Methotrexate (MTX) is a folic acid antagonist and cell cycle–specific antimetabolite. While still an important component of hematologic and solid tumour treatment regimens, MTX is now used in low doses for the treatment of many autoimmune disorders, including Crohn disease, rheumatoid arthritis, and psoriasis. Although MTX is generally well tolerated at low doses, serious and predictable organ toxicity, including myelosuppression, mucositis, hepatotoxicity, and kidney injury, can occur owing to drug interactions or changes in renal or hepatic function. This toxicity can be rapid and life threatening, even in patients taking oral doses as low as 5 to 25 mg of MTX per week.1
Trimethoprim-sulfamethoxazole (TMP-SMX) is a bacteriostatic antimicrobial used in the treatment and prevention of various infections, including urinary tract infections, otitis media, chronic bronchitis exacerbations, and Pneumocystis jiroveci pneumonia. Like MTX, TMP-SMX is an inhibitor of folic acid metabolism and can cause bone marrow suppression. Trimethoprim-sulfamethoxazole is also known to decrease the renal excretion of MTX. When used in combination, the potential for toxicity is substantial.
In this article, we report a clinically important interaction between MTX and TMP-SMX that resulted in severe mucositis, pancytopenia, and febrile neutropenia in a patient with Crohn disease who had been treated with long-term, low-dose MTX.
Case
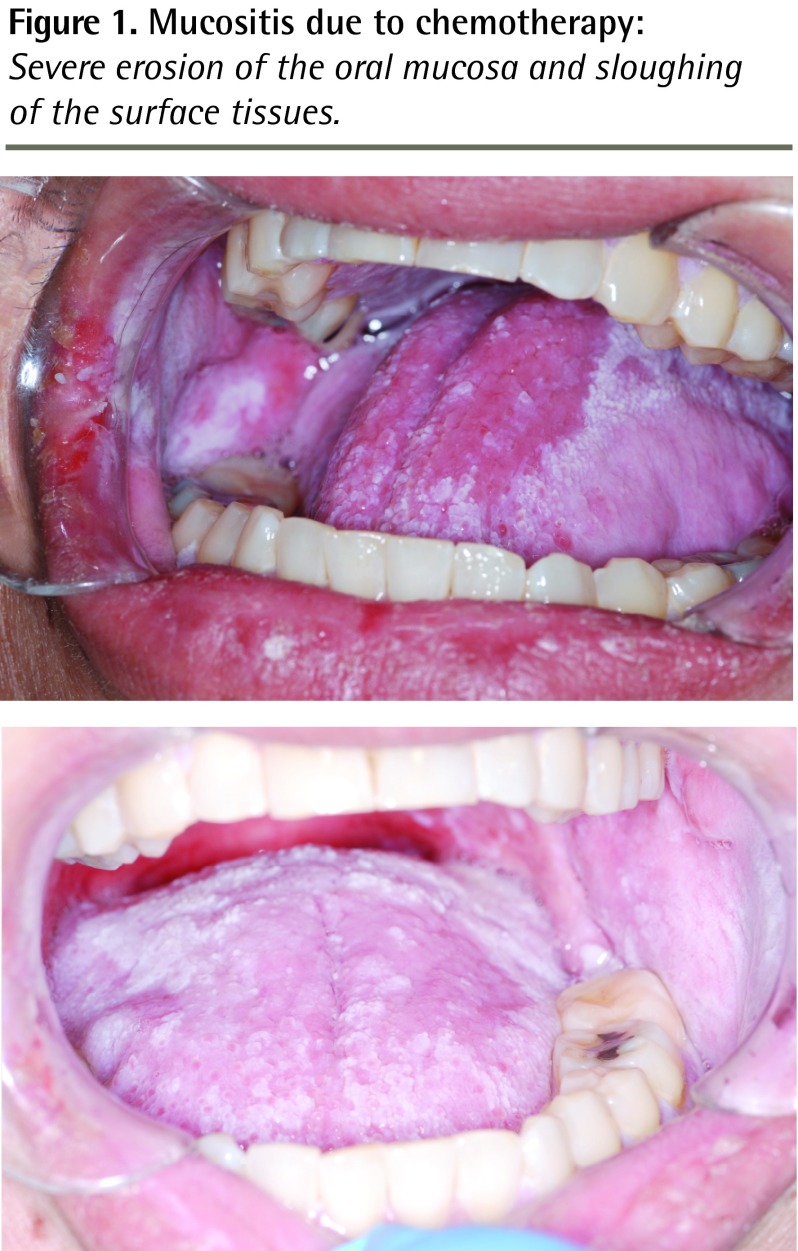
A 68-year-old woman with a 39-year history of Crohn disease had been treated with a stable dose of MTX (25 mg/wk), along with folic acid, for approximately 13 years. In response to abdominal pain and an increased frequency of bowel moments, oral prednisone was initiated at a recent hospital admission for what clinically appeared to represent a Crohn disease exacerbation. The patient was also prescribed TMP-SMX (combination of 160 mg of TMP, 800 mg of SMX 3 times weekly) for P jiroveci pneumonia prophylaxis while taking corticosteroids. The patient was discharged with a tapering dose of prednisone, and over the following weeks as an