Abstract
Background
Adverse drug events, the unintended and harmful effects of medications, are important outcome measures in health services research. Yet no universally accepted set of International Classification of Diseases (ICD) revision 10 codes or coding algorithms exists to ensure their consistent identification in administrative data. Our objective was to synthesize a comprehensive set of ICD-10 codes used to identify adverse drug events.
Methods
We developed a systematic search strategy and applied it to five electronic reference databases. We searched relevant medical journals, conference proceedings, electronic grey literature and bibliographies of relevant studies, and contacted content experts for unpublished studies. One author reviewed the titles and abstracts for inclusion and exclusion criteria. Two authors reviewed eligible full-text articles and abstracted data in duplicate. Data were synthesized in a qualitative manner.
Results
Of 4241 titles identified, 41 were included. We found a total of 827 ICD-10 codes that have been used in the medical literature to identify adverse drug events. The median number of codes used to search for adverse drug events was 190 (IQR 156–289) with a large degree of variability between studies in the numbers and types of codes used. Authors commonly used external injury (Y40.0–59.9) and disease manifestation codes. Only two papers reported on the sensitivity of their code set.
Conclusions
Substantial variability exists in the methods used to identify adverse drug events in administrative data. Our work may serve as a point of reference for future research and consensus building in this area.
Keywords: Patient Safety, Drug Safety, International Classification of Diseases, Administrative Data, Comparative Effectiveness and Safety Research
Introduction
The use of prescribed medications has risen dramatically in the past decades.1 In 2008, over 76% of Americans older than 60 years reported ingesting two or more prescribed medications daily, and 37% used five or more.1 Patients who use indicated medications appropriately can expect to derive benefit. Yet, a significant proportion will experience adverse drug events, the unintended and harmful effects resulting from medication use that are associated with suboptimal patient outcomes and increased health services utilization.2–7 Adverse drug reactions alone, a subset of adverse drug events that occur when drugs are used in therapeutic doses,8 cause 5–10% of acute care hospital admissions,9–12 prolong hospital stays,13 and may contribute to more potential years of life lost than all other injuries combined.14 Efforts to optimize medication use and reduce adverse drug events are therefore a public health priority.15
Adverse drug events that are encountered in clinical practice may vary substantially from those observed in pre-market clinical trials.16 Reasons include differences in patient populations, treatment indications (eg, off-label use), monitoring protocols, duration of drug exposure and compliance between the clinical practice setting and the controlled environment of clinical trials.6 16 17 Monitoring and evaluating health outcomes that are associated with the way medications are used in clinical practice is difficult, yet essential to understanding the ongoing safety and risk–benefit profiles of medications, and paramount to promoting their optimal use.
Administrative databases, electronic health records and disease registries contain a plethora of health data that can be used to ascertain health outcomes in clinical practice. These data are generally inexpensive, readily accessible and have been collected without interfering in the delivery of care. Thus, data from these sources are more likely to reflect the outcomes experienced by patients in the real-world clinical practice setting than the research setting, provided that the outcomes are appropriately identified and coded.16
Administrative databases worldwide, including in the USA, increasingly use the International Classification of Diseases (ICD) revision 10 system to classify diagnostic, health services utilization and death data. The ICD-10 coding dictionary enables coders to document adverse drug events in three ways: (1) by documenting the medication that caused an adverse drug event using ‘external injury cause codes’ (ie, Y40.0–59.9); (2) by documenting diagnoses that may be caused by a drug using ‘disease manifestation codes’ (eg, A04.7 Clostridium difficile colitis); and (3) by clustering an external injury cause code indicating the drug-related etiology with a disease manifestation code indicating the patient's diagnosis.18 Because a large number of disease manifestation codes exist that might be adverse drug event related (eg, gastric ulcer), variation exists among health researchers in the code sets and coding algorithms used to identify adverse drug events coded in ICD-10.
Our main objective was to synthesize a comprehensive set of ICD-10 codes used by health researchers to identify adverse drug events. Our secondary objective was to identify studies with ICD-10 coding algorithms for adverse drug events.
Methods
Data sources and searches
This was a qualitative systematic review of the literature. Ethics approval was not required because it did not involve the use of human subjects or medical records.
A professional librarian (MDW) and study author (CMH) developed a systematic search strategy that was adapted for, and applied to, the following electronic bibliographic databases: MEDLINE (1948–2011), EMBASE (1980–2011), International Pharmaceutical Abstracts ((IPA) 1970–2011), Web of Science (1980–2011), the Cochrane Database of Systematic Reviews (1993–2011) and the Cochrane Central Register of Controlled Trials ((CENTRAL) 1996–2011) (see supplementary appendix A, available online only, for Medline search). Our search strategy combined three concepts: the ICD coding system, adverse drug events, and health outcomes. We reviewed the scope notes for each search term in order to identify and incorporate previous indexing terms, alternative keywords, and appropriate MeSH terms. No language filters were applied.
We hand-searched the following medical journals for relevant studies and conference proceedings from 2000 onwards: the Milbank Quarterly, Health Technology Assessment Journal, Health Affairs, Medical Care, American Journal of Medical Care, and Quality & Safety in Health Care. We used 2000 as the start date as this was the year that ICD-10 was introduced in Canada. We did not search for any additional conference proceedings not published in the above journals, as we thought it would be unlikely for code sets to be published in abstract format. We conducted an electronic grey literature search using the search engine Google with the same search terms that we used for our electronic bibliographic database searches. We hand-searched the bibliographies of all relevant articles. In 2012, we conducted periodic environmental scans of the literature for newly published studies using auto alerts from MEDLINE, EMBASE and IPA. We contacted content experts and authors of relevant studies for any additional studies and for clarifications about their methodology and code sets.
Study selection
We included all studies reporting the use of the ICD-10 coding system to identify adverse drug events in adult patients. Studies had to report the ICD-10 code set (our outcome measure) or coding algorithms used to search the administrative data. We excluded studies reporting only pediatric data, as common manifestations of adverse drug events vary between adults and children. In addition, pediatric adverse drug events are more commonly the result of dosing errors or unintentional toxic ingestions compared to adult adverse drug events. We also excluded studies using other coding systems, written in languages other than English, French and German, reporting only adverse events to illicit drugs or intentional overdoses, and studies that we could not access.
One study author (AK) screened all titles for potential eligibility using predefined criteria. Any potentially relevant studies were retained for abstract review. Two study authors (AK and CMH) reviewed the abstracts of potentially relevant titles. If either or both of the authors felt the abstract was potentially relevant, the full text article was retrieved and reviewed independently by two authors (AK and CMH) for inclusion and exclusion criteria. All disagreements about study eligibility at the full text review stage were resolved by achieving consensus through discussion.
Data extraction and quality assessment
Two authors (AK and CMH) independently abstracted data from included studies using standardized and piloted abstraction forms. Any disagreements over data abstraction points were resolved by achieving consensus through discussion, after contacting study authors for clarification. Data abstractors were not blinded to authorship or journal.
