Abstract
Background & objectives:
Geographical variations are known to influence different aspects of endophthalmitis. We report the epidemiological, clinical and microbiological profile of patients with infectious endophthalmitis presented to a tertiary eye care centre in Odisha, India, and compare the results with published reports from other parts of India.
Methods:
Retrospective review of medical records of 107 patients, seen between December 2006 and January 2009 was done. All patients had undergone parsplana vitrectomy with intraocular antibiotics and the management was based on microbiological analysis of the vitreous fluid.
Results:
Forty six (43.0%) patients had post-operative (PO), 43 had post-traumatic (PT) and 18 (16.8%) had endogenous (EG) endophthalmitis. Males were predominant in all three types of endophthalmitis. Significantly younger individuals constituted PT group. While culture established microbial diagnosis in 45 patients (42%), direct microscopy was positive in 38 patients (35.5%). Fungal aetiology was found in 13 patients (PO-7, PT-4, EG-2) and bacteria accounted for 32. Similar to studies from north, central and south India, fungi and Gram-negative bacteria accounted for a large number of PO endophthalmitis cases. Two PT patients had polymicrobial infection. All Gram-positive bacteria were susceptible to vancomycin. Susceptibility to ceftazidime was variable among the Gram-negative bacteria. Best corrected visual acuity (BCVA) at presentation was less than 20/200 in majority (93%) of the patients. While the treatment outcome was variable in fungal and sterile endophthalmitis, the BCVA was either unchanged or improved in 100 per cent of bacterial endophthalmitis patients.
Interpretation & conclusions:
The spectrum of infection and outcome of infectious endophthalmitis in Odisha was similar to other parts of the country. Fungi and bacteria were involved in all three types of endophthalmitis. Empirical use of standard intravitreal therapy is recommended while emphasizing on vitreous biopsy for culture and sensitivity whenever possible.
Keywords: Diagnosis, endogenous, endophthalmitis, Odisha, post-operative, post-traumatic, treatment
The exogenous infective endophthalmitis occurs either after intraocular surgery or trauma and the endogenous endophthalmitis is usually the result of haematogenous spread of organisms to the eye from a site of infection elsewhere in the body or from contaminated catheters or needles. Almost all aspects of the disease have been dealt with in thousands of studies published including epidemiology, type of organisms, antibiotic susceptibility, diagnosis, prevention, treatment options, treatment outcome, etc. While majority of the reports have been from the USA, there are many reports from other countries that highlight the variation in epidemiology, organism type, their antibiotic susceptibility and treatment outcome in diverse settings1,2,3,4,5.
Several studies have been published from the premier ophthalmology institutes of India, reporting different aspects of endophthalmitis in the last two decades, most of which deal with post-operative endphthalmitis3,6,7,8. There are three reports that describe post-traumatic endophthalmitis in great detail4,9,10. These reports provide considerable insight into the clinical, epidemiological and microbiological aspects of post-operative and post-traumatic endophthalmitis, however, none of these deals with endogenous endophthalmitis. We present here the data of the first 107 patients of endophthalmitis who were referred to our tertiary eye care centre located in the State of Odisha in India. The aim was to analyze the clinical and microbiological data of patients who were clinically diagnosed as endophthalmitis on presentation. Apart from demographic features and predisposing factors, the treatment profile and outcome were described in patients with culture positive (bacterial, fungal) or negative post-operative, post-traumatic and endogenous endophthalmitis.
