Abstract
Background & objectives:
The prevalence of Graves’ ophthalmopathy (GO) varies widely in different ethnic groups. Indians have been reported to have a lower prevalence of Graves’ ophthalmopathy as compared to Caucasians of European origin, but data are sparse and inconclusive. We studied the prevalence, clinical features and association of GO in Indian patients with Graves’ disease attending a referral centre in north India.
Methods:
A prospective study was conducted on 235 consecutive newly referred north Indian patients with Graves’ disease presenting to a tertiary care centre in north India. All patients underwent a comprehensive ophthalmological examination as per the European Group on Graves’ Orbitopathy (EUGOGO) recommendations.
Results:
GO was diagnosed in 65 patients (prevalence 28%; 95% confidence interval 22-33%). The prevalence was similar in males (28%) and females (27%). It was mild in 83 per cent, moderate-severe in 15 per cent and sight-threatening in only 2 per cent of cases. Ophthalmopathy was clinically active in only two (3%) cases. Upper eyelid retraction was the most common manifestation (83%), followed by exophthalmos (75%). Extra-ocular muscle involvement (5%) and optic nerve dysfunction (2%) were uncommon. The risk of GO was 3.9- fold (95% confidence interval 1.1-13.6) higher in smokers compared to non-smokers. However, severity of disease in smokers was similar to non-smokers. On multivariate logistic regression analysis, GO was associated only with high thyrotropin receptor antibody titres and current smoking.
Interpretation & conclusions:
Among north Indian patients with GD studied at a referral center, the prevalence of GO was similar to Caucasians of European descent, but clinically active and severe ophthalmopathy was uncommon. More studies are needed to confirm these findings.
Keywords: Graves’ disease, Graves’ ophthalmopathy, Indians, smoking
Graves’ disease (GD) is a common autoimmune endocrine disorder characterized by thyroid hyperplasia and excessive thyroid hormone production1. Eye involvement, known as Graves’ ophthalmopathy (GO), is the most common extra-thyroidal manifestation of GD2,3. GO results from infiltration of the orbit by auto-reactive T-lymphocytes, proliferation of orbital fibroblasts and increased orbital fat2,3. This leads to periorbital oedema, lid retraction, proptosis, diplopia and in severe cases, diminution or loss of vision. Clinically recognized GO occurs in about 25-50 smoking cases of GD in Caucasians and may be sight-threatening in 3-5 per cent2,3,4.
Environmental (smoking and radioactive iodine) and genetic factors are important in the predisposition to GO4,5,6,7. Ethnicity is likely to play an important role in the development of GO4,7,8. In a study of 155 patients with GD (116 European, 39 Indian), patients of European descent had a 6.4 times higher risk of GO compared to Indians7. The presentation of GO also varies in different ethnic groups. Upper eyelid retraction and soft tissue involvement are reported as the commonest manifestations in Caucasians, in contrast to increased risk of exophthalmos and lower lid retraction in Asians9,10,11. Studies addressing the prevalence and risk factors of GO in a large population of Indian patients with GD are not available.
In view of the paucity of data, we studied the prevalence, presentation and risk factors for GO in north Indian patients with GD.
Material & Methods
Subjects: A total of 235 consecutive adult patients with GD (age at onset ≥18 yr) who were referred to the Endocrinology Clinic at the Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, over a period of 1.5 years (April 2009 to September 2010), were enrolled. The hospital is a government-subsidized, tertiary care referral centre in north Indian State of Uttar Pradesh. A diagnosis of GD was made on the basis of clinical features of thyrotoxicosis, elevated thyroid hormones, suppressed thyrotropin (TSH) and thyroid 99mTechnetium-pertechnetate scan evidence of diffuse homogeneous increased uptake in both lobes of the thyroid. All patients provided written informed consent, and institutional ethics committee approval was obtained prior to the start of the study.