outpatient, her abdominal pain progressed and her diarrhea worsened; she was also unable to ingest solid food owing to nausea, vomiting, and extremely painful mouth sores. Three weeks after initial discharge, she presented to hospital with dehydration and painful stomatitis (Figure 1). Her medications at the time of admission were as follows: 25 mg of MTX intramuscularly weekly; 160/800 mg of TMP-SMX 3 times weekly; 15 mg of prednisone daily; 1 mg of folic acid daily; 10 mg of alendronate daily; and 1000 IU of vitamin D daily. On admission, her bloodwork results revealed the following: a serum creatinine level of 184 μmol/L; a white blood cell count of 1.3 × 109/L; a hemoglobin level of 71 g/L (mean corpuscular volume of 80.4 fL); and a platelet concentration of 115 × 109/L. The patient was treated with intravenous (IV) fluid for rehydration, oral nystatin for a presumptive diagnosis of oral candidiasis, and IV morphine for mouth pain. Consultation with a hematologist was requested in order to assess the pancytopenia. Vitamin B12 deficiency was ruled out, and blood and urine cultures had negative results.
Figure 1.
Mucositis due to chemotherapy: Severe erosion of the oral mucosa and sloughing of the surface tissues.
Treatment and clinical interpretation
Methotrexate and TMP-SMX were stopped on day 3 of admission, and therapy with folinic acid (15 mg IV for 5 days) was given to antagonize the effects of MTX and reverse the patient’s marrow suppression. The prednisone taper was continued, and valacyclovir was initiated for the empiric management of stomatitis. Buccal swabs were negative for Candida, while a lip swab later grew herpes simplex in culture.
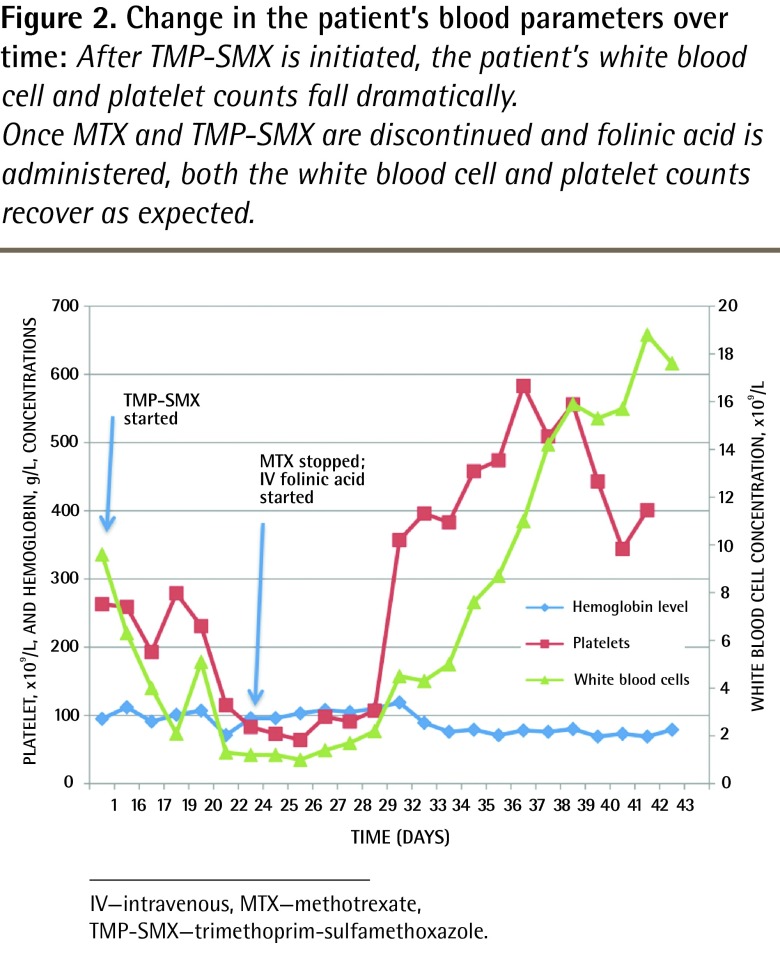
The white blood cell, hemoglobin, and platelet concentrations returned to baseline 5 days after folinic acid was initiated (Figure 2). The patient’s creatinine level normalized, and her diarrhea subsided. She was discharged from hospital when she was able to eat without pain. At her follow-up appointment with the gastroenterologist, she was started on infliximab for treatment of her Crohn disease.
