Abstract
Background & objectives:
Soil-transmitted helminths (STH) are a major public health problem in tropical and sub-tropical countries, affecting the physical growth and cognitive development in school-age children. This study was aimed to assess the prevalence and risk factors of STH infection among school children aged 6-14 yr in Vellore and Thiruvanamalai districts in south India.
Methods:
Children aged 6-14 yr, going to government and government aided schools (n=33, randomly selected) in Vellore and Thiruvanamalai districts were screened to estimate the prevalence of STH, and a case control study was done on a subset to assess the risk factors for the infection.
Results:
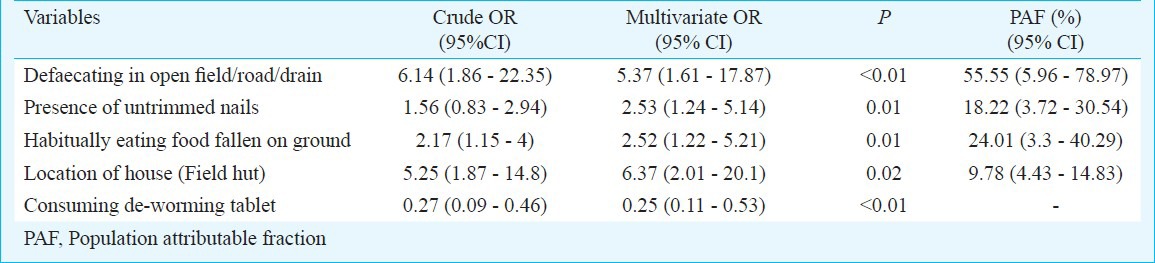
The prevalence of STH was 7.8 per cent, varying widely in schools from 0 to 20.4 per cent, in 3706 screened children. Hookworm (8.4%) rates were high in rural areas, while Ascaris (3.3%) and Trichuris (2.2%) were more prevalent among urban children. Consumption of deworming tablets (OR=0.25, P < 0.01) offered protection, while residing in a field hut (OR=6.73, P=0.02) and unhygienic practices like open air defaecation (OR=5.37, P < 0.01), keeping untrimmed nails (OR=2.53, P=0.01) or eating food fallen on the ground (OR=2.52, P=0.01) were important risk factors for STH infection.
Interpretation & conclusions:
Our study indicated that school children with specific risk factors in the studied area were vulnerable subpopulation with elevated risk of STH infection. Identifying risk factors and dynamics of transmission in vulnerable groups can help to plan for effective prevention strategies.
Keywords: India, risk factor analysis, school children, soil-transmitted helminths
The soil-transmitted helminths (STH) i.e. Ascaris lumbricoides, hookworms (Ancylostoma duodenale and Necator americanus) and Trichuris trichiura are among the most common gastrointestinal worm infestations in humans in both tropical and subtropical countries. The World Health Organization (WHO) estimates that more than two billion of the world's population is infected with STH1. A number of studies have suggested that even a moderate intensity of infection may result in delayed physical growth and impaired cognitive development, particularly among children of school-going age2,3, and STH infections are considered a leading cause of sickness, absenteeism and disability adjusted life years (DALYs) lost4. In India, the reported prevalence of STH ranges from 12.5-66 per cent, with varying prevalence rates for individual parasites5,6,7. The risk of acquiring STH infection and higher prevalence cannot be attributed to just one factor, but is due to the coexistence and amalgamation of various biological, social, behavioural and environmental factors like poverty, substandard living conditions and lack of personal hygiene, both at the individual and the community level. Studies in other tropical countries have postulated that the environment and behaviour of local residents influence the rates of infection8,9.