Material & Methods
A retrospective chart review of 107 patients who had presented in the outpatient clinic of the L.V. Prasad Eye Institute, Bhubaneswar, Odisha, India, between December 2006 and January 2009 with a clinical diagnosis of endophthalmitis was done. The study protocol was approved by the institutional review board and written informed consent was obtained from all patients. All patients had undergone complete eye examination under slit lamp biomicroscopy following collection of history and demographic details. Ultrasound B scan was done in eyes when media opacity (corneal oedema, anterior chamber exudates, vitreous exudates) precluded fundus view with indirect ophthalmoscope. Following complete pre-operative evaluation all patients were subjected to parsplana vitrectomy with intraocular antibiotics with or without corticosteroids injection. At the time of vitrectomy, undiluted vitreous sample (0.2-0.5 ml) was collected from all cases prior to intraocular injection, using a syringe attached to the vitrectomy canula and the sample was sent immediately to the microbiology laboratory. Additional surgical procedures were performed whenever indicated. The vitreous sample was processed within 30 min in the laboratory as per institutional protocol published earlier11. Drops of vitreous were used to make three smears (stained with Gram, Giemsa and Calcofluor white stain) for microscopic examination and inoculation of six culture media [5% sheep blood agar, 5% sheep blood chocolate agar, brain heart infusion (BHI) broth, thioglycollate broth, Robertson's cooked meat broth and Sabouraud dextrose agar (SDA), Hi media, Mumbai]. All media were incubated at 37°C except Sabouraud dextrose agar that was held at 27°C in BOD incubator. Chocolate agar was incubated in 5 per cent CO2. Any bacterial growth was identified using API system (bioMerieux, France) and fungal identification was based on colony characteristics and microscopic features. All culture media were held for two weeks in case of no growth before declaring the sample as sterile. Only unequivocal or significant culture results were considered11. The criteria to determine significance of a culture included (i) confluent growth in any solid media; and/or (ii) growth in more than one medium; and/or (iii) growth in one medium with presence of organism in direct microscopy. All bacterial isolates were tested for their susceptibility to a battery of antibiotics by Kirby-Bauer disc diffusion method12. Susceptibility for fungal isolates was not performed.
After collection of vitreous sample all patients received intravitreal vancomycin 1.0 mg/0.1 ml and ceftazidime 2.25 mg/0.1 ml, as per the institutional antibiotic policy. Post-operative treatment of the patients consisted of systemic and topical antibiotics. Patients with fungal infection were given intravitreal amphotericin B (5 μg/0.1 ml). The patients were examined post-operatively on days 1, 3, 7 and weekly thereafter for one month or more based on the response to therapy. Final visual acuity at not less than one month follow up was included in the analysis.
Geographical location of the patients, prior history, presenting visual acuity, type of intervention, length of follow up, microscopy and culture results of the vitreous sample, antibiotic susceptibility of the bacterial isolates and final outcome were analysed. Chi square test for proportions was applied for all comparisons and a P <0.05 was considered significant.
Results
Of the 107 patients, 43 (40.2%) were post-traumatic, 46 (43.0%) post-operative and 18 (16.8%) were endogenous. Of the 46 post-operative cases, eight (17.3%) patients had undergone cataract surgery in-house while the remaining were referred from outside. All cases of endogenous and post-traumatic endophthalmitis were referred from outside. The patients came from all over Odisha State, especially in and around Bhubaneswar situated in Khurda district. Six patients (5.6%) came from the neighbouring States of West Bengal, Assam and Andhra Pradesh.
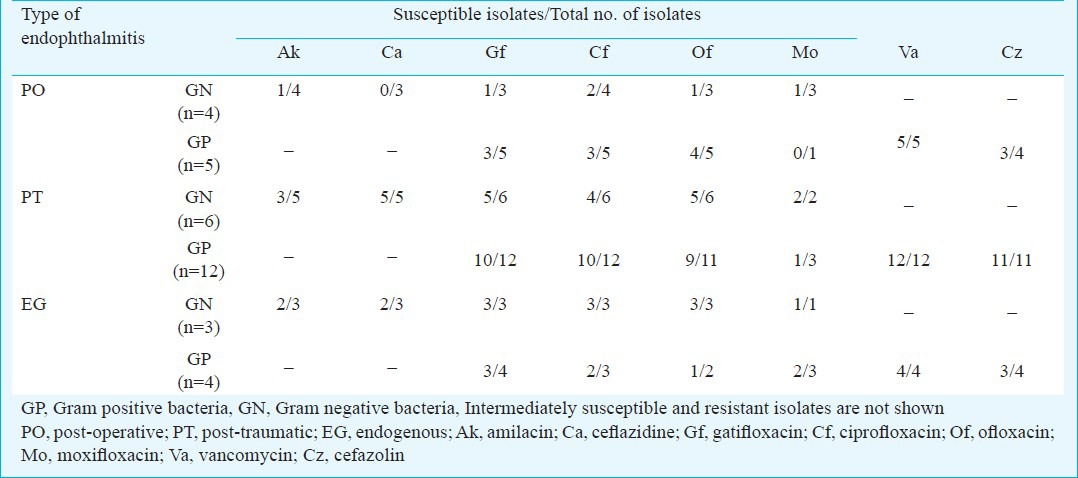
The demographic details of the patients in different groups are given in Table I. The mean age of patients with post-operative endophthalmitis at 51.8 yr was higher than that of patients with post-traumatic (22.1 yr) and endogenous endophthalmitis (30.9 yr), however, the difference was not significant. Table II provides the analysis of microscopy and culture findings of the vitreous from all patients. Distribution of types of bacteria and fungi causing endophthalmitis is given in Table III. Culture was positive in 45 patients (42%), and microscopy was positive in 38 patients (35.5%). The distribution of infecting organisms was as follows: 31 patients had monobacterial infection, 12 patients had monofungal infection and two patients had polymicrobial infection (bacteria and fungus-1, two bacterial species-1). Of the 34 bacterial isolates, 13 (38.2%) were Gram-negative and 21 (61.8%) were Gram-positive. The results of antibiotic susceptibility testing are shown in Table IV for Gram-negative and Gram-positive bacteria.