Figure 2.
Change in the patient’s blood parameters over time: After TMP-SMX is initiated, the patient’s white blood cell and platelet counts fall dramatically.
Once MTX and TMP-SMX are discontinued and folinic acid is administered, both the white blood cell and platelet counts recover as expected.
IV—intravenous, MTX—methotrexate, TMP-SMX—trimethoprim-sulfamethoxazole.
Before her acute illness, the patient’s hematologic profile on a stable weekly dose of MTX had been unremarkable, aside from long-standing normocytic, normochromic anemia consistent with chronic inflammation (Figure 2). Although MTX levels were not available, the recent addition of TMP-SMX, especially in the setting of dehydration and renal impairment, made toxicity from the combination of MTX and TMP-SMX the most likely diagnosis.
Discussion
Methotrexate and TMP-SMX are commonly prescribed medications that, when used in combination, might be associated with life-threatening organ toxicity, especially in patients with renal or hepatic impairment. With up to 37% of patients discontinuing MTX owing to toxicity or intolerability,1 it is imperative that treatment be optimized in order to avoid adverse effects and patient dissatisfaction. Familiarity with the metabolism and side effects of these drugs and their potential for toxicity is essential for their safe clinical use.
Methotrexate is susceptible to drug interactions because of its reliance on renal excretion and its high degree of protein binding. Ninety percent of MTX is excreted unchanged in the urine within 48 hours.2,3 Although minimal, active metabolism of MTX occurs via 2 pathways: a degree of intestinal bacterial metabolism occurs to produce inactive metabolite4; and hepatic metabolism produces 7-hydroxymethotrexate, which has the potential to cause nephrotoxicity.3,4 As most of MTX is eliminated unchanged, any condition or drug that impairs renal blood flow will impair MTX elimination and increase the potential for toxicity.3 In addition to prerenal acute kidney injury, drugs that have the potential to impair renal function and increase toxicity include nonsteroidal anti-inflammatory drugs and weak organic acids such as penicillins, cephalosporins, piperacillin, TMP-SMX, and acetylsalicylic acid.2–4 Second, 50% of MTX is bound to albumin and easily displaced by other drugs that bind to albumin with high affinity, such as sulfonamides (eg, TMP-SMX), salicylates, and phenytoin.2,3 When used in combination with MTX, these drugs increase both the concentration of free MTX and its potential for toxicity. Common signs of MTX toxicity include mucositis, stomatitis (Figure 1), diarrhea, marrow suppression (Figure 2), and renal impairment, as observed in our patient.4 Methotrexate can also cause elevations in liver enzymes.4
The concomitant use of MTX and TMP-SMX might result in severe systemic toxicity through multiple mechanisms. First, because both agents inhibit dihydrofolate reductase,4,5 the combination of these 2 drugs substantially reduces folate metabolism and increases the potential for myelosuppression. Second, both sulfonamides and MTX have the potential to cause nephrotoxicity, and the resulting renal impairment can itself lead to elevated levels of either or both drugs. Third, owing to competitive protein binding and reduced tubular secretion, the addition of TMP-SMX increases free serum levels of MTX by approximately 30% and decreases MTX excretion by half.6
Given the multiple indications for long-term, low-dose MTX and the frequency with which antibiotics, such as penicillin derivatives or TMP-SMX, are used in clinical practice, health care providers must be familiar with the seriousness of this and other clinically important drug interactions involving MTX (Table 1).7,8 Several case reports,7,9–14 as well as a recent systematic review7 of the literature, highlight severe toxicity with concurrent use of MTX and TMP-SMX. Although well described, this interaction continues to occur.