In 2001, the World Health Assembly passed a resolution urging the Member States to control the morbidity of STH infections through large-scale use of anthelminthic drugs for school-aged children in less developed countries10. The wide spread use of anthelminthics has shown remarkable reduction in the burden of STH in south India11. However, there is a risk of developing drug resistance as a result of frequent treatment with anthelminthics, as there is evidence of widespread drug resistance in the nematodes of livestock who undergo cycles of treatment10. Although periodic treatment with anthelminthics for the control of intestinal parasitic infection is highly effective and inexpensive, careful study of epidemiology of STHs is needed before making large scale periodic treatment schedules12. This study was undertaken to understand the current epidemiological pattern of STH and to assess the risk factors associated with STH infection in school children in southern India.
Material & Methods
Study area and population: The study was conducted in the rural areas of Kaniyambadi block and urban town area of Vellore, and Polur block of Thiruvanamalai, two adjacent districts of Tamil Nadu in south India. Vellore district covers a total of 6077 km2 area, with a population of 3,477,317 whereas Thiruvanamalai district covers a total of 6191 km2 area with a population of 2,186,125. Approximately, 17.1 per cent of the total population in the two districts were school aged children13.
Screening for STH: Assessment of the prevalence of STH infection was undertaken as part of a partially WHO funded multi-country evaluation of the efficacy of albendazole in STH treatment14. Between December 2008 and August 2009, children aged 6-14 yr, studying in 33 randomly selected government and government aided schools (15 from Vellore and 18 from Thiruvanamalai) were screened for the presence of STH in their stool samples. The schools which were located under the jurisdiction of a Municipal Corporation were considered urban and those under a Gram panchayat were considered rural. Each school catered to a unique geographic location of one village/area. Permission for the survey was obtained from the educational authorities of the two districts and the school administration, following which health education about STHs, transmission, ill effects and prevention was given to all the children studying in the participating schools. After the educational programme, a participant information sheet and an informed consent form in Tamil were provided to all eligible children. A child was enrolled into the study only after at least one parent provided written informed consent. The study protocol was approved by the Institutional Review Board of Christian Medical College (CMC), Vellore.
All enrolled children were provided with a screw-capped plastic container to collect their stool sample. The following day, a field worker visited the child's home to collect the container. All stool samples were transferred to the laboratory at the Christian Medical College, Vellore, within 4 h of collection.
Laboratory procedure: Saline and iodine wet preparations were examined for the presence of nematode ova. All positive stool samples were re-examined by the McMaster egg counting technique to quantify the number of eggs per gram of stool15.
Case-control study: Risk factors for STH infection were assessed in a sub-sample of 180 children. Any child with an egg count of >150 eggs per gram faeces (EPG) was considered a case, while controls were children with no helminthic eggs in their stool. As this study was the part of a multi-country evaluation of the efficacy of albendazole in STH treatment, the cut-off of >150 EPG was fixed as recommended in the anthelminthic resistance guidelines of World Association for Advancement of Veterinary Parasitology16. A total of 90 cases were randomly selected from the list of children who fulfilled the case status and 90 controls were randomly selected after matching for the district and residence in rural/urban area. Parents/guardians of these children were interviewed using structured questionnaires developed for the study and piloted before use after obtaining consent.
Statistical analysis: Data were entered in Epi-Info 2002 (CDC, Atlanta, GA) and analyzed using STATA 10.0 (STATA Corp. TX, USA) software. The overall and individual prevalence rates (PR) were calculated and compared between districts and rural and urban areas using χ2 tests. Co-linearity between exposure variables was assessed using χ2 tests. Confidence intervals (95% CI) were calculated using the Taylor linearized method17, which takes into account of potential effect of clustering due to the nature of sampling scheme. Logistic regression analysis was performed to assess the risk factors for acquisition of STH infection. At first, the effect of each exposure variable on the outcome was assessed using univariate logistic regression analysis. The variables significant at P=0.20 level were then considered for the multivariate analysis using a backward step-wise model. Both univariate and multivariate analyses were done for STH infection among cases and controls (n=180). Variables with P < 0.05 were considered significant and were retained in the final model. The population attributable fraction (PAF) along with 95% CI was calculated using the maximum likelihood estimation method18. The PAF shows the burden of infection that can be prevented by removal or modification of the risk factor.