Study procedure: In each patient, the initial screening for GO was performed by an endocrinologist. Cases with any evidence of ophthalmopathy/activity were then examined in detail by an ophthalmologist for determining the clinical activity and severity. The findings were classified as per the European Group on Graves’ Orbitopathy (EUGOGO) recommendations - preliminary case record form12,13. The diagnosis of GO was based on the criteria of Bartley and Gorman14. A measurement of ≥20 mm using a Hertel's exophthlamometer was diagnosed as exophthalmos. The above cut-off has been previously validated in healthy north Indian population15. Clinical activity of GO was classified as per clinical activity score (CAS) recommended by EUGOGO12,13. A CAS of 0-2 was considered inactive and 3-7 active GO12,13,16. Severity of GO was classified into mild, moderate-severe and sight-threatening based on the EUGOGO classification13.
Assays: Serum total T3, total T4 and TSH were estimated by chemiluminescence immunoassay (Immulite 1000, Siemens, USA). Serum TSH receptor antibodies (TRAb) concentrations were determined with a second-generation enzyme immunoassay (Medizym T.R.A., Medipan GmbH, Dahlewitz/Berlin, Germany). A value of TRAb ≥1.5 IU/l was considered positive. The analytical sensitivity of the assay was 0.5 IU/l. Serum thyroid peroxidase antibodies (TPOAb) were measured using radioimmunoassay (Immunotech, Prague, Czech Republic). A cut-off value of TPOAb ≥20 IU/ml was considered positive. The analytical sensitivity was 2 IU/ml.
Statistical analysis: Continuous variables are reported as mean ± standard deviation (SD) or median (inter-quartile range). The Student's t test or Mann-Whitney U test was used for comparison of continuous variables as appropriate. The chi-square test was used to compare categorical variables. Univariate binary logistic regression analysis was performed to study the presence or absence of GO (dependent variable) with various risk factors including age at onset of GD, gender, current smoking (in males only), domestic smoke exposure, serum T4 and T3, TRAb titre and TPOAb titre. Risk factors were analyzed as follows: age at onset (in units of 10 years), T4 (units of 10 nmol/l), T3 (units of 1 nmol/l), TRAb and TPOAb (highest vs. lowest quartile). Variables which were significant in univariate analysis were included in a forward multiple logistic regression analysis. A two-tailed P value <0.05 was considered significant. Statistical analyses were performed using the SPSS software package (version 15.0; SPSS Inc., Chicago, IL, USA).
Results
The baseline characteristics of the patients are summarized in Table I. All patients belonged to the State of Uttar Pradesh or adjoining regions. The patients had a median age at onset of 36 yr (inter-quartile range 29-45 yr) and 161 (69%) were females. Thirteen (18%) males were current smokers (median 5 pack-years, range 2-45 pack-years) while five were ex-smokers (stopped for >12 months). None of the females gave a history of smoking. Environmental smoke exposure due to domestic wood fuel use was elicited in 40 (17%) of patients. GD was newly diagnosed in 53 (23%) patients, 81 (34%) had a duration <6 months and 101 (43%) patients presented with relapse of GD. Most patients 231 (98%) were on carbimazole and only 2 (1%) had received radioactive iodine. At the time of enrolment, 178 (76%) patients were thyrotoxic, 47 (20%) euthyroid and 10 (4%) hypothyroid on therapy. Graves’ dermopathy and acropachy were rare (3 and 1 patient, respectively). Associated autoimmune disease was present in 7 (3%) and family history of autoimmune thyroid disease in 22 (9%) cases.
Table I.