We are unaware of any validated quality assessment scales to measure the quality of non-comparator, retrospective population-based cohort studies.19 Therefore, we adapted relevant quality-assessment criteria from the GRACE guidelines and the York Centre for Dissemination and Reviews that were intended for population-based comparative effectiveness studies and reviews of adverse effects (table 1).19 20
Table 1.
Quality assessment criteria adapted from York Centre of Dissemination Reviews and the GRACE quality assessment checklist for this review of non-comparator cohort studies19 20
| 1. Was the primary outcome(s) defined in a manner that was independent of the code set? | Yes | The primary outcome(s) was defined in a manner that was independent from the code set |
| No | The primary outcome(s) was not defined in a manner that was independent from the code set (ie, the definition was based on the ICD-10 codes used for searching) | |
| NR | Not reported | |
| 2. Were methods for identifying the appropriate ICD-10 codes to reflect the primary outcome reported, and was the search comprehensive? | Yes | The methods for identifying the code set were explicit and comprehensive (eg, through literature review or mapping of pharmacovigilance terms to ICD-10 codes). It is unlikely that significant gaps in the code set exist |
| No | The methods for identifying the code set was not reported, and the code set is not likely to be comprehensive | |
| NR | Not reported | |
| 3. Did the authors provide data or reference other work to allow the reader to understand how well the primary outcome was ascertained within the same data source(s) using the ICD-10 code set they chose (ie, sensitivity, specificity of the code set)? | Yes | The primary outcome was validated based on medical chart abstraction with clear definitions (eg, a formal medical record review of a sample of charts was done with adjudication of the primary diagnosis by a committee and the code set had reasonable sensitivity and specificity for identifying the primary outcome), or the code set was validated by linking and comparing existing data from various sources to ensure consistency and accuracy (eg, prospective registry compared with administrative data). Alternatively, previous work validated the code set, and the code set was likely to identify the stated primary outcome |
| No | No data were reported, and no other work referenced, to suggest that the code set adequately identified the primary outcome | |
| NR | Not reported | |
| 4. Were analyses conducted to test assumptions about the causal link between drug exposure and the disease, and how this uncertainty may have influenced the study results? | Yes | Analyses were reported to evaluate the impact of uncertain causality on the study results (eg, analyses to test the impact of including codes for diagnoses that are likely, but not exclusively drug-induced, ie, Clostridium difficile colitis), and may not have been cluster coded with external cause codes |
| No | No analysis was done to test the assumptions about the causal link between drug exposure and the disease manifestation |
ICD, International Classification of Diseases.
Data synthesis and analysis
We synthesized the data in a qualitative manner, with two authors (CMH and AK) reviewing all data extraction forms and re-reading primary manuscripts. A third author reviewed all tables and figures for accuracy (LR), and all authors subsequently critically reviewed the manuscript for content and accuracy. We adapted causality ratings for individual ICD-10 codes from two previous publications, and modified them by adding the rating ‘unlikely’ (U) for codes that other authors used to identify adverse drug events in the literature that we felt unlikely to have indicated an adverse drug event (table 2).21 22 We also added the rating ‘vaccine’ (V) for codes that were vaccine related. Two study authors (CMH and JS) independently, and blinded to one another's ratings, assigned causality scores to ICD-10 codes without previously assigned causality ratings, and came to consensus through discussion about any disagreement.
Table 2.
| Code category | Definition | Examples | |
|---|---|---|---|
| Code | Code description | ||
| A1 | The ICD-10 code description includes the phrase ‘induced by medication/drug’ | J70.2 | Acute drug-induced interstitial lung disorders |
| A2 | The ICD-10 code description includes the phrase ‘induced by medication or other causes’ | 142.7 | Cardiomyopathy due to drugs and other external agents |
| T88.7 | Unspecified adverse event of drug or medicament | ||
| B1 | The ICD-10 code description includes the phrase ‘poisoning by medication’ | T36 | Poisoning by systemic antibiotics |
| B2 | The ICD-10 code description includes the phrase ‘poisoning by or harmful use of medication or other causes’ | X44 | Accidental poisoning by, and exposure to, other and unspecified drugs, medicaments and biological substances |
| C | Adverse drug event deemed to be very likely although the ICD-10 code description does not refer to a drug | L51.2 | Toxic epidermal necrolysis |
| D | Adverse drug event deemed to be likely although the ICD-10 code description does not refer to a drug | N17 | Acute renal failure with tubular necrosis |
| E | Adverse drug event deemed to be possible although the ICD-10 code dictionary does not refer to a drug | K25 | Gastric ulcer |
| U | Adverse drug event deemed unlikely | I49.0 | Ventricular fibrillation and flutter |
| V | Vaccine-associated adverse event | A80.0 | Acute paralytic poliomyelitis, vaccine-associated |
The causality ratings were modified for the purposes of this systematic review. We added category U for ICD-10 codes that have been used by others to identify adverse drug events, but which we felt were unlikely to be adverse drug event related. We also added category V to indicate codes that may be vaccine-related.
ICD, International Classification of Diseases.
Descriptive statistics were provided as averages with 95% CI, or medians with IQR. We calculated the interrater agreement of causality ratings assigned to ICD-10 codes, by collapsing causality ratings into a category indicating that an adverse drug event was very likely (categories A1, A2, B1, B2 and C), and a category in which adverse drugs events were deemed unlikely (categories D, E, U and V) based on the previous literature.21 22 We calculated κ scores with 95% CI as a measure of agreement beyond chance alone.
