorbent assay (ELISA) kits (Detect HIV-1/2, BioChem Immunosystems Inc., Montreal, Canada; UBI HIV 1/2 EIA, Beijing United Biomedical Co. Ltd., Beijing, China) to detect antibodies to HIV-1 and HIV-2, as per the WHO strategy9. A diagnosis of TB
was made as per the Revised National Tuberculosis Control Programme (RNTCP) and WHO criteria for smear-positive pulmonary TB, smear-negative pulmonary TB (PTB), or extrapulmonary TB (EPTB)10,11. Briefly, a sputum smear-positive pulmonary TB case was a patient with two or more initial sputum smear examinations positive for acid fast bacilli (AFB), or one sputum smear examination positive for AFB plus radiographic abnormalities consistent with active pulmonary TB. A patient having symptoms suggestive of TB with at least three sputum examinations negative for AFB, and radiographic abnormalities consistent with active pulmonary TB was classified as smear-negative TB; and EPTB referred to TB of organs other than the lungs which was substantiated by one culture-positive specimen from an extrapulmonary site, or histological or radiological findings. Sputum smear examination was done at the designated microscopy centre following the RNTCP guidelines10.
Detailed clinical examination was done at enrolment and repeated every month as the patients came to the ART clinic for follow up, and collection of antiretroviral drugs in accordance with the guidelines of NACO. The timing of highly active antiretroviral therapy (HAART) initiation was decided as per the NACO guidelines, and the regimen comprised two nucleoside reverse transcriptase inhibitors (zidovudine or stavudine plus lamivudine) and one non-nucleoside reverse transcriptase inhibitor (efavirenz or nevirapine)12. Patients with TB were offered treatment free of cost from Directly Observed Treatment Short-Course (DOTS) centre in accordance with the RNTCP of Ministry of Health and Family Welfare, Government of India10. New cases received Category I treatment (thrice-weekly intermittent treatment with rifampicin, isoniazid, pyrazinamde and ethambutol in the intensive phase followed by the administration of rifampicin and isoniazid in the continuation phase); whereas, retreatment cases received Category II treatment (thrice-weekly intermittent treatment with rifampicin, isoniazid, pyrazinamde, ethambutol and streptomycin for 2 months followed by rifampicin, isoniazid, pyrazinamde and ethambutol for 1 month followed by rifampicin, isoniazid and ethambutol). Majority of patients were on DOTS, whereas some patients preferred to take daily therapy (taking the alternate day regimen daily). The intensified TB-HIV package was implemented and patients were given co-trimoxazole prophylaxis therapy in the clinic from 2009 onwards. The study protocol was approved by the ethics committee of the institute.
The operational definitions used for sputum positive cure, treatment completed, failure, defaulter were according to the RNTCP guidelines10. Briefly, a patient registered as pulmonary smear-positive, who completed treatment and had negative smear results on two occasions, one of which was at end of treatment was classified as cured; a patient with pulmonary smear-positive TB with no smear results at the end of treatment, and smear-negative or extrapulmonary TB patients completing treatment were classified as completed treatment. Patients registered as pulmonary smear-positive CAT I, who was smear-positive at five months or registered as pulmonary smear-positive CAT II (retreatment), and were smear-positive at five months or later of CAT II treatment, or registered as pulmonary smear-negative or EPTB, but were smear positive any time during treatment were classified as treatment failure. Patients not taking drugs for more than two months consecutively any time after starting treatment were classified as defaulters. The TB treatment outcomes were assessed as ‘favourable’ (cure and treatment completed) and ‘unfavourable’ (default, failure and dead). Retreatment cases were those having history of previous TB treatment of more than one month.
CD4+ cell counts were performed by flow cytometry at baseline and every six months thereafter in accordance with the NACO guidelines12. Plasma HIV viral load estimation was not done in the National Programme. Drug susceptibility testing (DST) for tuberculosis was not performed routinely due to resource constraints. Medical social workers ensured regular visits of the patients to the DOTS and ART clinics. During each visit the patients were evaluated for clinical improvement, drug toxicity and development of new opportunistic infections. Adherence to HAART (95% of drugs taken) was assessed during each visit by pill count, and through counselling, patients were motivated to adhere to the therapy. Patients were contacted telephonically or their houses were visited in case they failed to turn up for their scheduled visits.