Characteristics of Graves’ disease (GD) with and without ophthalmopathy
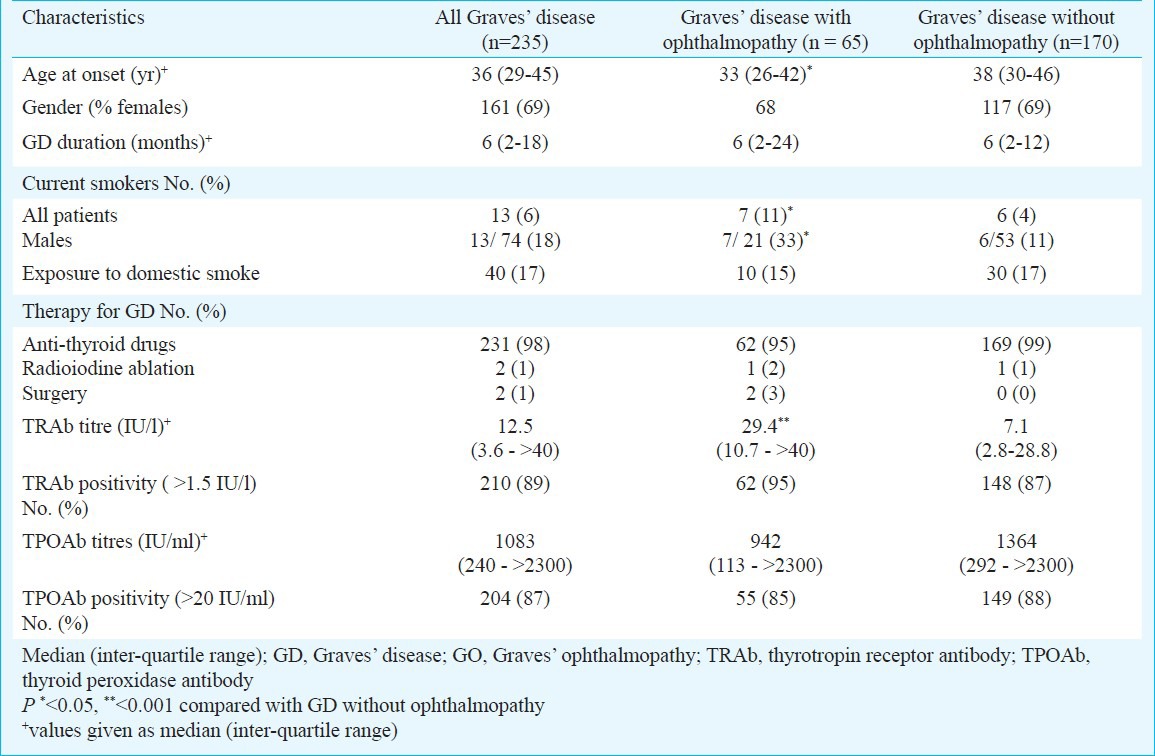
GO was present in 65 patients (prevalence 28%, 95% CI 22-33%). If lower lid retraction was also included as a diagnostic criterion, the number of subjects with GO (n=75) increased to 32 per cent. The prevalence was similar in males (28%, 95% CI 18-39%) and females (27%; 95% CI 20-34%). The female: male ratio in GO was 2.1:1, which was similar to the ratio in GD (2.2:1).
Subjects with GO had an earlier age at onset of GD as compared with patients without GO (33 vs. 38 yr, P <0.05) (Table I). The onset of GO was simultaneous with onset of GD in 49 (75%), within the preceding 12 months in 6 (10%) and after 12 months in 10 (15%) of cases.