Table I.
Demographic details of 107 patients included in the study
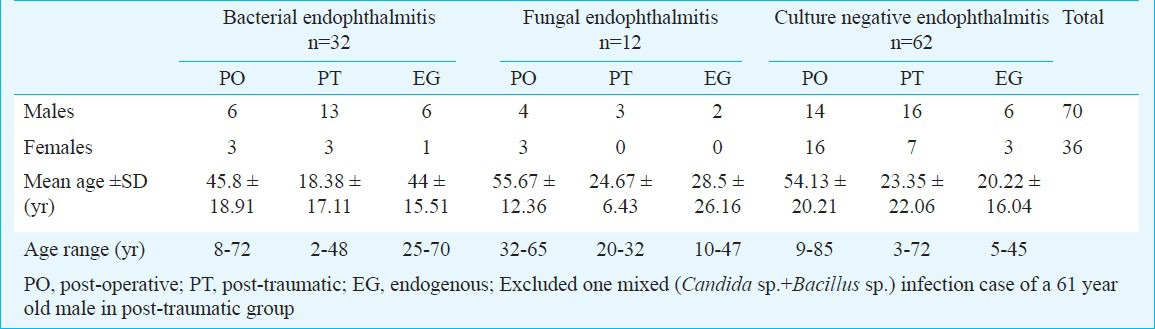
Table II.
Results of microscopy and culture of vitreous samples from all patients with endophthalmitis
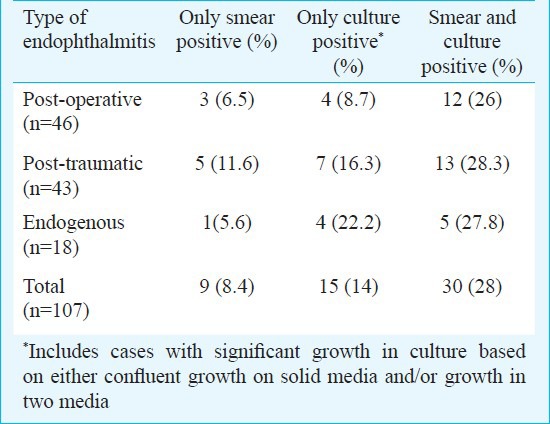
Table III.
Microbiological findings of vitreous samples from 45 patients with post-operative, post-traumatic and endogenous endophthalmitis yielding significant growth of bacteria or fungus in culture
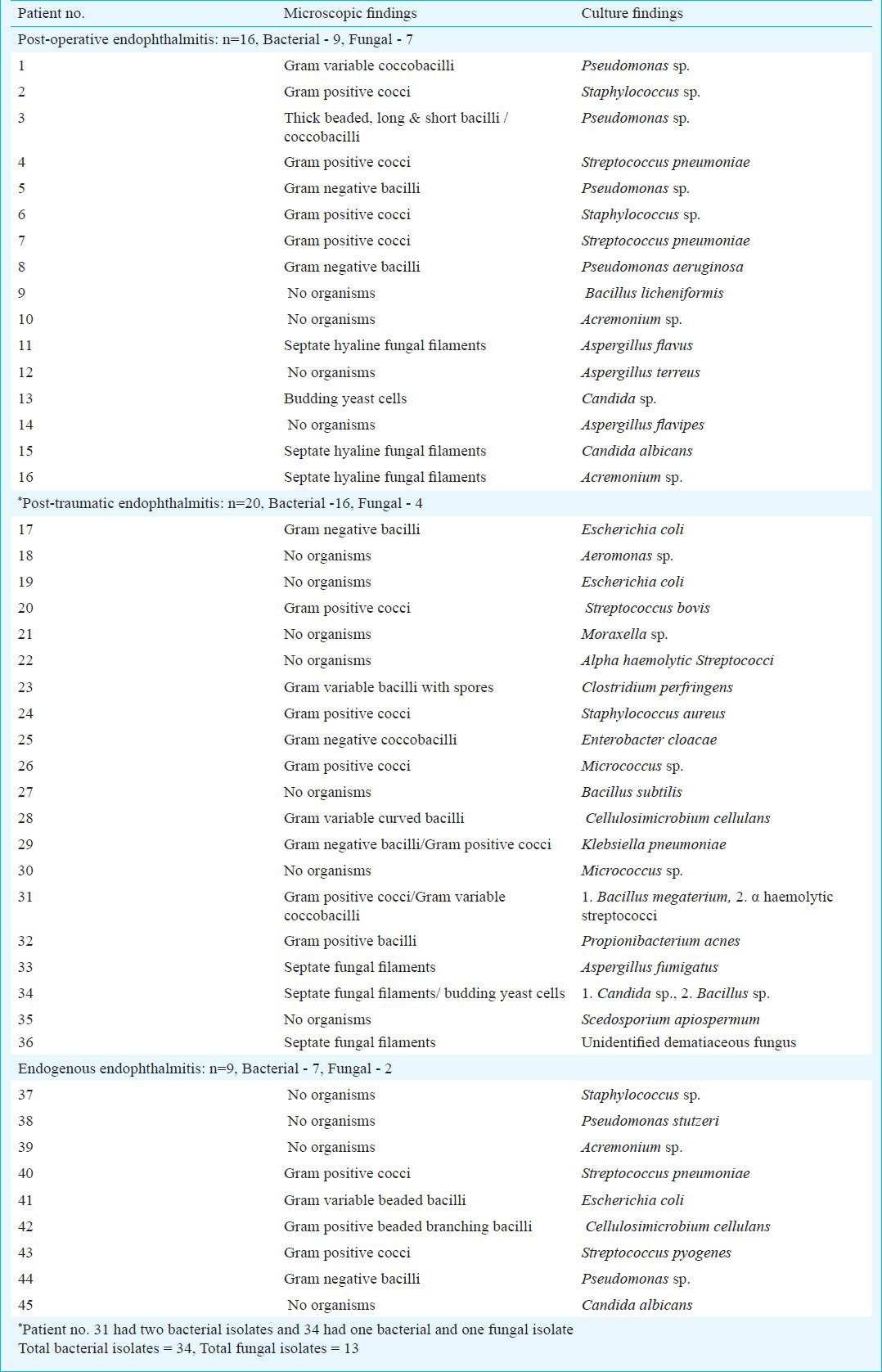
Table IV.
Antibiotic susceptibility profile of bacterial isolates from different types of endophthalmitis (n=34)
Best corrected visual acuity (BCVA) at presentation was less than 20/200 in 94 out of 101 (93%, data missing-6) patients. The presenting BCVA of the patients in various groups was compared with the final BCVA after treatment. All patients had a minimum follow up of at least one month. Thirteen patients (12.1%) were lost to follow up. Fig. 1A shows the comparison of presenting and final BCVA of 32 patients with bacterial endophthalmitis (single bacterial isolate - 31, two bacterial isolates - 1), and 12 patients with fungal endophthalmitis. One patient with mixed fungal and bacterial infection was excluded from this analysis. Although the mean presenting BCVA of patients with bacterial endophthalmitis (LogMAR 2.75) was less than the eyes with fungal endophthalmitis (LogMAR 2.35), the final visual recovery was better in bacterial than fungal (LogMAR 1.78 and 1.93, respectively) endophthalmitis. The difference was, however, not significant. Presenting