Results
Study characteristics and study quality
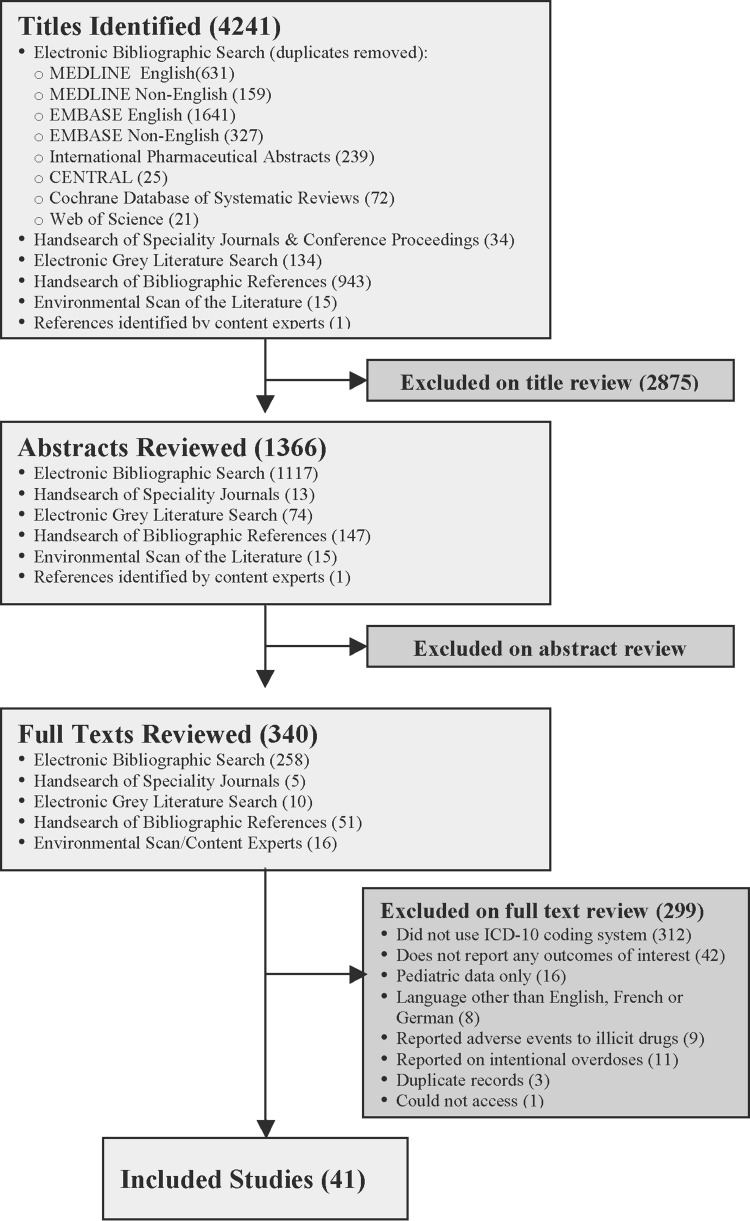
Our search revealed 4241 titles, of which 41 met our inclusion and exclusion criteria (figure 1). Sixteen studies were conducted in Europe,21–36 13 in North America,14 37–48 nine in Australia,49–57 and three in Asia (tables 3 and 4).58–60 The majority of included studies were non-comparator retrospective studies that used administrative data to ascertain the prevalence of adverse drug events in population-based cohorts. Twenty-eight studies examined adverse drug events in general as the main outcome measure,14 21–26 29 32–40 42 43 49–54 56–58 and 13 examined drug or drug class-specific adverse drug events.27 28 30 31 41 44–48 55 59 60 Eleven of 28 studies reported explicit methods for identifying the ICD-10 code set they used.21–23 34–36 38 40 42 53 58
Figure 1.
Flow diagram of included studies.
Table 3.
Characteristics of 28 studies looking at adverse drug events in general, that is, events that were not specific to any drug or disease category
| Study | Country | Setting | Design | Data source | Main objective | Main outcome and definition | No. codes | Methods to identify ICD-10 codes | Sample size | Frequency of outcome measure |
|---|---|---|---|---|---|---|---|---|---|---|
| Malpass et al53 | Australia | NR | Review | NR | To describe an ADE monitoring system | ADE: NR | 318i AM |
Mapped an adverse event classification system to ICD-10. | NR | NR |
| Cox et al26 | England | Hospital | RS | Admin and PV | To compare ADR reports in administrative and PV data | ADR: NR | 175 | NR | 21 635 records | 0.2% of admissions due to ADR |
| Runciman et al54 | Australia | Hospital | Review | Admin, trial, drug use, chart review and VS | To review information about ADE and medication errors in Australia | ADR: Noxious and unintended response to a drug used at doses for prophylaxis, diagnosis or therapy of disease or modification of function. ADE: ADR, harm from medication errors and underuse |
175 AM |
NR | NR | ▸ ADR: occur in 1% of admissions. ▸ ADE: occur in 2–4% of admissions |
| Waller et al35 | England | Hospital | RS | Admin | To describe records coded as drug-induced and assess their utility for research | ADR: NR | 243 | Codes containing ‘drug-induced’, diagnoses ‘due to’ a drug, ‘clearly implying’ an ADR and Y40–59 | 53.8M records | 0.4% of admissions due to ADR |
| CDC37 | USA | NR | RS | VS | To describe trends in poisoning deaths | Death from ingestion, inhalation or exposure to pharmaceuticals, illicit drugs and chemicals37 | 137 | NR | NR | 5.0–7.8 deaths/100 000 population |
| Wysowski43 | USA | NR | Letter | VS | To study deaths attributed to therapeutic drug use | Death attributed to drugs used therapeutically | 4 | NR | 604 records | NR |
| Moneret et al32 | NR | NR | Review | NR | To review the epidemio-logy of anaphylaxis | Anaphylaxis | 6 | NR | NR | NR |
| Burgess et al49 | Australia | Hospital | Case series | Admin data | To examine trends in ADR-related admissions in people ≥60 years | ADR: Noxious and unintended response to a drug that occurs at doses normally used in humans | 200 AM |
NR | NR | 0.8% of admissions associated with ADR |
| Barrow et al23 | England | Hospital | RS | Admin and PV | To compare ADR in admin data with PV reports | ADR: NR | 37 | Used codes identified by Waller et al35 | NR | NR |
| Lugardon et al29 | France | Hospital | RS | Admin and PV | To estimate the incidence of serious ADR in hospital | ADR: Noxious and unintended response to a drug used at doses for prophylaxis, diagnosis, or therapy of disease or modification of physiological function | 299 | NR | 261 records | 2.9% of admissions associated with ADR |
| Wysowski42 | USA | NR | RS | VS | To identify prescription drugs associated with >1000 deaths/year | Death due to a prescription drug | NRii | Seven disease manifestation codes and codes listing prescription drugs as cause | NR | NR |
| Zhang et al57 | Australia | Hospital | RS | Admin, VS and census | To examine trends in repeat ADR causing hospitalization in elderly | Hospitalization for ADR. ADR: Noxious and unintended response to a drug at doses normally used in humans |
175 AM |
NR | 37 296 records | 30.3% of ADR-related admissions were repeat events |
| Patel et al34 | England | Hospital | RS | Admin | To examine trends in hospital admissions associated with ADR | ADR: NR | 245 | Codes containing ‘drug-induced’, indicating a diagnosis ‘due to’ a drug, and codes Y40–59 | 88M records | 0.5% of admissions due to ADR |
| Phillips et al14 | USA | NA | RS | VS | To describe trends in fatal medication errors | ADE: Preventable deaths resulting from accidental overdose, wrong drug given or taken in error, and other accidents in the use of drugs | 180 | NR | 50M death records | 0.4% of deaths due to fatal medication errors |
| Hwang et al 58 | Korea | Hospital | RS | Chart review | To evaluate an electronic ADE monitoring system | ADE: Injury from a medical intervention related to a drug | 326 | Codes corresponded to ADE described in four previous studies on ADE monitoring systems73–76 | 598 patients | 31% of patients admitted to hospital |
| Benkhaial et al24 | Germany | Hospital | RS | Admin | To assess the value of ICD-10 codes to identify drug allergies | Drug allergy: NR | 35 GM |