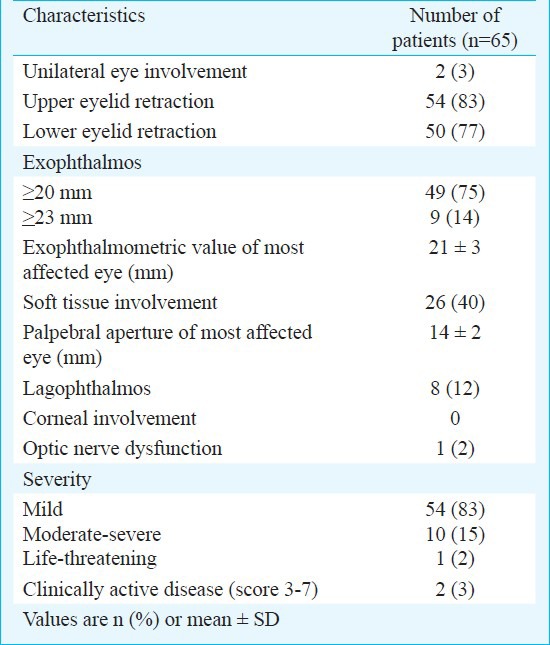
The ophthalmological findings in patients with GO are shown in Table II. GO was bilateral in 97 per cent (n=63) of patients. Upper eyelid retraction was the most common manifestation 54 (83%), followed by exophthalmos 49 (75%) and soft tissue involvement 26 (40%). Extra-ocular muscle involvement 3 (5%) and optic nerve dysfunction 1 (2%) were uncommon. GO was mild in 54 cases (83%), moderate-severe in 10 (15%) and sight-threatening (due to optic nerve dysfunction) in only 1 patient (2%). Most patients 63 (97%) had clinically inactive GO, of whom 32 (49%) had a CAS of 1-2. Only two patients (3%) had active GO with CAS 4 and 5 and required immunosuppressive therapy with systemic steroids.
Table II.
Clinical characteristics of Graves’ ophthalmopathy in north Indians
TRAb and TPO antibodies: TRAb were detected in 210 (89%) GD patients (Table I). Median TRAb titres were significantly higher in patients with GO as compared to those without GO (29.4 IU/l vs. 7.1 IU/l, P <0.001). The prevalence of GO progressively increased with increasing TRAb titres (Figure). However, median TRAb titres did not differ between patients with moderate-severe and mild disease (18.3 vs. 29.6 IU/l). TPOAb positivity was detected in 87 per cent of patients with GD. Median titers of TPOAb were similar in patients with or without GO (Table I).
Fig.
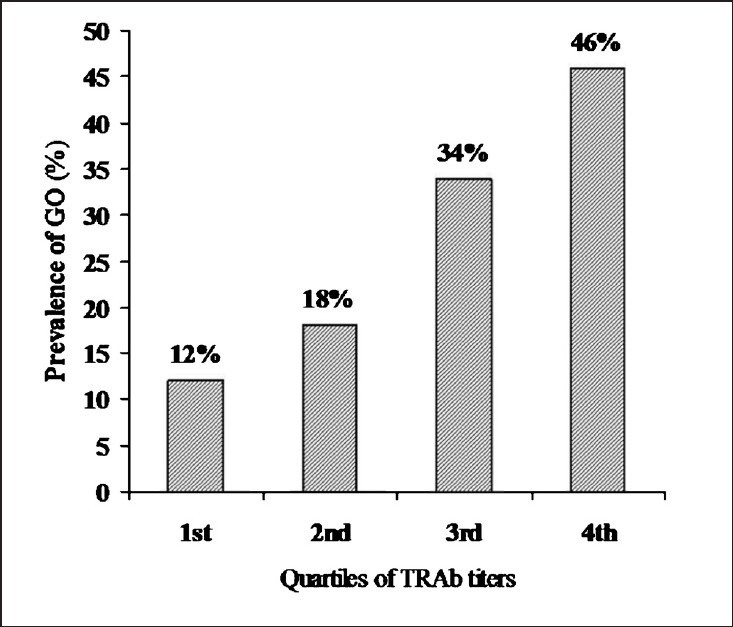
Prevalence of Graves’ ophthalmopathy in relation to TRAb titres. GO, Graves’ ophthalmopathy; TRAb, thyrotropin receptor antibodies; TRAb titres: Quartiles: <3.5 IU/l, 3.5-12.5 IU/l, 12.5-40 IU/l, >40 IU/l. Prevalence in quartile, P <0.01 (chi-square test).