Results
A total of 3706 children from the 33 schools were screened, of whom 290 (PR, 95% CI: 7.8%, 5.3-10.4%) children had STH infection. Two hundred and thirty three children were positive for hookworm (PR, 95% CI: 6.3%, 3.5-9%), whereas 45 children were positive for A. lumbricoides (PR, 95% CI: 1.2%, 0.3-2.1%) and 30 for T. trichiura (PR, 95% CI: 0.8%, 0.1-1.5%), respectively. There was no significant difference in the overall prevalence rates of STH; however, the prevalence rates of the individual parasites between Vellore and Thiruvanamalai districts were significantly different (Table I). The prevalence (95% CI) of STH in rural areas was 9 per cent (5.9-12.1%), whereas in the urban areas it was 4.8 per cent (1.6-8.0%); this difference was not significant. Prevalence of individual helminths differed significant between rural and urban areas. Hookworm infestations were more prevalent among rural children, whereas A. lumbricoides and T. trichiura were more prevalent among urban children (Table II). Also, there was a wide variation in the prevalence of STH across various schools ranging from 0-20.4 per cent, suggestive of a strong cluster effect (Fig.).
Table I.
Prevalence rates of total and individual soil-transmitted helminths (STH) in Vellore and Thiruvanamalai districts
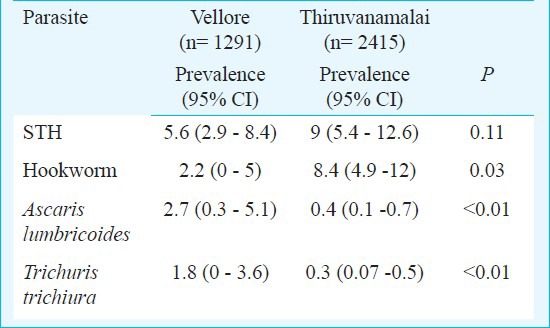
Table II.
Prevalence rates of total and individual soil-transmitted heminths (STH) in rural and urban area
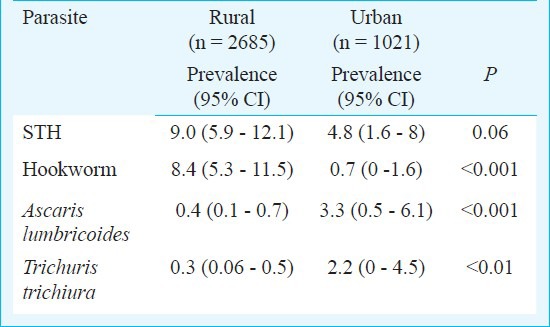
Fig.
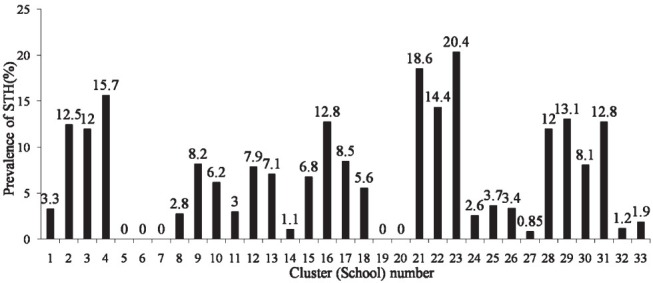
School specific prevalence rates of soil transmitted helminths (STH) across 33 schools in Vellore and Thiruvanamalai districts. Design effect for STH = 8.08.
Of the 166 children who had faecal egg counts of >150 EPG, 90 (54.2%) were selected as cases. Similarly, 90 (2.6%) of 3416 children without any helminthic ova in their stool were selected as controls. The commonest helminth identified from cases were hookworms (n=67, 37.2%), followed by A. lumbricoides (n=18, 10.0%) and T. trichiura (n=16, 8.9%). The mean (SD) age of cases and controls was 11.7 (2.5) and 11.2 (2.2) years, respectively. There were more males in the cases (63.3 versus 52.2%), but this difference was not significant.