NR | 200 records | 9% of records indicating an allergy |
| Hodgkin-son et al51 | Australia | Hospital | RS | Admin and PV | To compare ADR identification using coding surveillance with spontaneous reporting | ADR: Noxious and unintended response to a drug that occurs at doses used for prophylaxis, diagnosis, or therapy, or modification of physiological function | 175 AM |
NR | 12 414 records | 4.5% of admissions associated with ADR |
| Wu40 | Canada | ED | RS | Admin data | To estimate the incidence of ADR-related ED visits and admissions for patients >65 years | ADR: Injury resulting from a medical intervention relating to a drug. | 245 CA |
Used codes identified by Patel et al34 | 966 232 records | 0.8% of ED visits were ADR-related |
| Zhang et al56 | Australia | Hospital | RS | Admin, VS and census | To identify factors that predict repeat hospital admission for ADR in older adults | ADR: Harmful or unpleasant reaction related to a drug that predicts hazard from future use and warrants prevention, treatment, dose change or withdrawal | 175 AM |
NR | 28 548 patients | 17.7% of ADR-related admissions were repeat events |
| Jackson et al52 61 | Australia | Hospital | RS | Admin | To develop a tool to monitor hospital-acquired diagnoses | Hospital acquired diagnosis. ADE: NR |
279iii AM |
Codes Y40–59 and codes with a C prefix, indicating a hospital acquired condition | 126 940 records | NR |
| Wu et al36 | England | Hospital | RS | Admin | To examine trends in hospital admissions associated with ADR | ADR: Undesirable effect of a drug beyond its anticipated therapeutic effects | 260 | Codes containing ‘ADR’, ‘drug-induced’, ‘due to drug’, ‘due to medication’, ‘drug allergy’ and Y40–59 | 59.7M records | 0.9% admissions associated with an ADR |
| Bergman et al25 | Sweden | Hospital | RS | Admin and PV | To examine trends in the use of the Y57.9 code for ADR reporting | ADR: Unintended effect of therapeutic use of drugs | 1 | NR | NR | 500 ADR reports/million in population |
| Stausberg and Hasford21, Stausberg77 | Germany | Hospital | RS | Admin | To examine the utility of ICD-10 coded diagnoses in admin data to identify ADE among inpatients | ADE: Unfavorable medical event that occurred in association with the use of a medication, and that may be causally related to the medication | 502*> GM |
Literature search for ADE, identified previously used codes,78 and applied screening criteria of and data from a PV center. Mapped ADE to ICD-10 | 12M records | ▸ 0.7% admissions due to an ADE ▸ 5.3% admissions possibly due to ADE |
| Stausberg and Hasford22 | Germany | Hospital | RS | Admin | To examine the frequency of ADE-related admissions and hospital-acquired ADE | ADE: Injury resulting from a medical intervention related to a drug including errors and ADR | 505iv GM |
Literature search for ADE, identified previously used codes,78 and applied screening criteria of and data from a PV center. Mapped ADE to ICD-10 | 48M records | ▸ 0.5–0.7% of admissions due to ADE ▸ 5% of admissions possibly due to ADE |
| Osmont et al33 | France | Hospital | RS | Admin | To evaluate ICD-10 queries to identify serious ADR | ADR: NR | NR | NR | NR | NR |
| Hauck and Zhao50 | Australia | Hospital | RS | Admin | To examine the association between ADR and hospital length of stay | ADR: NR | 206 AM |
NR | 206 489 records |
3.4% risk of ADR for 2-day admission |
| Shepherd et al39 | USA | NA | RS | VS | To examine trends in mortality attributed to ADR using US VS data | ADR: Noxious and unintended response to a medication used at doses administered for diagnosis, prophylaxis or treatment | 175 | NR | NR | 0.1 deaths from ADR/100 000 in population |
| Hohl et al submitted38 | Canada | ED | RS | Admin and prospect data | To measure proportion of ADE-related ED visits identifiable in admin data | ADE: Untoward and unintended symptoms, signs or abnormal laboratory values from medication use | 650 | Adapted previously established code set21 22 with others found through literature review | 1574 records | 14.0% of ED visits ADE related |
iUse of the AM modification likely, although unable to verify with authors.
iiOnly codes associated with >1000 deaths and/or >1000 total mentions per year were listed.
iiiJackson et al describe the CHADx algorithms to identify hospital acquired diagnoses, including adverse drug events (ADE). The CHADx code set and algorithms are published on the Australian Commission on Quality and Safety in Healthcare website. Jackson et al. recommend searching for ADE using disease manifestation codes clustered with external injury cause codes (Y40–59). C-prefixes are codes that were introduced in the Victorian addition of the Australian Modification of ICD-10.
ivThe difference in the number of codes used by Stausberg et al. has to do with the splitting of code E66.1 (in ICD-10-German Modification 2006) into the four codes: E66.10, E66.11, E66.12 and E66.19 (in ICD-10-German Modification 2008).
ADE, adverse drug event; Admin, administrative; ADR, adverse drug reaction; AM, Australian modification; CDC, Centers for Disease Control and Prevention; ED, emergency department; GM, German modification; ICD, International Classification of Diseases; M, million; NR, not reported; pros, prospective; PV, pharmacovigilance; RS, retrospective; VS, vital statistics.
Table 4.