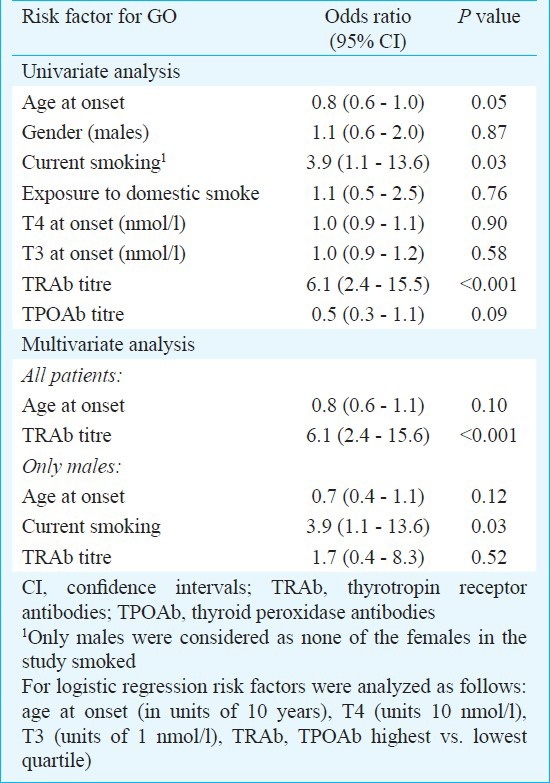
Risk factors for GO: The prevalence of GO in males was higher in current smokers (i.e. within 1 year of assessment of > 2 pack-years) than non-smokers (56 vs. 23%, P <0.05). Current smoking (i.e. within 1 year of assessment of > 2 pack-years) was associated with a 3.9-fold (95% CI 1.1-13.6) risk of GO. However, the severity of GO did not differ between smokers and non-smokers (prevalence of moderate-severe GO 14 vs.17%, respectively). Also, there was no association of wood smoke inhalation and GO. On univariate logistic regression analysis, taking GO as a categorical variable, a younger age at onset of GD, TRAb titre, and current smoking (only assessed in males) were positively associated with GO (Table III). On multivariate logistic regression analysis, which included all patients with GD, only TRAb titres were positively associated with GO (OR for highest vs. lowest quartile 6.1, 95% CI 2.4 -15.6, P <0.001). On a multivariate analysis performed only in males, current smokers had a significantly higher risk of GO vs. non-smokers (OR 3.9; 95% CI 1.1-13.6, P=0.03).
Table III.
Logistic regression analysis of Graves’ ophthalmopathy (GO) in north Indians with Graves’ disease
Discussion
In the current study of 235 consecutive north Indian subjects with GD presenting to a single center, the prevalence of GO was similar to that described in Caucasians of European descent7, but the disease was clinically inactive and of mild severity in most instances.
Among patients with GD, the prevalence of GO varies widely in different ethnic groups, with frequencies ranging from 8-56 per cent7,8,11,17. These studies have used different clinical settings and diagnostic criteria for GO and comparisons between studies must be interpreted cautiously. The prevalence of GO documented in our study (28%), was similar to previous studies in white patients (25-50%)7,17. In a previous study from the United Kingdom which included 39 Indians, a lower prevalence of GO was reported in Indians (8%) as compared to Europeans (42%)7. In a retrospective study on surgically treated Indian patients with GD, GO was noted in 58 per cent18. However, there was a significant bias in this study as GO formed a major indication for thyroid surgery. In another report, the prevalence of GO was 40 per cent among Indians residing in Malaysia. However, only 10 Indian subjects were included in this study11.