and final BCVA in patients with Gram-positive (n=19) and Gram-negative (n=13) bacterial infection is shown in Fig. 1B. The pre- and post-intervention BCVA of bacterial, fungal and culture negative endophthalmitis cases for the three types of endophthalmitis are shown in Figs 2-4. In the endogenous endophthalmitis group, data on pre- and post-treatment visual acuity were available for 6 bacterial, 2 fungal and 7 culture endophthalmitis patients (Fig. 2). Post-intervention BCVA remained unchanged or improved by one line or more for 6 of 6 bacterial, 1 of 2 fungal and 6 of 7 culture negative endophthalmitis patients. Analysis of visual acuity before and after treatment, available for 43 post-operative endophthalmitis (Fig. 3) patients (bacterial-8, fungal-7, culture negative-28) showed unchanged or improved BCVA after treatment in 8 of 8 bacterial, 5 of 7 fungal and 26 of 28 culture negative endophthalmitis patients. Post-intervention BCVA data of post-traumatic endophthalmitis (Fig. 4) available in 35 cases (bacterial-9, fungal-3, culture negative- 23) showed unchanged or improved BCVA in 9 of 9 bacterial, 2 of 3 fungal and 20 of 23 culture negative endophthalmitis patients. Infection was not controlled in two patients with fungal infection and the eyes needed evisceration (PO-1, Aspergillus flavipes, PT-1, Aspergillus fumigatus). Data of two patients with polymicrobial infection in post-traumatic group are not included in the figures. The presenting BCVA in both these cases was PL PR inaccurate and remained same after treatment.