Statistical analysis: For all the patients attending the ART centre, the clinical and investigation details were recorded in a file and the data were entered in a pre-designed proforma. Continuous data were presented as mean ± standard deviation (for normally distributed variables) or median and interquartile range or IQR (for variables influenced by extreme values). Categorical data were presented as numbers with proportions. Medians were compared using Kruskal-Wallis test. For the purpose of assessment of outcomes, the patients who had completed treatment but had less than 12 months post-treatment follow up were excluded from the analysis. However, all death, default and failure cases were included for analysis. The variables of patients with ‘favourable’ and ‘unfavourable’ treatment outcomes were compared initially through univariate analysis and subsequently with logistic regression analysis to identify the independent predictors of treatment outcome. Variables with P <0.01 in univariate analysis were included for logistic regression model. All tests were two-sided, and P <0.05 was considered significant. All analyses were done using SPSS (version 17) (SPSS Inc., USA).
Results
Of the 2612 patients registered in the clinic during the study period, 1754 met the inclusion criteria. HIV-TB co-infection was diagnosed in 583 (33.2%) patients. Active TB at diagnosis of HIV was present in 538 (30.7%), while 45 (2.6%) patients were diagnosed with TB while on HAART. The demographic, clinical and laboratory profile of these patients are shown in Table I. EPTB was diagnosed in 372 (63.8%) patients [76 (20.4%) had disseminated TB]; whereas, pulmonary TB was diagnosed in 211(36.2%) patients. The disease classification and the CD4 counts are shown in Table II. There was no significant difference in median CD4 counts between patients with PTB and EPTB. ATT related adverse events were reported in 100 (17.1%) patients; drug induced hepatitis (DIH) observed in 93 (15.9%) patients, was the commonest adverse event.
Table I.
Demographic, clinical and laboratory profile of HIV-infected patients with (n=583) and without TB (n=1171)
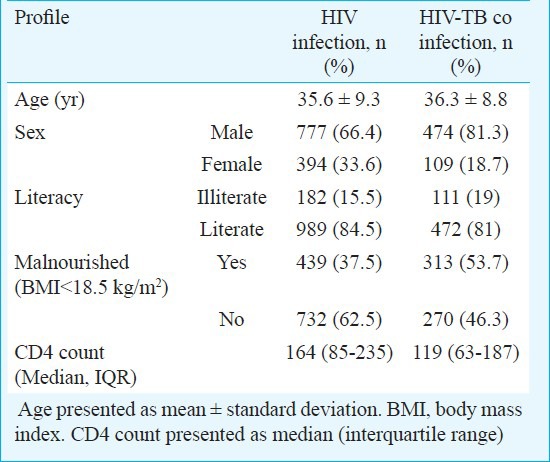
Table II.
Disease classification and CD4 counts in HIV-infected patients with TB (n=583)

TB treatment outcome in 431 patients is shown in Table III. For the assessment; 124 patients who were either on treatment or had completed treatment but had less than 12 months follow-up, and 28 patients who had complete baseline evaluation, but were transferred out to their respective local ART centres, were excluded from analysis (Figure). “Favourable outcome” was observed in 332 (77%) patients; 122 (75.3%) having PTB and 210 (78%) having EPTB. Among PTB patients, sputum positives had lower success rate compared to sputum negative group (70.9 vs 77.6%); mainly attributed to higher rates of default among patients with sputum positive PTB. At 1-year post-treatment follow up, 12 (3.6%) patients had disease relapse.
Table III.
Treatment outcomes of TB in HIV-infected patients (n=431)
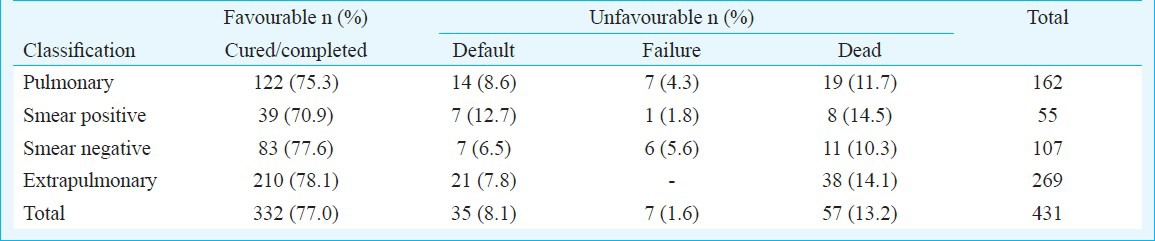
Fig.