In our study the disease was mild in most patients with GO (83%) and of moderate or sight-threatening severity was seen in only 15 and 2 per cent patients, respectively. Only 3 per cent patients had clinically active disease requiring immunosuppressive therapy. In contrast, the EUGOGO described 152 European patients with GO where 28 per cent had severe and 33 per cent moderate disease10. The disease was clinically active in 60 per cent. In a report of 159 German patients with GO followed for 12 months, 53 per cent had severe disease and 84 per cent were treated with steroids19. Possible explanations for the reduced severity of GO in our patients could be lower prevalence of smoking and prior radioactive iodine use. However, since smokers in our study had a similar severity of GO compared to non-smokers, genetic susceptibility is also likely to play a role. It is unlikely that the decreased severity is due to a referral bias since all patients seen in the ophthalmology department were also referred to the Endocrinology clinic. The severity of GO/CAS is known to vary with the duration of the disease. However, since most of our patients were unable to provide an accurate time of onset of eye symptoms, we did not include this variable in our analysis for CAS.
Variability in clinical presentation of GO has been noted in different ethnic groups. Upper eyelid retraction, exophthalmos and soft tissue involvement were the common manifestations of GO in our study, which was consistent with a prior study in Indians20. Diplopia (5%) was uncommon in our study when compared to a previous report in Caucasians9. In a multi-ethnic Asian study, exophthalmos and lower lid retraction were the most common signs11. In contrast, soft tissue inflammatory signs were observed with greatest frequency (75%) in the EUGOGO study10. The reason for this variability is unclear.
Among various environmental factors associated with increased risk of GO, smoking has been consistently noted4,5,7. While the prevalence of cigarette smoking observed in our patients was low, male smokers had a high risk of GO on multivariate logistic regression analysis. This risk was similar to that reported in previous studies in other Caucasians3,4. However, an association of smoking with greater severity of GO was not apparent, possibly because of small number of patients with GO who were current smokers.
As noted in studies in other ethnic groups, higher TRAb titres were found in patients and GD with GO as compared to those without ophthalmopathy3,19,21. A progressively increasing prevalence of GO was observed with increasing TRAb titres and TRAb titres were independent risk factor for GO. In contrast to a prior Asian study, TPOAb negativity was not associated with GO in our study21.
The current study was conducted on patients presenting to an academic referral center which receives a broad and representative spectrum of patients with GD for management. Hence, while it is possible that the features of GO noted may not be fully representative of that in the community, it is likely that they will not differ greatly from that seen in general practice.
In conclusion, our results indicated that while the prevalence of GO in north Indians patients with GD seen at our referral center was similar to that reported in the Caucasians of European origin, clinically active and severe GO was uncommon. Further studies are needed to ascertain if these differences are a result of genetic or environmental differences in these racial groups.
References
- 1.Brent GA. Clinical practice. Graves’ disease. N Engl J Med. 2008;358:2594–605. doi: 10.1056/NEJMcp0801880. [DOI] [PubMed] [Google Scholar]
- 2.Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med. 2009;360:994–1001. doi: 10.1056/NEJMcp0806317. [DOI] [PubMed] [Google Scholar]
- 3.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726–38. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stan MN, Bahn RS. Risk factors for development or deterioration of Graves’ ophthalmopathy. Thyroid. 2010;20:777–83. doi: 10.1089/thy.2010.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton J, Kelly SP, Harrison RA, Edwards R. Cigarette smoking and thyroid eye disease: a systematic review. Eye (Lond) 2007;21:1135–45. doi: 10.1038/sj.eye.6702603. [DOI] [PubMed] [Google Scholar]