Table 1.
Common drug-drug interactions with MTX
| DRUGS* | ADVERSE REACTION | SUPPORTING EVIDENCE |
|---|---|---|
| TMP-SMX | Bone marrow suppression, renal toxicity | ≥ 17 case reports and 1 retrospective case-control study |
| Penicillins and cephalosporins (eg, amoxicillin, cephalexin) | Bone marrow suppression | ≥ 5 case reports and multiple interaction studies |
| NSAIDs (eg, high-dose ASA, indomethacin, ibuprofen) | Bone marrow suppression | ≥ 30 case reports, 2 retrospective observational studies, and multiple interaction studies |
ASA—acetylsalicylic acid, MTX—methotrexate, NSAIDs—nonsteroidal anti-inflammatory drugs, TMP-SMX—trimethoprim-sulfamethoxazole.
Rare drug-drug interactions have also been reported with MTX and quinolones, macrolides, retinoids, proton pump inhibitors, isoniazid, ranitidine, probenecid, and theophylline; however, the reliability and clinical importance of these potential interactions are substantially uncertain.
Several factors likely contribute to the persistence of this preventable drug interaction. An under-appreciation for the potential toxicity of these agents, especially when used at low doses, appears to exist.7 Weekly MTX dosing administered in the doctor’s office might circumvent pharmacy-based safety processes designed to identify potential drug interactions; this was a contributing factor in the case presented in this report.
It is unknown whether short courses of TMP-SMX for conditions such as urinary tract infections are safe for patients taking long-term MTX, and clinical trials to inform practice are unlikely to be conducted. In the setting of uncomplicated cystitis in women, an alternative agent such as nitrofurantoin should be considered. Empiric and definitive therapy should be guided by local resistance patterns and the results of culture and susceptibility testing. An infectious disease specialist should be consulted if clinical uncertainty exists regarding local bacterial resistance patterns or the use of alternative agents. If TMP-SMX use is required, short courses (up to 3 days) are recommended. Baseline laboratory testing that includes a complete blood count, liver enzymes, and serum creatinine levels, along with follow-up testing in 5 to 7 days, should be considered. If pancytopenia is identified, consultation with a hematologist should be considered. Folic acid supplementation would not be expected to prevent dangerous drug interactions or to effectively treat them once they occur. Folinic acid, a reduced derivative of folic acid that supplies the necessary cofactor antagonized by MTX, does reverse the block in folate metabolism induced by both MTX and TMP-SMX, but would be expected to reduce the effectiveness of these agents to treat the conditions for which they were initiated.
Conclusion
Concomitant use of MTX and TMP-SMX, even in low doses, can result in serious systemic toxicity characterized by pancytopenia, oral mucositis, and nephrotoxicity. Health care providers should be mindful of this and other potential drug interactions with the introduction of new prescription or nonprescription medications. The persistence of cases of serious toxicity due to the combined use of MTX and TMP-SMX, despite published reports, not only highlights the need for education and effective knowledge translation strategies, but also signals the need for improved system processes designed to prevent this interaction from occurring.
EDITOR’S KEY POINTS
Methotrexate (MTX) is a commonly used medication for conditions such as Crohn disease, rheumatoid arthritis, and psoriasis.
Concomitant use of MTX and trimethoprim-sulfamethoxazole can result in life-threatening myelosuppression, mucositis, and nephrotoxicity.
Clinicians caring for patients taking MTX must be aware of this important and dangerous drug interaction. System processes designed to prevent this interaction from occurring are needed.
POINTS DE REPÈRE DU RÉDACTEUR
Le méthotrexate (MTX) est un médicament couramment utilisé pour des problèmes comme la maladie de Crohn, l’arthrite rhumatoïde et le psoriasis.
L’utilisation concomitante de MTX et de triméthoprim-sulfaméthoxazole peut causer une myélosuppression, une mucosite et une néphrotoxicité potentiellement mortelles.