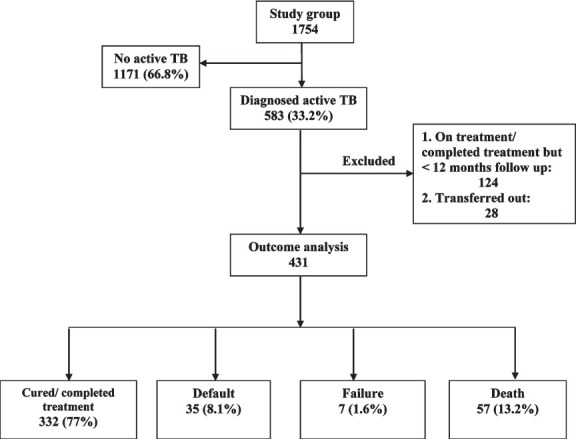
Flow chart showing the study profile.
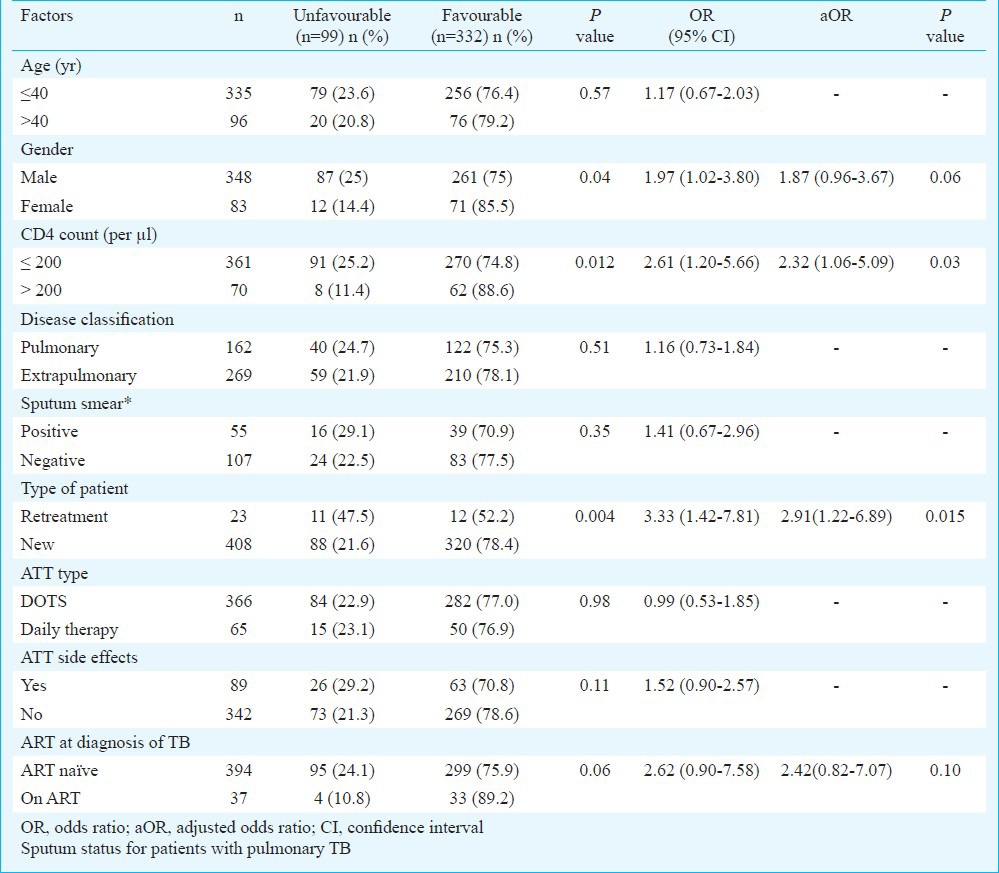
The variables compared between the groups with “favourable” and “unfavourable” outcomes were CD4 counts, disease classification (PTB/EPTB), sputum smear status in PTB patients, type of patient (retreatment/new), ATT type (DOTS/daily therapy), ATT related side effects, and initiation of ART at the time of diagnosis of TB (ART naïve/on ART). Table IV shows the univariate analysis of various factors; the associations with P <0.01 were included in the logistic regression model. In the logistic regression analysis factors independently associated with poor outcome were ‘CD4 count <200/μl [aOR 2.32, CI (1.06-5.09)] and ‘retreatment’ [aOR 2.91, CI (1.22-6.89)].