In the univariate analysis, poor living conditions, i.e., a “kuccha” house (house with thatched roof) a house with cow dung flooring or residing in a “field hut” (thatched house surrounded by agricultural land, away from village residential areas), and improper hygienic and sanitary practices like habitually eating food that has fallen on the ground or open air defaecation were found to be significant risk factors. On the other hand, consumption of deworming tablets was associated with significant protection from the risk of acquiring STHs (Table III). The consumption of deworming tablet was ascertained by asking parents if the child had consumed a deworming tablet in the previous six months.
Table III.
Unmatched univariate analysis for risk factors of STH
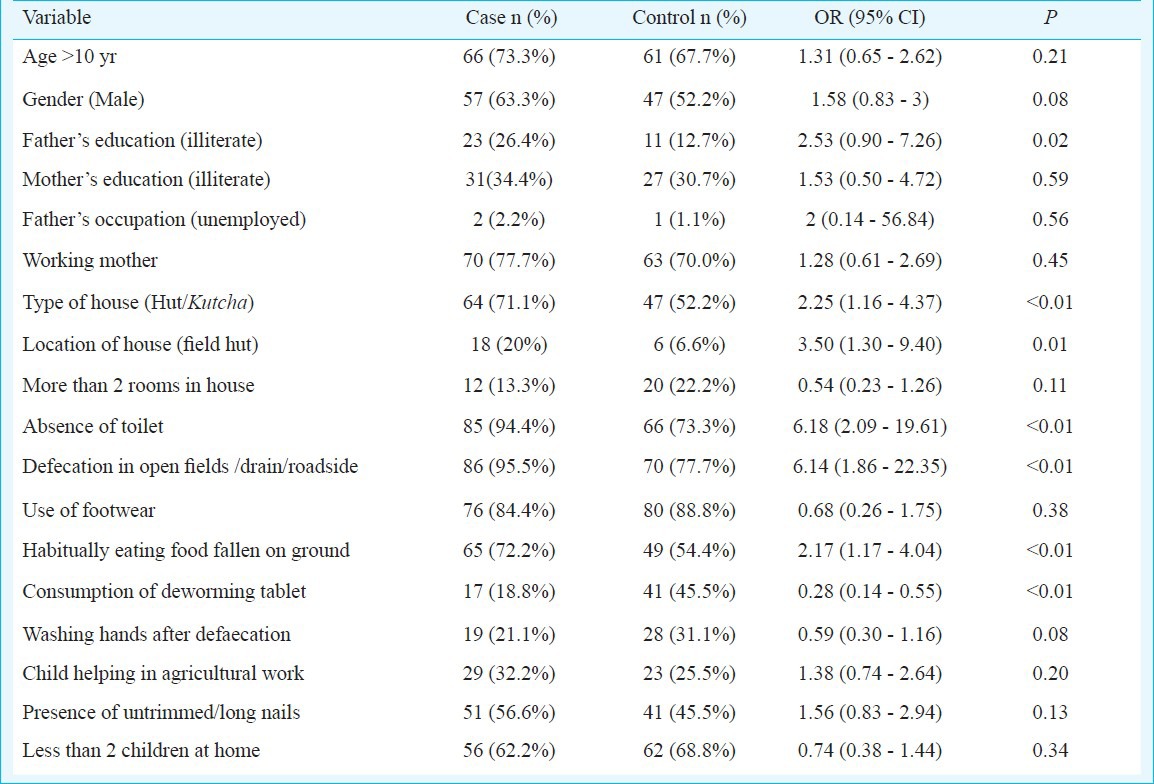
In the multivariate analysis, residing in a field-hut, open air defaecation, habitually eating food that has fallen on the ground remained independent risk factors for acquiring STH infection and consumption of deworming tablets remained protective (Table IV). The PAF for open air defaecation was highest at 55.55 per cent (5.96-78.97%), followed by eating food fallen on the ground 24.01 per cent (3.3-40.29%), the presence of untrimmed nails 18.22 per cent (3.72-30.54%), and residing in fields huts 9.78 per cent (4.43-14.83%).