Characteristics of 13 studies looking at drug or disease-specific adverse drug events, in order of publication year
| Study | Country | Setting | Design | Data source | Main objective | Main outcome and definition | No. codes | Methods to identify ICD-10 codes | Sample size | Frequency of outcome measure reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Gaus et al28 | Germany | Outpatients | RS case crossover | Admin and drug use | To illustrate case crossover methodology to identify ADR using bleeding complications as an example | Bleeding complications | 84 | NR | 320 644 records | 3.5 episodes of bleeding/100 years observation |
| Wysowski41 | USA | NR | RS | Drug use and VS | To determine the number, rate and types of deaths attributed to x-ray contrast media | Death from contrast agents | 3 | NR | NR | 1.1–1.2 deaths/million doses |
| Wysowski44 | USA | NR | RS | Admin, drug use, PV and VS | To compile and analyze data on the prevalence of bleeding related to warfarin | Warfarin-related deaths | 1 | NR | 0.4–0.5 deaths/100 000 population | |
| Sims et al47 | USA | NR | RS | Admin, drug use and vital stats | To examine the utility of ADR surveillance methods that combine and analyze multiple data sources | Methadone-related death | 1 | NR | NR | 0.8–4.3 deaths/100 000 population |
| Myers et al46 | Canada | Hospital | RS | Admin and chart review | To validate coding algorithms for acetaminophen overdose and hepatotoxicity | Admission for acetaminophen toxicity | 16 | NR | 1776 cases | NR |
| Molokhia et al31 | France | Hospital | RS | Admin and PV | To estimate the incidence and reporting rate of nonfatal drug-induced LQTS leading to VT and/or death | Drug-induced LQTS | 3 | NR | 861 cases | 10.9 cases/million population/year |
| Elalamy et al27 | France | Hospital | RS | Admin and laboratory surveillance | To estimate the average cost of one episode of HIT in France | HIT | 3 | NR | 50 958 records | 0.9% of admissions |
| Lyytikainen 200930 | Finland | Admin data | RS | Admin and VS | To determine the prevalence of CDAD in hospitalized patients | Admission associated with CDAD | 2 | NR | NR | 16–34 cases/100 000 population |
| Li et al45 | USA | Hospital | RS | Admin and VS | To examine the epidemiology of anesthesia-related deaths | Anesthesia-related death | 46v | Lit review and ICD-10 search | NR | 8.2 deaths/million surgical discharges |
| Treeprasertsuk et al60 | Thailand | Hospital | RS | Admin | To examine the incidence and complications of antimicrobial induced liver injury in hospitalized patients | Drug-induced liver injury | 4 | NR | 237 970 records | 0.03% of admitted patients |
| Rhee et al59 | Korea | Hospital | RS case control | Admin | To quantify the risk of digoxin toxicity with concomitant use of diuretics | Admission for digoxin toxicity | 1 | NR | 104 075 records | 61.5 cases/100 000 admissions |
| Sood et al55 | Australia | Hospital | RS | Admin | To examine the epidemiology, outcomes and burden of acetaminophen poisoning | Admission for acetaminophen poisoning | 2 AM |
NR | NR | 39–46 cases/100 000 admissions |
| Wysowski et al48 | USA | NR | RS | PV, admin, VS, drug use and surveillance | To determine the incidence of serious anaphylactic reactions to parenteral iron | Anaphylaxis due to parenteral iron | 2vi | NR | NR | 0.1–0.3 deaths/million doses sold |
vIncludes one code unrelated to ADE, ADR (eg, Y65.3 Endotracheal tube wrongly placed).
viThe authors used surveillance data from the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance (NEISS-CADES) and the Drug Abuse Warning Network (DAWN) Live!. Codes indicating anesthesia-related events that were not medication-relation have been omitted.
Admin, administrative; ADR, adverse drug reaction; AM, Australian modification; CDAD, Clostridium difficile-associated disease; ED, emergency department; HITS, heparin-induced thrombocytopenia; ICD, International Classification of Diseases; LQTS, long QT syndrome; NR, not reported; PV, pharmacovigilance; RS, retrospective; VS, vital statistics; VT, ventricular tachycardia.
We found a total of 827 individual ICD-10 codes that have been used in the health literature to identify adverse drug events (see supplementary appendix B, available online only, for the complete list of codes). Of these, 175 were external injury cause codes (Y40.0–59.9), and 652 disease manifestation codes. Only 13 disease manifestation codes only appeared in combination with a clustered code (table 5).52 61 Among studies examining adverse drug events in general, the median number of codes used was 190 (IQR 156–289). Seven studies used the external injury cause codes Y40.0–59.9 only,25 26 39 51 54 56 57 five studies used disease manifestation codes only,23 24 32 33 37 and 16 studies used a combination of both types of codes.14 21 22 29 34–36 38 40 42 43 49 50 52 53 58 Only one guideline recommended the use of algorithms to search for clustered codes, specifying external injury cause codes that should be clustered with specific disease manifestation codes in order to identify known adverse drug events.52 This list of clustered codes can be accessed freely online.61 The most common disease manifestation codes used are listed in table 6. Two authors independently assigned causality ratings to each ICD-10 code that had not previously had a causality rating assigned. The κ statistic as a measure of interrater agreement was 0.88 (95% CI 0.78 to 0.97).
Table 5.
Disease manifestation codes that were used only as clustered codes and never as stand-alone codes
| ICD-10 code | Description |
|---|---|
| D68.8 | Other specified coagulation defects |
| F05 | Delirium, not induced by alcohol and other psychoactive substances |
| I95 | Hypotension |
| L21 | Seborrhoeic dermatitis |
| L26 | Exfoliative dermatitis |
| L27 | Dermatitis due to substances taken internally |
| L28 | Lichen simplex chronicus and prurigo |
| L30 | Other dermatitis |
| R20 | Disturbances of skin sensation |
| R23 | Other skin changes |
| R40 | Somnolence, stupor and coma |
| R41 | Other symptoms and signs involving cognitive functions and awareness |
| R44 | Other symptoms and signs involving general sensations and perceptions |
ICD, International Classification of Diseases.
Table 6.
Top 15 disease manifestation codes used to identify adverse drug events in all studies
| ICD-10 code | Description |
|---|---|
| T88.6 | Anaphylactic shock due to the adverse effect of a drug |
| T88.7 | Unspecified adverse effect of a drug |
| N14.1 | Nephropathy induced by drugs, medicaments and biological substances |
| D59.0 | Drug-induced autoimmune hemolytic anemia |
| D59.2 | Drug-induced non-autoimmune hemolytic anemia |
| D61.1 | Drug-induced aplastic anemia |
| J70.4 | Drug-induced interstitial lung disorders |
| K71 | Toxic liver disease with cholestasis |
| K71.1 | Toxic liver disease with hepatic necrosis |
| K71.2 | Toxic liver disease with acute hepatitis |
| K71.6 | Toxic liver disease with hepatitis, not elsewhere classified |
| K71.9 | Toxic liver disease, unspecified |
| L56.1 | Drug photoallergic response |
| N14.2 | Nephropathy induced by unspecified drug, medicament or biological substance |
| T88.3 | Malignant hyperthermia due to anesthesia |
ICD, International Classification of Diseases.