Fig. 1.
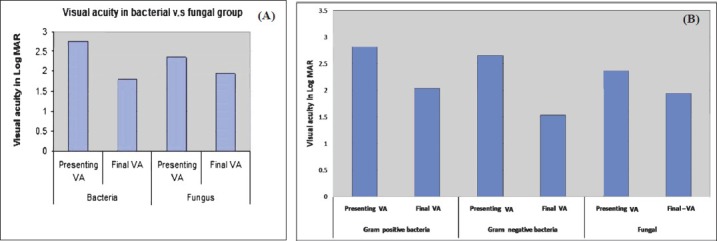
Comparison of presenting visual acuity and final visual acuity between (A) patients with bacterial (n=32), fungal (n=12), (B) Gram-negative (n=19) and Gram-positive (n=13) bacterial endophthalmitis.
Fig. 2.
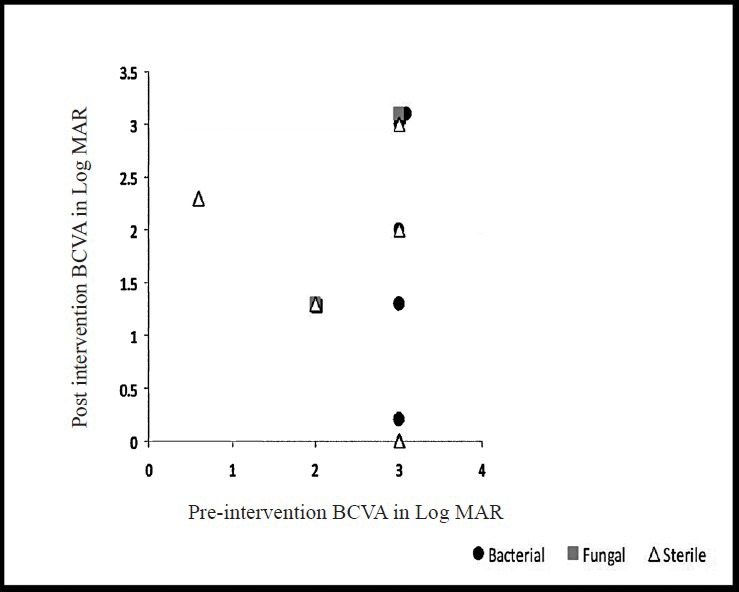
Comparison of pre- and post-intervention BCVA (LogMAR) in endogenous endophthalmitis (n=15, bacterial-6, fungal-2, sterile-7, missing data=3).
Fig. 4.
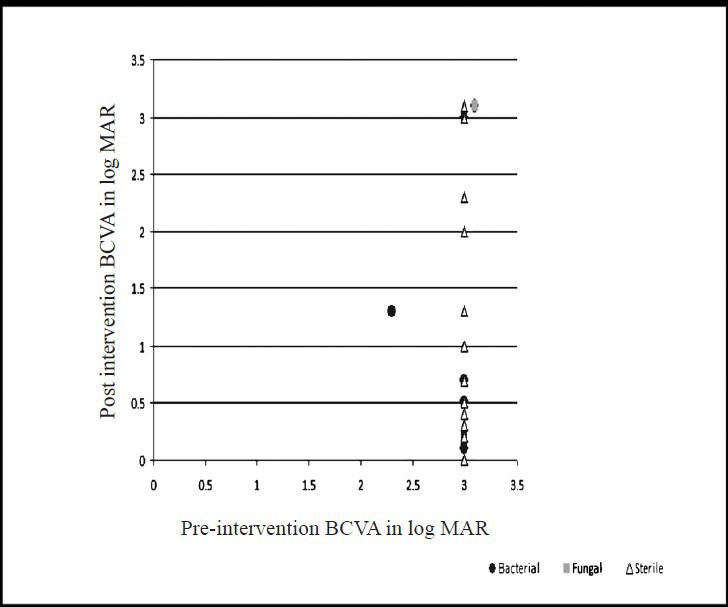
Comparison of pre-and-post intervention BCVA (LogMAR) in post-traumatic endophthalmitis (n=35, bacterial-9, fungal-3, sterile-23, missing data=8).