Table IV.
Multivariate logistic regression analysis for significant risk factors of soil transmitted helminths (STH)
Discussion
The epidemiology of STH in India is changing. An earlier study from Vellore found 22.8 per cent of all stool samples positive for hookworm and 0.8 per cent positive for Ascaris19. Another study on children aged 9-10 yr, conducted in the same region during 2000-2003, reported a prevalence of 60 per cent11. Contrary to these findings, the prevalence estimated from the current study was much lower at 7.8 per cent. This could partially be attributed to a single-day mass drug administration campaign introduced in specific districts of Tamil Nadu from 2001 during which 100 mg diethylcarbamazine and 400 mg albendazole were co-administered to all residents of the district annually20. Another reason for this could be an underestimation of prevalence due to the use of a less sensitive screening technique such as wet smear. This could be one of the limitations of our study, although the previous higher estimates from Vellore were based on similar laboratory methods.
Data comparing STH in urban and rural settings are very few, and those that exist indicate a complicated picture. In this study, a clear rural-urban difference in the prevalence of STH was observed, with Ascaris and Trichuris more prevalent among urban children and hookworm more among rural children. It has been suggested that A. lumbricoides and T. trichiura are more prevalent in urban areas21, and that the higher prevalence can be attributed to overcrowding, lack of adequate water and improper sanitation22.
STH infection can occur in the entire community or in a few individuals as clusters. In this study, differences in the prevalence of STH across schools were noted, suggesting a strong cluster effect. In an Ethiopian study, it was hypothesized that the differences in prevalence among different communities might be associated with environmental sanitation, water supply, and the socio-economic status of the households23. This phenomenon of clustering can hamper attempts to control the infection, as individuals with heavy infection are likely to reintroduce it into the community. Although mass drug administration is the cornerstone of disease control programmes, additional measures like changes in the behavioural, social, economic and environmental factors are also required to bring about a reduction in their prevalence24.
In this study, living in a field hut was found to be an important risk factor for acquisition of STH infection, with children residing in such huts six times more likely to be infected than others. In rural south India, people residing in huts in the fields, far away from the main village are socio-economically deprived, and children walk barefoot through “faecal fields” that surround the village because open air defaecation is a common practice25. In a study from Turkey, children living in shanty areas had a higher risk of STH infection than those living in towns3. Another study from Egypt found that children living in villages near a river or desert, considered to have low levels of services such as sewage, garbage disposal and water supply, had a higher risk of infection than town residents2. In the present study, defaecating in open fields was found to be a major risk factor for STH infection. From the PAF value, one can expect 55.55 per cent reduction in the acquisition of infection if this practice is abandoned. It has been observed that even if only a few individuals in the household used a toilet, the contamination of immediate environment and farmlands surrounding the household was less intense than around households without a functional toilet26.
It has been postulated that intestinal parasites spread through poor hygienic practices, evidenced by contaminated finger nails and unclean hands27,28. In this study, children with long/untrimmed nails were found to have a higher risk of STH infection. Also, it was noted that children who habitually picked up and ate food from the ground had a higher risk of STH infection, again highlighting the importance of hygiene in preventing infection. Treatment with anthelminthic drugs reduces the transmissibility of the parasite by reducing worm load and shedding of eggs29, with a single dose of anthelminthics resulting in cure rates of 88 per cent for A. lumbricoides and 78 per cent for hookworm30. A study on the efficacy of a mass drug administration programme from south India revealed that periodically administering albendazole reduced the STH burden by 77 per cent11. In this study, children reporting consuming deworming tablets had 75 per cent protection against STH infection.