Among studies looking for drug or drug class-specific adverse events, all studies reported the entire code set they used. The median number of codes that was used was three (IQR 1.5–10) (table 4). One study used external injury cause codes only,44 six studies used disease manifestation codes only,28 30 31 46 47 59 and six studies used a combination of both types of codes without any requirement for the codes to be clustered.27 41 45 48 55 60
Quality assessments
Among the 28 studies looking at adverse drug events in general, 19 reported an explicit definition of their primary outcome measure that was independent of the ICD-10 code set.14 21 22 25 29 32 36–40 42 43 49 51 54 56–58 Of these, 9 reported definitions for adverse drug reactions,29 36 39 40 49 51 56 57 62 5 definitions for adverse drug events,14 21 22 38 58 and one explicit definitions for both.54 Three studies used death as a result of poisoning or prescription drug use as primary outcome.37 42 43 Among the 13 studies on drug or drug class-specific adverse events, all provided definitions for their primary outcome measure with 4 using drug-induced deaths41 44 45 47 and 4 hospital admission due to an adverse drug event.30 46 55 59
Among the 28 studies looking at adverse drug events in general, 11 provided methods for their selection of ICD-10 codes.21–23 34–36 38 40 42 53 58 Methods included searching the ICD-10 code dictionary for diagnoses that could be attributable to medications (ie, gastric ulcer) and/or phrases (ie, ‘drug-induced’), empiric study of existing pharmacovigilance reports and mapping of events to the ICD-10 code dictionary,21 22 53 and literature review.21 22 34–36 Others adopted code sets that had previously been established by other authors.23 38 40 58
Only two studies estimated the sensitivity with which their code set may have ascertained the desired outcomes.38 40 Wu et al40 compared records containing adverse drug event-related diagnoses between two administrative databases. The authors’ premise was that for patients admitted to hospital through the emergency department for a drug-related diagnosis, the emergency department discharge and hospital admitting diagnoses should be similar. Using an ICD-10 code set containing 245 drug-related codes, including the external injury cause codes, Wu et al40 found that 15% of drug-related emergency department visits leading to hospital admission were coded with a corresponding admitting diagnosis. Wu et al40 estimated the specificity of their code set, and found it to be 99.7%. In comparison, in a study including both admitted and discharged emergency department patients, in which adverse drug events were identified prospectively by pharmacists and physicians, 6.8% of prospectively identified adverse drug events were identifiable using an ICD-10 code set consisting of diagnoses rated as definitely, very likely or likely to be related to medications.38 When the code set was broadened to include lower likelihood codes, 28.1% were identifiable with little drop in the code set's specificity (98.7–87.7%).
Three studies considered the uncertainty of the causal link between drug exposure and the adverse event, and provided analyses allowing the reader to ascertain the impact that this may have had on the study results.21 22 38
Discussion
Our objective was to synthesize a comprehensive set of ICD-10 codes and coding algorithms that have been used by health researchers to identify adverse drug events in administrative health data. Among 41 published studies, we found 827 ICD-10 codes that have been used for this purpose. There was a large degree of variability in the number and types of codes used between studies, and only one published guideline recommended the use of algorithms to identify external injury cause codes clustered with disease manifestation codes. Of the reviewed studies, two provided estimates of the code set's sensitivity and specificity.
Adverse drug events represent a growing public health concern.3 In the USA, adverse drug events represent the fourth to sixth leading cause of death, and are a frequent cause of unplanned hospitalizations, emergency department visits and ambulatory care encounters.5 10 63–65 The focus of the US$1 billion federal private–public initiative, Partnership for Patients, is to reduce hospital-acquired conditions by 40% and hospital readmissions by 20% by the end of 2013.66
In order to accomplish this target, the Partnership for Patients has identified the reduction of in-hospital adverse drug events as a priority. One intervention that the Partnership for Patients is promoting to accomplish this goal is medication reconciliation, a health systems intervention aimed at decreasing adverse drug events that result from the inaccurate transfer of medication information.67 68 However, to date little research has been conducted to describe and rank possible etiologies of adverse drug events, and as a result, it is largely unknown to what extent inaccurate transfer of medication information contributes to the development of clinically significant adverse drug events. Thus, it is not surprising that a recent systematic review of 26 controlled studies failed to find an effect of medication reconciliation on downstream health services use, mortality or cost.69 This example underscores the need for further development of innovative, evidence-based and effective patient safety strategies to reduce adverse drug events, and for their evaluation on health outcomes before their implementation outside of the research setting.
In order to inform the development of strategies to reduce preventable adverse drug events, the burden of disease in different healthcare settings and patient populations and their common etiologies need to be understood. This will help to prioritize and rationalize the development and evaluation of emerging strategies to prevent commonly occurring events associated with health services use and cost. Modifiable risk factors that can be targeted in carefully designed health systems interventions need to be identified. These may be related to medications, medication classes, treatment protocols, prescribing patterns, patient groups, provider groups, healthcare settings, and models of care, some of which may assist in developing successful interventions. Once developed, strategies are likely to benefit from refinement to enhance their feasibility of implementation and their performance. Finally, their impact on health outcomes and cost must be evaluated and compared with that of other health interventions in order to guide rational resource allocation and optimize health value for expenditure.
Population-level administrative health data that can be linked with medication dispensing data may represent a rich source of health information for this type of work. Adverse drug event data from this source may offer accessible and standardized population-level data over long time periods, enabling analysis of time trends, prescribing patterns, and comparisons across healthcare settings.16 70 However, no consensus presently exists among health researchers on how to identify adverse drug events reliably within such data sources, leading to substantial variability in the methods used for their identification.
Our study is the first systematically to review the health literature to synthesize a comprehensive set of ICD-10 codes previously used to identify adverse drug events. Previous studies have identified code sets by relying on ad hoc reviews of the literature, and mapping of drug-related diagnoses and pharmacovigilance case reports to the ICD-10 code dictionary. Most have adopted and used code sets developed by previous authors without conducting any validation studies to understand their sensitivity or specificity. When examining the code sets, common manifestations of adverse drug events have often been omitted (eg, E16.2 hypoglycemia), while the codes of rare events are commonly used (eg, T88.3 malignant hyperthermia due to anesthesia). This is problematic, as multiple studies relied entirely on disease manifestation codes to identify drug-related diagnoses. The omission of common manifestations of adverse drug events from their code sets based on the assumption that they might be associated with low positive predictive values would probably have dramatically influenced the numbers and types of adverse drug events found.
There is general agreement among health researchers that adverse drug events are underreported in administrative data, and that the effect of coding quality on adverse drug event identification is poorly understood.23 34 38–40 71 Based on our review, it is also possible that the use of incomplete code sets for adverse drug events may be a contributing factor. We found only two studies that evaluated the sensitivity of their ICD-10 code sets for adverse drug events, and both were low.38 40 Therefore, validation of a more comprehensive set of adverse drug event-related ICD-10 codes is necessary to try and enhance the sensitivity of the code sets used, while retaining specificity. This work needs to be conducted in a variety of care settings (eg, hospital vs ambulatory care), on a variety of adverse drug event types (eg, adverse drug reactions vs non-adherence), on different grades of adverse drug event severity (eg, severe vs mild), and by syndrome (eg, intracerebral hemorrhage vs epistaxis). Different clinical practice settings may influence the diagnostic performance of the code set(s) that is/are used, and may require refinements of the code sets used. Finally, it may be that administrative data may be well suited to tracking and investigating some consistently coded and identifiable adverse outcomes (eg, bleeding events), but not all manifestations of drug-related events (eg, delirium). Thus, we cannot recommend the adoption of our proposed code set without validation and further refinement. Instead, we present a comprehensive list of codes that we hope will provide the basis for further investigation, debate and consensus building in this area.