- 6.Acharya SH, Avenell A, Philip S, Burr J, Bevan JS, Abraham P. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review. Clin Endocrinol (Oxf) 2008;69:943–50. doi: 10.1111/j.1365-2265.2008.03279.x. [DOI] [PubMed] [Google Scholar]
- 7.Tellez M, Cooper J, Edmonds C. Graves’ ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol (Oxf) 1992;36:291–4. doi: 10.1111/j.1365-2265.1992.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Lee SY, Yoon JS. Risk factors associated with the severity of thyroid-associated orbitopathy in Korean patients. Korean J Ophthalmol. 2010;24:267–73. doi: 10.3341/kjo.2010.24.5.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, et al. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:284–90. doi: 10.1016/s0002-9394(14)70276-4. [DOI] [PubMed] [Google Scholar]
- 10.Prummel MF, Bakker A, Wiersinga WM, Baldeschi L, Mourits MP, Kendall-Taylor P, et al. Multi-center study on the characteristics and treatment strategies of patients with Graves’ orbitopathy: the first European Group on Graves’ Orbitopathy experience. Eur J Endocrinol. 2003;148:491–5. doi: 10.1530/eje.0.1480491. [DOI] [PubMed] [Google Scholar]
- 11.Lim SL, Lim AK, Mumtaz M, Hussein E, Wan Bebakar WM, Khir AS. Prevalence, risk factors, and clinical features of thyroid-associated ophthalmopathy in multiethnic Malaysian patients with Graves’ disease. Thyroid. 2008;18:1297–301. doi: 10.1089/thy.2008.0044. [DOI] [PubMed] [Google Scholar]
- 12.Wiersinga WM, Perros P, Kahaly GJ, Mourits MP, Baldeschi L, Boboridis K, et al. European Group on Graves’ Orbitopathy (EUGOGO) Clinical assessment of patients with Graves’ orbitopathy: the European Group on Graves’ Orbitopathy recommendations to generalists, specialists and clinical researchers. Eur J Endocrinol. 2006;155:387–9. doi: 10.1530/eje.1.02230. [DOI] [PubMed] [Google Scholar]
- 13.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, et al. European Group on Graves’ Orbitopathy (EUGOGO) Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158:273–85. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 14.Bartley GB, Gorman CA. Diagnostic criteria for Graves’ ophthalmopathy. Am J Ophthalmol. 1995;119:792–5. doi: 10.1016/s0002-9394(14)72787-4. [DOI] [PubMed] [Google Scholar]
- 15.Sodhi PK, Gupta VP, Pandey RM. Exophthalmometric values in a normal Indian population. Orbit. 2001;1:1–9. doi: 10.1076/orbi.20.1.1.2647. [DOI] [PubMed] [Google Scholar]
- 16.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 1997;47:9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 17.Bednarczuk T, Hiromatsu Y, Fukutani T, Jazdzewski K, Miskiewicz P, Osikowska M, et al. Association of cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) gene polymorphism and non-genetic factors with Graves’ ophthalmopathy in European and Japanese populations. Eur J Endocrinol. 2003;148:13–8. doi: 10.1530/eje.0.1480013. [DOI] [PubMed] [Google Scholar]
- 18.Bhansali SK, Chandalia HB. Thyrotoxicosis - surgical management in the era of evidence-based medicine: experience in Western India with 752 cases. Asian J Surg. 2002;25:291–9. doi: 10.1016/S1015-9584(09)60194-9. [DOI] [PubMed] [Google Scholar]
- 19.Eckstein AK, Plicht M, Lax H, Neuhäuser M, Mann K, Lederbogen S, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91:3464–70. doi: 10.1210/jc.2005-2813. [DOI] [PubMed] [Google Scholar]
- 20.Khurana AK, Sunder S, Ahluwalia BK, Malhotra KC, Gupta S. A clinico-investigative profile in Graves’ ophthalmopathy. Indian J Ophthalmol. 1992;40:56–8. [PubMed] [Google Scholar]
- 21.Khoo DH, Ho SC, Seah LL, Fong KS, Tai ES, Chee SP, et al. The combination of absent thyroid peroxidase antibodies and high thyroid-stimulating immunoglobulin levels in Graves’ disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid. 1999;9:1175–80. doi: 10.1089/thy.1999.9.1175. [DOI] [PubMed] [Google Scholar]