Identification of high risk pockets and locally relevant risk factors could provide vital clues to the transmission of a pathogen in the community, and help formulate a more focused preventive approach. Although, mass drug treatment with albendazole was initiated in 2001 in Vellore district, our study identified school children with specific risk factors as vulnerable sub-populations with an elevated risk of STH infection. Strategic planning and targeting the at-risk groups with concerted health education and awareness campaigns could go a long way in reducing the burden of STH in such communities.
References
- 1.Geneva: WHO; 2012. World Health Organization (WHO). Eliminating soil transmitted helminthiases as a public health problem in children. Progress Report 2001-2010 and Strategic Plan 2011-2020. [Google Scholar]
- 2.Curtale F, Pezzotti P, Saad YS, Aloi A. An analysis of individual, household, and environmental risk factors for intestinal helminth infection among children in Qena Governorate, Upper Egypt. J Trop Pediatr. 1999;45:14–7. doi: 10.1093/tropej/45.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Ostan I, Kilimcioglu AA, Girginkardesler N, Ozyurt BC, Limoncu ME, Ok UZ. Health inequities: lower socio-economic conditions and higher incidences of intestinal parasites. BMC Public Health. 2007;7:342. doi: 10.1186/1471-2458-7-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtale F, Pezzotti P, Sharbini AL, al Maadat H, Ingrosso P, Saad YS, et al. Knowledge, perceptions and behaviour of mothers toward intestinal helminths in Upper Egypt: implications for control. Health Policy Plan. 1998;13:423–32. doi: 10.1093/heapol/13.4.423. [DOI] [PubMed] [Google Scholar]
- 5.Ramesh GN, Malla N, Raju GS, Sehgal R, Ganguly NK, Mahajan RC, et al. Epidemiological study of parasitic infestations in lower socio-economic group in Chandigarh (north India) Indian J Med Res. 1991;93:47–50. [PubMed] [Google Scholar]
- 6.Singh P, Gupta ML, Thakur TS, Vaidya NK. Intestinal parasitism in Himachal Pradesh. Indian J Med Sci. 1991;45:201–4. 200. [PubMed] [Google Scholar]
- 7.Singh S, Raju GV, Samantaray JC. Parasitic gut flora in a north Indian population with gastrointestinal symptoms. Trop Gastroenterol. 1993;14:104–8. [PubMed] [Google Scholar]
- 8.Mahfouz AA, el-Morshedy H, Farghaly A, Khalil A. Ecological determinants of intestinal parasitic infections among pre-school children in an urban squatter settlement of Egypt. J Trop Pediatr. 1997;43:341–4. doi: 10.1093/tropej/43.6.341. [DOI] [PubMed] [Google Scholar]
- 9.Tomono N, Anantaphruti MT, Jongsuksuntigul P, Thongthien P, Leerapan P, Silapharatsamee Y, et al. Risk factors of helminthiases among school children in southern Thailand. Southeast Asian J Trop Med Public Health. 2003;34:264–8. [PubMed] [Google Scholar]
- 10.Horton J. Global anthelmintic chemotherapy programs: learning from history. Trends Parasitol. 2003;19:405–9. doi: 10.1016/s1471-4922(03)00171-5. [DOI] [PubMed] [Google Scholar]
- 11.Rajendran R, Sunish IP, Mani TR, Munirathinam A, Arunachalam N, Satyanarayana K, et al. Community-based study to assess the efficacy of DEC plus ALB against DEC alone on bancroftian filarial infection in endemic areas in Tamil Nadu, south India. Trop Med Int Health. 2006;11:851–61. doi: 10.1111/j.1365-3156.2006.01625.x. [DOI] [PubMed] [Google Scholar]
- 12.Fallah M, Mirarab A, Jamalian F, Ghaderi A. Evaluation of two years of mass chemotherapy against ascariasis in Hamadan, Islamic Republic of Iran. Bull World Health Organ. 2002;80:399–402. [PMC free article] [PubMed] [Google Scholar]