Due to the multiple ways in which adverse drug events may be coded (ie, by using external injury cause codes only, disease manifestation codes only, clusters of codes, or a combination of these methods), methodologies need to be developed to avoid double counting. Two of the studies we reviewed concluded that double counting was indeed possible when searching for adverse drug events using a combination of external injury cause codes and disease manifestation codes, and that this occurred in up to 15% of records.35 36 Similarly, studies need to be conducted to understand to what extent the use of disease manifestation codes (ie, E87.1 hyponatremia), which may indicate an adverse drug event or a non-drug-related event, may influence the sensitivity and specificity of the code set. To date, only one study has compared the sensitivity and specificity of narrower and broader code sets, and compared them to an independent prospective criterion standard in emergency department administrative data.38 In that study, while the broader code set led to higher sensitivity (6.8% vs 28.1%), broadening the code set had little impact on the code sets’ specificity.38 Unfortunately, the study did not examine coding quality to determine which steps during the patient care and coding trajectory may have contributed most to the under-coding of adverse drug events. Finally, methods to identify and understand the extent to which adverse events related to prescription medication use may be coded using codes that do not distinguish between prescription drugs and drugs of abuse (eg, F11 mental and behavioral disorders due to the use of opioids) need to be developed.
The most widely used definition for adverse drug events is ‘harm caused by the use of a drug’.2 72 In this study, we presented all definitions as reported by the study authors, as these may have led to variability in the code sets used. The existing inconsistency in the operational definition of adverse drug events needs to be addressed before being able to achieve consensus on a common code set(s), and may enhance the consistency with which adverse drug events are identified and reported, and thus comparability between studies. Given this limitation, we provided a comprehensive list of definitions and a corresponding code set that may serve as a point of reference for consensus building.
We did not attempt to meta-analyze data on the prevalence of adverse drug events, as this was not the objective of our study. In addition, significant differences in the ICD-10 code sets used to find adverse drug events are likely to result in significant heterogeneity between studies, and any differences that are found may simply be due to the methods used to identify them.
Ongoing national adaptations of the ICD-10 coding systems have introduced additional variability in the coding of adverse drug events that we were unable to account for. Not all studies described explicitly which national adaptation and versions of the coding dictionary they based their code set on. Some adaptations, for example, the German modification, may use additional two-decimal subcategorizations of individual disease manifestation codes that allow more refined coding than other systems. At present, the USA only uses ICD-10 coding for mortality reporting, explaining why all the US studies reported only on events related to death. Thus, while the majority of code categories are comparable across coding systems, the variability between national adaptations and coding versions used needs to be taken into account before application of any code set.
Additional limitations of our work are that we only reviewed publications in English, French and German. We also did not search extensively for abstracts or conference proceedings, as we thought we would be unlikely to find code sets published in these formats. We applied the only causality rating system that we are aware of for adverse drug event-related ICD-10 codes.21 22 While the causality rating system is based on clinical reasoning, and therefore inherently subjective, it may provide health researchers with a framework with which to start incorporating the certainty/uncertainty of drug-related causes to diagnoses identified in ICD-10. Therefore, we applied the previously proposed causality categories to additional ICD-10 codes that we identified through our review.
In conclusion, in this study we have synthesized a set of ICD-10 codes that have been used by health researchers to identify adverse drug events in administrative health data. Our code provides a basis for future work in establishing comprehensive and agreed-upon code sets that can be validated and refined for future work in this area.
Supplementary Material
Footnotes
Contributors: All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: CMH and JS. Acquisition of data: CMH, AK and LR. Analysis and interpretation of data: CMH and AK. Drafting of the manuscript: CMH. Critical revision of the manuscript for important intellectual content: All authors. Administrative, technical or material support: LR. Study supervision: CMH.
Competing interests: CMH is supported by a New Investigator Award from the Canadian Institutes of Health Research, and is a member of the Drug Safety and Effectiveness Network.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The ICD-10 code set is available from the authors.
Use of the AM modification likely, although unable to verify with authors.
Only codes associated with >1000 deaths and/or >1000 total mentions per year were listed.
Jackson et al describe the CHADx algorithms to identify hospital acquired diagnoses, including adverse drug events (ADE). The CHADx code set and algorithms are published on the Australian Commission on Quality and Safety in Healthcare website. Jackson et al. recommend searching for ADE using disease manifestation codes clustered with external injury cause codes (Y40–59). C-prefixes are codes that were introduced in the Victorian addition of the Australian Modification of ICD-10.
The difference in the number of codes used by Stausberg et al. has to do with the splitting of code E66.1 (in ICD-10-German Modification 2006) into the four codes: E66.10, E66.11, E66.12 and E66.19 (in ICD-10-German Modification 2008).
Includes one code unrelated to ADE, ADR (eg, Y65.3 Endotracheal tube wrongly placed).
The authors used surveillance data from the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance (NEISS-CADES) and the Drug Abuse Warning Network (DAWN) Live!. Codes indicating anesthesia-related events that were not medication-relation have been omitted.
References
- 1.Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: US prescription drug data for 2007–2008. NCHS Data Brief 2010. http://www.cdc.gov/nchs/data/databriefs/db42.pdf (accessed 9 Feb 2013).
- 2.Nebeker J, Barach P, Samore M. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Ann Intern Med 2004;140:795–801 [DOI] [PubMed] [Google Scholar]
- 3.Bates D. Drugs and adverse drug reactions. How worried should we be? JAMA 1998;279:1216–17 [DOI] [PubMed] [Google Scholar]
- 4.Slone Epidemiology Center. Patterns of Medication Use in the United States 2004: a Report from the Slone Survey. 2004. http://www.bu.edu/slone/SloneSurvey/AnnualRpt/SloneSurveyWebReport2006.pdf (accessed April 2010).
- 5.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003;289:1107–16 [DOI] [PubMed] [Google Scholar]
- 6.Tamblyn R. Evidence-based utilization of prescription drugs: challenges and directions for the future in Canada. Institute for Research on Public Policy, 2001
- 7.Hohl CM, Nosyk B, Zed P, et al. Outcomes of emergency department patients presenting with adverse drug events. Ann Emerg Med 2011;58:270–9 [DOI] [PubMed] [Google Scholar]
- 8.WHO. International drug monitoring: the role of national centres. Report of a WHO Meeting, Vol 498 Geneva: World Health Organization; 1972:1–44 [PubMed] [Google Scholar]
- 9.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004;329:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarou J, Pomeranz B, Corey P. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998;279:1200–5 [DOI] [PubMed] [Google Scholar]
- 11.Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 2008;42:1017–25 [DOI] [PubMed] [Google Scholar]
- 12.Wiffen P, Gill M, Edwards J, et al. 2002. Adverse drug reactions in hospital patients. A systematic review of the prospective and retrospective studies. Bandolier extra. http://www.medicine.ox.ac.uk/bandolier/extraforbando/adrpm.pdf.