Fig. 3.
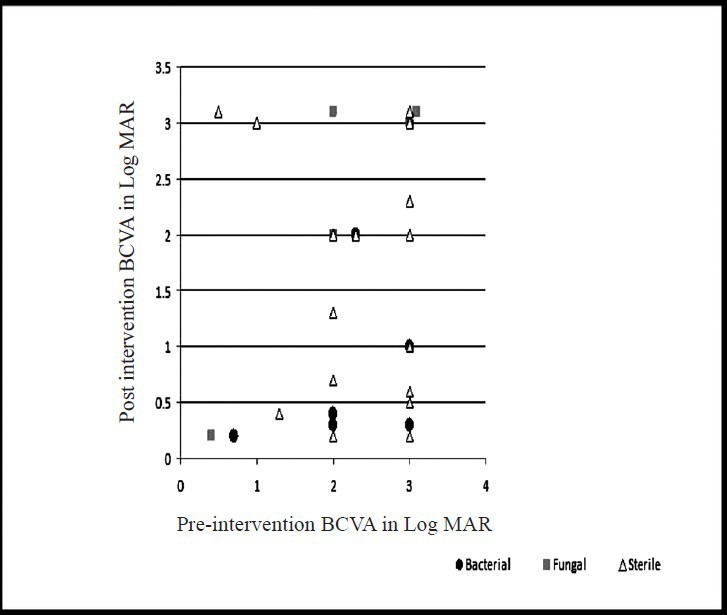
Comparison of pre- and post-intervention BCVA (LogMAR) in post-operative endophthalmitis (n=43, bacterial-8, fungal-7, sterile-28, missing data=3).
Discussion
Published in 1975, Forster et al13 described 54 cases of suspected endophthalmitis seen between July 1969 through March 1975. Several reports have been published from north, south and central India on post-operative endophthalmitis3,6,7,8, and one on post-traumatic endophthalmitis9. We have earlier reported on the independent risk factors for poor visual outcome in patients with post-operative and post-traumatic endophthalmitis in a multivariate analysis10. Of these, only two have analyzed the visual outcome of the patients after treatment7,8. The data presented here nearly represent whole of the eastern State of Odisha because all patients had been referred from various parts of Odisha except six who came from the neighbouring States. Eight patients who developed post-operative endophthalmitis after cataract surgery in this hospital also belonged to Odisha.
There were more males than females in all the groups except culture negative post-operative group. While the male predominance in post-traumatic group could be due to greater chances of exposure of males to trauma due to outdoor activities, the reason for male preponderance in other two groups could possibly be due to more males willing to travel when referred or could be socio-economic. For reasons that young males are more actively involved in outdoor activities, the mean age of patients in post-traumatic group was lower than other two groups.
The vitreous culture was positive in 42 per cent cases and direct microscopy was positive in 35.5 per cent cases. None of the patients had received intraocular antibiotics prior to presentation although some had received systemic antibiotics. Extensive variation in culture positivity in clinically diagnosed endophthalmitis cases is known and has been reported14,15,16. This varies from 38 to 44 per cent in Indian subjects with post-operative endophthalmitis6,7 and is marginally higher in post-traumatic endophthalmitis4. A variety of causes such as delay in processing the sample, low microbial load, sequestered microorganisms, prior antibiotic therapy, etc. might be responsible for low culture positivity; molecular methods such as polymerase chain reaction (PCR) have been recommended for the diagnosis of endophthalmitis. In the present study, the vitreous samples were processed immediately for microscopy and culture, ruling out delay in processing as a cause for low positivity.