Les cliniciens qui soignent des patients prenant du MTX doivent être au courant de cette importante et dangereuse interaction médicamenteuse. Il faut établir dans le système des processus conçus pour prévenir la survenance de cette interaction.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs
Competing interests
None declared
References
- 1.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68(7):1100–4. doi: 10.1136/ard.2008.093690. Epub 2008 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertino J, Cleary J, Ortiz T, Lane A, Supko JG, Ryan D. Cytotoxic agents. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill Global Education Holdings; 2011. Available from: www.accessmedicine.com/content.aspx?aID=16680251. Accessed 2013 Dec 14. [Google Scholar]
- 3.Bannwarth B, Péhourcq F, Schaeverbeke T, Dehais J. Clinical pharmacokinetics of low-dose pulse methotrexate in rheumatoid arthritis. Clin Pharmacokinet. 1996;30(3):194–210. doi: 10.2165/00003088-199630030-00002. [DOI] [PubMed] [Google Scholar]
- 4.Crom WR, Evan WE. Methotrexate. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied pharmacokinetics: principles of therapeutic drug monitoring. 3rd ed. New York, NY: Lippincott Williams & Wilkins; 1992. [Google Scholar]
- 5.Herbert V. Trimethoprim-sulfamethoxazole: metabolism of folic acid in man. J Infect Dis. 1973;128(Suppl):601–6. doi: 10.1093/infdis/128.supplement_3.s601. [DOI] [PubMed] [Google Scholar]
- 6.Ferrazzini G, Klein J, Sulh H, Chung D, Griesbrecht E, Koren G. Interaction between trimethoprim-sulfamethoxazole and methotrexate in children with leukemia. J Pediatr. 1990;117(5):823–6. doi: 10.1016/s0022-3476(05)83351-7. [DOI] [PubMed] [Google Scholar]
- 7.Bourré-Tessier J, Haraoui B. Methotrexate drug interactions in the treatment of rheumatoid arthritis: a systematic review. J Rheumatol. 2010;37(7):1416–21. doi: 10.3899/jrheum.090153. [DOI] [PubMed] [Google Scholar]
- 8.Van Roon EN, van den Bemt PM, Jansen TL, Houtman NM, van de Laar MA, Brouwers J. An evidence-based assessment of the clinical significance of drug-drug interactions between disease-modifying antirheumatic drugs and non-antirheumatic drugs according to rheumatologists and pharmacists. Clin Ther. 2009;31(8):1737–46. doi: 10.1016/j.clinthera.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Groenendal H, Rampen FH. Methotrexate and trimethoprim-sulphamethoxazole—a potentially hazardous combination. Clin Exp Dermatol. 1990;15(5):358–60. doi: 10.1111/j.1365-2230.1990.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas MH, Gutterman LA. Methotrexate toxicity in a patient receiving trimethoprim-sulfamethoxazole. J Rheumatol. 1986;13(2):440–1. [PubMed] [Google Scholar]
- 11.Thomas DR, Dover JS, Camp RD. Pancytopenia induced by the interaction between methotrexate and trimethoprim-sulfamethoxazole. J Am Acad Dermatol. 1987;17(6):1055–6. doi: 10.1016/s0190-9622(87)80490-5. [DOI] [PubMed] [Google Scholar]
- 12.Jeurissen ME, Boerbooms AM, van de Putte LB. Pancytopenia and methotrexate with trimethoprim-sulfamethoxazole. Ann Intern Med. 1989;111(3):261. doi: 10.7326/0003-4819-111-3-261_1. [DOI] [PubMed] [Google Scholar]
- 13.Maricic M, Davis M, Gall EP. Megaloblatic pancytopenia in a patient receiving concurrent methotrexate and trimethoprim-sulfamethoxazole treatment. Arthritis Rheum. 1986;29(1):133–5. doi: 10.1002/art.1780290118. [DOI] [PubMed] [Google Scholar]
- 14.Thevenat JP, Ristori JM, Cure H, Mizony MH, Bussiera JL. Pancytopenia during treatment of rheumatoid arthritis with methotrexate after administration of trimethoprim-sulfamethoxazole [article in French] Presse Med. 1987;(30):1487. [PubMed] [Google Scholar]