Table IV.
Univariate and multivariate analysis of factors associated with poor TB treatment outcome (n=431)
Discussion
TB was diagnosed in 33.2 per cent patients with HIV infection. The estimated annual risk of reactivation among those co-infected with HIV and TB is about 5 to 8 per cent, with a cumulative lifetime risk of 30 per cent or more; compared to a cumulative lifetime risk of 5-10 per cent in HIV-negative adults4. TB is the most common life-threatening opportunistic infection in patients with HIV/AIDS in developing countries with about 25 to 65 per cent patients with HIV/AIDS having the disease1,4,13,14,15,16.
Extrapulmonary TB was more common than pulmonary TB, consistent with the findings in other studies17,18,19. In a study from south India, higher proportion of patients had pulmonary TB in a district6; however, the discrepancy was attributed to under-reporting of extrapulmonary TB cases by peripheral health centres due to the limited diagnostic facilities. Advanced immunosuppression at presentation and high burden of extrapulmonary TB pose significant diagnostic challenges for resource-limited settings in India and newer diagnostic tests are urgently required that are not only sensitive and specific but easy to use in programme settings.
The overall rate of favourable outcome to anti-tuberculosis treatment was 77 per cent. Studies from resource-constrained settings have shown a success rate of 66-75 per cent6,8,20 The mortality rate while on treatment with ATT was high (13.2%), consistent with other studies from resource-constrained settings6,7,8. High rate of default is a major problem in the management of these patients in the programme21. Adverse drug reactions (22.8% among defaulters), initial symptomatic improvement, social stigma and lack of awareness of the disease could have been the contributory factors.
Patients with advanced immunosuppression at presentation were at increased risk for poor outcome, consistent with the literature22. Retreatment cases were also associated with poor outcome. Though drug susceptibility test (DST) was not done routinely in the Programme setting, prior suboptimal therapy may lead to multidrug resistant (MDR) TB, a known risk factor for poor outcome23. Further, various factors including increased susceptibility to tuberculosis, increased opportunity to acquire TB due to overcrowding, exposure to patients with MDR-TB during hospital visits, and suboptimal therapeutic levels of anti-tuberculosis drugs due to malabsorption may potentially increase the chances of MDR-TB in these patients24.
The limitations of the study were non availability of DST routinely under the Programme, a high rate of attrition and lack of effective measures of retrieval of these patients, and inherent weakness associated with any retrospective study.
In conclusion, our results indicate towards an urgent need to strengthen the information, education, communication activities and expand the ART services to meet the requirements of early testing and initiation of HAART. The findings also highlight the importance for performing DST for patients starting retreatment regimen to improve outcome. Prospective studies are required to assess the efficacy of short course chemotherapy regimens on outcome of patients with HIV-TB co- infection including relapse rates on a long term follow up.
Acknowledgment
Authors thank the National AIDS Control Organization (NACO) and Delhi State AIDS Control Society (DSACS) for providing Integrated Counselling and Testing Centre (ICTC) facility, drugs and other support staff for management of these patients. Authors are also thankful to All India Institute of Medical Sciences hospital, New Delhi, for providing administrative and other logistic support for the study. We also thank residents, nursing and other staff involved in the management of these patients.