The spectrum of organisms partly depends on the geographical location of the patient. The Endophthalmitis Virectomy Study (EVS)16 conducted in the United States reported 94 per cent Gram-positive cocci and 6 per cent Gram-negative bacilli in post-operative endophthalmitis; the published Indian literature reported 10-54 per cent Gram-positive cocci, 26-42 per cent Gram-negative and 16-22 per cent fungal infection in post-operative endophthalmitis3,6,7. One Indian study has reported unusually high (60%) Nocardia infection in post-operative endophthalmitis8. In an earlier study, we found the prevalence of Nocardia in post-operative endophthalmitis to be less than 1 per cent3. There were no cases of Nocardia infection in the present study, and there was almost equal involvement of bacteria and fungi in cases of post-operative endophthalmitis with high prevalence of Gram-negative bacteria. Bacteria predominated in post-traumatic and endogenous endophthalmitis groups and involved a large number of species. Fungi were isolated from all types of endophthalmitis and 9 of the 13 fungal isolates were filamentous. Polymicrobial infections have been reported more often from post-traumatic endophthalmitis4 than post-operative3,7 and two cases in this study were also in post-traumatic group.
Intravitreal antibiotics with or without vitrectomy and intravitreal corticosteroid are the current standards of care in bacterial endophthalmitis. It is also considered important to empirically inject antibiotics against Gram-positive organisms (vancomycin) and Gram-negative organisms (ceftazidime). Intravitreal antifungal medication (amphotericin B) with vitrectomy is current standard of care in fungal endophthalmitis. In this study 100 per cent of the Gram-positive bacteria were sensitive to vancomycin and the susceptibility of Gram-negative bacteria to ceftazidime was variable. Earlier studies from different parts of India have reported similar results3,4,6,7. Hence, one could expect good response in Gram-positive bacterial endophthalmitis compared to either Gram-negative or fungal infections. However, we did not find such a difference in this study. Final BCVA was in fact marginally better in patients with Gram-negative infection which may be due to the small sample size.
Compared to bacterial endophthalmitis, the treatment outcome of fungal endophthalmitis cases was poorer (though statistically not significant) in this study despite specific therapy. This is attributed in some measure to the low dosage of amphotericin B that can be safely administered intraocularly and not so favourable response of filamentous fungi to amphotericin B17,18,19. The trend of better response to treatment in bacterial endophthalmitis and poorer response of fungal endophthalmitis was seen in all types of endophthalmitis.
We have earlier reported good visual outcome in presumed non-infectious endopthalmitis20. In this study also the post-intervention BCVA was superior when the vitreous samples was negative in culture and the patients were empirically treated as bacterial endophthalmitis. This implied that while some cases might have been sterile, those infective were probably of bacterial origin.
In conclusion, this study in the Odisha State in eastern India demonstrates that the spectrum of infection and outcome following the standard of care in all forms of endophthalmitis is no different from other parts of India. The State has a peculiar climatic condition of extreme dry weather in north Odisha (similar to north India) and extreme humid weather in south coastal Odisha (similar to south coastal India). This does not seem to have affected the endophthalmitis infection spectrum unlike some of the other eye infections such as microsporidial keratoconjunctivitis unique to this region21. Hence, based on our results empirical use of standard intravitreal therapy (vancomycin and ceftazidime in bacterial endophthalmitis; amphotericin B in fungal endophthalmitis) may be recommended though vitreous biopsy for culture and sensitivity should always be insisted on.
Acknowledgment
Authors acknowledge the Hyderabad Eye Research Foundation, Hyderabad for financial assistance.