- 13.Chennai: State Project Directorate, Sarva Shiksha Abhiyan; 2011. [accessed on January 30, 2011]. Sarva Shiksha Abhiyan. General Educational Statistics of Tamil Nadu. Available from: http://www.ssa.tn.nic.in/Statistics.htm . [Google Scholar]
- 14.Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, Bethony JM, et al. Assessment of the antihelminthic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis. 2011;5:e948. doi: 10.1371/journal.pntd.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levecke B, Behnke JM, Ajjampur SS, Albonico M, Ame SM, Charlier J, et al. A comparison of the sensitivity and fecal egg counts of the McMaster egg counting and Kato-Katz thick smear methods for soil-transmitted helminths. PLoS Negl Trop Dis. 2011;5:e1201. doi: 10.1371/journal.pntd.0001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levecke B, De Wilde N, Vandenhoute E, Vercruysse J. Field validity and feasibility of four techniques for the detection of Trichuris in simians: a model for monitoring drug efficacy in public health? PLoS Negl Trop Dis. 2009;3:e366. doi: 10.1371/journal.pntd.0000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreuter F, Valliant R. A survey on survey statistics: what is done and can be done in Stata. Stata J. 2007;7:1–21. [Google Scholar]
- 18.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49:865–72. [PubMed] [Google Scholar]
- 19.Kang G, Mathew MS, Rajan DP, Daniel JD, Mathan MM, Mathan VI, et al. Prevalence of intestinal parasites in rural southern Indians. Trop Med Int Health. 1998;3:70–5. doi: 10.1046/j.1365-3156.1998.00175.x. [DOI] [PubMed] [Google Scholar]
- 20.Sunish IP, Rajendran R, Mani TR, Munirathinam A, Dash AP, Tyagi BK. Vector control complements mass drug administration against bancroftian filariasis in Tirukoilur, India. Bull World Health Organ. 2007;85:138–45. doi: 10.2471/BLT.06.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crompton DW, Savioli L. Intestinal parasitic infections and urbanization. Bull World Health Organ. 1993;71:1–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–61. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alemu A, Atnafu A, Addis Z, Shiferaw Y, Teklu T, Mathewos B, et al. Soil transmitted helminths and Schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. BMC Infect Dis. 2011;11:189. doi: 10.1186/1471-2334-11-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lansdown R, Ledward A, Hall A, Issae W, Yona E, Matulu J, et al. Schistosomiasis, helminth infection and health education in Tanzania: achieving behaviour change in primary schools. Health Educ Res. 2002;17:425–33. doi: 10.1093/her/17.4.425. [DOI] [PubMed] [Google Scholar]
- 25.Gopal S, Sarkar R, Banda K, Govindarajan J, Harijan BB, Jeyakumar MB, et al. Study of water supply & sanitation practices in India using geographic information systems: some design & other considerations in a village setting. Indian J Med Res. 2009;129:233–41. [PubMed] [Google Scholar]
- 26.Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: Systematic review and meta-analysis. PLoS Med. 2012;9:e1001162. doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ismid S, Rukmono B. collected papers on the control of soil transmitted helminthiases II. Vol. 5. Tokyo: Asian Parasite Control Organisation; 1983. Nail and dust examination for helminth eggs in orphanages; pp. 1–53. [Google Scholar]
- 28.Hoa NTV, Noda S, Uga S, Thuan LK, Aoki Y, Fujimaki Y. Parasite egg contamination of hands in a suburban area of Hanoi, Vietnam. Trop Med Health. 2010;38:75–9. [Google Scholar]
- 29.Geneva: WHO; 2002. World Health Organization (WHO). The World Health Report 2002. Reducing risk, promoting healthy life. [Google Scholar]
- 30.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–48. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]