References
- 1.Sharma SK, Kadhiravan T, Banga A, Goyal T, Bhatia I, Saha PK. Spectrum of clinical disease in a series of 135 hospitalised HIV-infected patients from north India. BMC Infect Dis. 2004;4:52. doi: 10.1186/1471-2334-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes CB, Losina E, Walensky RP, Yazdanpanah Y, Freedberg KA. Review of human immunodeficiency virus type 1-related opportunistic infections in sub-Saharan Africa. Clin Infect Dis. 2003;36:652–62. doi: 10.1086/367655. [DOI] [PubMed] [Google Scholar]
- 3.Harries A, Maher D, Graham S. 2nd ed. Geneva: World Health Organization; 2004. TB/HIV: a clinical manual. WHO/HTM/TB/2004. 329. [Google Scholar]
- 4.Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis & management. Indian J Med Res. 2005;121:550–67. [PubMed] [Google Scholar]
- 5.Geneva: WHO; 2011. World Health Organization. Global tuberculosis control: WHO report 2011; pp. 9–27. [Google Scholar]
- 6.Vijay S, Kumar P, Chauhan LS, Rao SV, Vaidyanathan P. Treatment outcome and mortality at one and half year follow-up of HIV infected TB patients under TB control programme in a district of South India. PLoS One. 2011;6:e21008. doi: 10.1371/journal.pone.0021008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaminathan S, Deivanayagam CN, Rajasekaran S, Venkatesan P, Padmapriyadarsini C, Menon PA, et al. Long term follow up of HIV-infected patients with tuberculosis treated with 6-month intermittent short course chemotherapy. Natl Med J India. 2008;21:3–8. [PubMed] [Google Scholar]
- 8.Tripathy S, Anand A, Inamdar V, Manoj MM, Khillare KM, Datye AS, et al. Clinical response of newly diagnosed HIV seropositive & seronegative pulmonary tuberculosis patients with the RNTCP Short Course regimen in Pune, India. Indian J Med Res. 2011;133:521–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Joint United Nations Programme on HIV/AIDS (UNAIDS)-WHO. Revised recommendations for the selection and use of HIV antibody tests. Wkly Epidemiol Rec. 1997;72:81–7. [PubMed] [Google Scholar]
- 10.New Delhi: Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2005. Technical and operational guidelines for tuberculosis control. [Google Scholar]
- 11.3rd ed. Geneva: WHO; 2003. World Health Organization. Treatment of tuberculosis: guidelines for national programmes. WHO/CDS/TB/2003313. [Google Scholar]
- 12.New Delhi, India: NACO; 2007. National AIDS Control Organization (NACO). Antiretroviral therapy guidelines for HIV infected adults and adolescents including post-exposure prophylaxis; pp. 1–125. [Google Scholar]
- 13.Arora VK, Kumar SV. Pattern of opportunistic pulmonary infections in HIV sero-positive subjects: observations from Pondicherry, India. Indian J Chest Dis Allied Sci. 1999;41:135–44. [PubMed] [Google Scholar]
- 14.Kumarasamy N, Solomon S, Jayaker Paul SA, Venilla R, Amalraj RE. Spectrum of opportunistic infections among AIDS patients in Tamil Nadu, India. Int J STD AIDS. 1995;6:447–9. doi: 10.1177/095646249500600615. [DOI] [PubMed] [Google Scholar]
- 15.Gothi D, Joshi JM. Clinical and laboratory observations of tuberculosis at a Mumbai (India) clinic. Postgrad Med J. 2004;80:97–100. doi: 10.1136/pmj.2003.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–53. [PubMed] [Google Scholar]
- 17.Chaisson RE, Schecter GF, Theuer CP, Rutherford GW, Echenberg DF, Hopewell PC. Tuberculosis in patients with the acquired immunodeficiency syndrome. Clinical features, response to therapy, and survival. Am Rev Respir Dis. 1987;136:570–4. doi: 10.1164/ajrccm/136.3.570. [DOI] [PubMed] [Google Scholar]
- 18.Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–7. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 19.Zumla A, Malon P, Henderson J, Grange JM. Impact of HIV infection on tuberculosis. Postgrad Med J. 2000;76:259–68. doi: 10.1136/pmj.76.895.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal U, Kumar A, Behera D. Profile of HIV associated tuberculosis at a tertiary institute in setting of free anti-retroviral therapy. J Assoc Physicians India. 2009;57:685–90. [PubMed] [Google Scholar]
- 21.Sharma SK, Dhooria S, Prasad KT, George N, Ranjan S, Gupta D, et al. Outcomes of antiretroviral therapy in a northern Indian urban clinic. Bull World Health Organ. 2010;88:222–6. doi: 10.2471/BLT.09.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dheda K, Lampe FC, Johnson MA, Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004;190:1670–6. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 23.Alpert PL, Munsiff SS, Gourevitch MN, Greenberg B, Klein RS. A prospective study of tuberculosis and human immunodeficiency virus infection: clinical manifestations and factors associated with survival. Clin Infect Dis. 1997;24:661–8. doi: 10.1093/clind/24.4.661. [DOI] [PubMed] [Google Scholar]
- 24.Sharma SK, Mohan A. Multidrug-resistant tuberculosis. Indian J Med Res. 2004;120:354–76. [PubMed] [Google Scholar]