References
- 1.Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK, Doft BH, et al. Spectrum and susceptibilities of microbiologic isolates in the endophthalmitis vitrectomy study. Am J Ophthalmol. 1996;122:1–17. doi: 10.1016/s0002-9394(14)71959-2. [DOI] [PubMed] [Google Scholar]
- 2.Fisch A, Salvanet A, Prazuck T, Forestier F, Gerband L, Cosca G, et al. Epidemic of infective endophthalmitis in France. Lancet. 1991;338:1373–6. [PubMed] [Google Scholar]
- 3.Kunimoto DY, Das T, Sharma S, Jalali S, Majji A, Gopinathan U, et al. Microbiologic spectrum and susceptibility of isolates. Part I. Postoperative endophthalmitis. Am J Ophthalmol. 1999;128:240–2. doi: 10.1016/s0002-9394(99)00112-9. [DOI] [PubMed] [Google Scholar]
- 4.Kunimoto DY, Das T, Sharma S, Jalali S, Majji A, Gopinathan U, et al. Microbiologic spectrum and susceptibility of isolates. Part II. Posttraumatic endophthalmitis. Am J Ophthalmol. 1999;128:242–4. doi: 10.1016/s0002-9394(99)00113-0. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YQ, Wang WJ. Treatment outcomes after pars plana vitrectomy for endogenous endophthalmitis. Retina. 2005;25:746–50. doi: 10.1097/00006982-200509000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Anand AR, Therese L, Madhavan HN. Spectrum of etiological agent of postoperative endophthalmitis and antibiotic susceptibility of bacterial isolates. Indian J Ophthalmol. 2000;48:123–8. [PubMed] [Google Scholar]
- 7.Gupta A, Gupta V, Gupta A, Dogra MR, Panday SS, Ray P, et al. Spectrum and clinical profile of post cataract surgery endophthalmitis in north India. Indian J Ophthalmol. 2003;51:139–45. [PubMed] [Google Scholar]
- 8.Lalitha P, Rajagopalan J, Prakash K, Ramasamy K, Prajna NV, Srinivasan M. Postcataract endophthalmitis in south India: Incidence and outcome. Ophthalmology. 2005;112:1884–9. doi: 10.1016/j.ophtha.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Vasumathy V, Nirmalan PK, Ramasamy K, Prakash K, Namperumalsamy P. Clinicomicrobiological profile and visual outcome of posttraumatic endophthalmitis at a tertiary eye care centre in south India. Indian J Ophthalmol. 2006;54:5–10. doi: 10.4103/0301-4738.21607. [DOI] [PubMed] [Google Scholar]
- 10.Das T, Kunimoto DY, Sharma S, Jalali S, Majji AB, Rao TN, et al. Relationship between clinical presentation and visual outcome in postoperative and posttraumatic endophthalmitis in south central India. Indian J Ophthalmol. 2005;53:5–16. doi: 10.4103/0301-4738.15298. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Jalali S, Adiraju MV, Gopinathan U, Das T. Sensitivity and predictability of vitreous cytology, biopsy, and membrane filter culture in endophthalmitis. Retina. 1996;16:525–9. doi: 10.1097/00006982-199616060-00010. [DOI] [PubMed] [Google Scholar]
- 12.Bauer AW, Kirby WMM, Sherris JC, Truck M. Antibiotic susceptibility testing by standard single disk method. Am J Pathol. 1964;45:493–6. [PubMed] [Google Scholar]
- 13.Forster RK, Zachary IG, Cottingham AJ, Norton EWD. Further observations on the diagnosis, etilogy and treatment of endophthalmitis. Tr Am Ophthalmol Soc. 1975;73:221–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Therese KL, Anand AR, Madhavan HN. Polymerase chain reaction in the diagnosis of bacterial endophthalmitis. Br J Opthalmol. 1998;82:1078–82. doi: 10.1136/bjo.82.9.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okhravi N, Adamson P, Caroll N, Dunlop A, Matheson MM, Towler HM. PCR-based evidence of bacterial involvement in eyes with suspected intraocular infection. Invest Ophthalmol Vis Sci. 2000;41:3474–9. [PubMed] [Google Scholar]
- 16.Results of Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol. 1995;113:1479–96. [PubMed] [Google Scholar]
- 17.Marangon FB, Miller D, Giaconi JA, Alfonso EC. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004;137:820–5. doi: 10.1016/j.ajo.2003.11.078. [DOI] [PubMed] [Google Scholar]
- 18.Benz MS, Scott IU, Flynn HW, Jr, Unonius N, Miller D. Endophthalmitis isolates and antibiotic sensitivities: A six year review of culture proven cases. Am J Ophthalmol. 2004;137:38–42. doi: 10.1016/s0002-9394(03)00896-1. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Gupta V, Dogra MR, Chakrabarti A, Ray P, Ram J, et al. Fungal endophthalmitis after a single intravenous administration of presumably contaminated dextrose infusion fluid. Retina. 2000;20:262–8. [PubMed] [Google Scholar]
- 20.Jalali S, Das T, Gupta S. Presumed non-infectious endophthalmitis after cataract surgery. J Cat Refract Surg. 1996;22:1492–7. doi: 10.1016/s0886-3350(96)80153-2. [DOI] [PubMed] [Google Scholar]
- 21.Das S, Sharma S, Sahu SK, Nayak SS, Kar S. New antimicrobial spectrum of epidemic keratoconjunctivitis: clinical and laboratory aspects of an outbreak. Br J Ophthalmol. 2008;92:861–2. [PubMed] [Google Scholar]