- 13.Classen D, Pestotnik S, Evans S, et al. Adverse drug events in hospitalized patients. JAMA 1997;277:301–6 [PubMed] [Google Scholar]
- 14.Phillips DP, Barker GEC, Eguchi MM. A steep increase in domestic fatal medication errors with use of alcohol and/or street drugs. Arch Intern Med 2008;168:1561–6 [DOI] [PubMed] [Google Scholar]
- 15.The WHO Research Priority Setting Working Group. Global priorities for research in patient safety (first edition). Geneva: World Health Organization, 2008 [Google Scholar]
- 16.Suissa S, Garbe E. Primer: administrative health databases in observational studies of drug effects—advantages and disadvantages. Nat Clin Pract Rheumatol 2007;3:725–32 [DOI] [PubMed] [Google Scholar]
- 17.Agency for Healthcare Research and Quality. Developing a Protocol for Observational Comparative Effectiveness Research (OCER): A User's Guide (XXX under Contract No. XXX HHSA) 2012. http://www.effectivehealthcare.ahrq.gov/ehc/products/440/1067/AHRQ_CER_Protocol_User_s_Guide_DRAFT-COPY_AllChapters.pdf [DOI] [PubMed]
- 18.World Health Organization. International statistical classification of diseases and related health problems 10th revision. 2004; Second, Volume 3. http://apps.who.int/classifications/icd10/browse/2010/en (accessed 14 Jul 2011).
- 19.Centre for Reviews and Dissemination. Systematic reviews: CDR's guidance for undertaking reviews in health care. 3rd edn. York: York Publishing Services Ltd, 2009 [Google Scholar]
- 20.Dreyer NA, Schneeweiss S, McNeil BJ, et al. GRACE Principles: recognizing high-quality observational studies of comparative effectiveness. Am J Manag Care 2010;16:467–71 [PubMed] [Google Scholar]
- 21.Stausberg J, Hasford J. Identification of adverse drug events: the use of ICD-10 coded diagnoses in routine hospital data. Dtsch Arztebl Int 2010;107:23–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stausberg J, Hasford J. Drug-related admissions and hospital-acquired adverse drug events in Germany: a longitudinal analysis from 2003 to 2007 of ICD-10-coded routine data. BMC Health Serv Res 2011;11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrow P, Waller P, Wise L. Comparison of hospital episodes with ‘drug-induced’ disorders and spontaneously reported adverse drug reactions. Br J Clin Pharmacol 2005;61:233–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benkhaial A, Kaltschmidt J, Weisshaar E, et al. Prescribing errors in patients with documented drug allergies: comparison of ICD-10 coding and written patient notes. Pharm World Sci 2009;31:464–72 [DOI] [PubMed] [Google Scholar]
- 25.Bergman U, Mejyr S, Holm L, et al. Pharmacovigilance and patient safety—experiences from a Regional Pharmacovigilance Centre (RPVC) in Stockholm, Sweden. Basic Clin Pharmacol Toxicol 2010;107:111 [Google Scholar]
- 26.Cox AR, Anton C, Goh CHF, et al. Adverse drug reactions in patients admitted to hospital identified by discharge ICD-10 codes and by spontaneous reports. Br J Clin Pharmacol 2001;52:337–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elalamy I, Gal GL, Nachit-Ouinekh F, et al. Heparin-induced thrombocytopenia: an estimate of the average cost in the hospital setting in France. Clin Appl Thromb Hemost 2009;15:428–33 [DOI] [PubMed] [Google Scholar]
- 28.Gaus W, Westendorf J, Diebow R, et al. Identification of adverse drug reactions by evaluation of a prescription database, demonstrated for “Risk of Bleeding”. Methods Inf Med 2005;5:697–703 [PubMed] [Google Scholar]
- 29.Lugardon S, Desboeuf K, Fernet P, et al. Using a capture–recapture method to assess the frequency of adverse drug reactions in a French university hospital. Br J Clin Pharmacol 2006;62:225–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyytikäinen O, Turunen H, Sund R, et al. Hospitalizations and deaths associated with Clostridium difficile infection, Finland, 1996–2004. Emerg Infect Dis 2009;15:761–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molokhia M, Pathak A, Lapeyre-Mestre M, et al. Case ascertainment and estimated incidence of drug-induced long-QT syndrome: study in southwest France. Br J Clin Pharmacol 2008;66:386–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moneret-Vautrin DA, Morisset M, Flabbee J, et al. Epidemiology of life-threatening and lethal anaphylaxis: a review. Allergy 2005;60:443–51 [DOI] [PubMed] [Google Scholar]
- 33.Osmont M, Bayat S, Polard E, et al. ICD-10 based queries to detect serious adverse drug reactions: 1-year evaluation in Rennes University Hospital. Fundam Clin Pharmacol 2011;25:21 [Google Scholar]
- 34.Patel H, Bell D, Molokhia M, et al. Trends in hospital admissions for adverse drug reactions in England: analysis of national hospital episode statistics 1998–2005. BMC Clin Pharmacol 2007;7:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waller P, Shaw M, Ho D, et al. Hospital admissions for ‘drug-induced’ disorders in England: a study using the Hospital Episodes Statistics (HES) database. Br J Clin Pharmacol 2004;59:213–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu T-Y, Jen M-H, Bottle A, et al. Ten-year trends in hospital admissions for adverse drug reactions in England 1999–2009. JRSM 2010;103:239–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Unintentional and undetermined poisoning deaths-11 states, 1990–2001. MMWR 2004;53:233. [PubMed] [Google Scholar]
- 38. Hohl CM, Kuramoto L, Yu E, et al. Evaluating Adverse Drug Event Reporting in Administrative Data from Emergency Departments: A Validation Study. BMC Health Services Research. In press. [DOI] [PMC free article] [PubMed]
- 39.Shepherd G, Mohorn P, Yacoub K, et al. Adverse drug reaction deaths reported in United States vital statistics, 1999–2006. Ann Pharmacother 2012;46:169–75 [DOI] [PubMed] [Google Scholar]
- 40.Wu C. Adverse drug reactions in the Emergency Department Population in Ontario: analysis of National Ambulatory Care Reporting System and Discharge Abstract Database 2003–2007. Toronto: Graduate Department of Health Policy, Management and Evaluation, University of Toronto, 2009 [Google Scholar]
- 41.Wysowski DK. Deaths attributed to X-ray contrast media on U.S. death certificates. AJR Am J Roentgenol 2006;186:613. [DOI] [PubMed] [Google Scholar]
- 42.Wysowski DK. Surveillance of prescription drug-related mortality using death certificate data. Drug Saf 2007;30:533–40 [DOI] [PubMed] [Google Scholar]
- 43.Wysowski DK, Nourjah P. Analyzing prescription drugs as causes of death on death certificates. Public Health Rep 2004;119:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use. Arch Intern Med 2007;167:1414–19 [DOI] [PubMed] [Google Scholar]
- 45.Li G, Warner M, Lang B, et al. Epidemiology of anesthesia-related mortality in the United States, 1999–2005. Anesthesiology 2009;110:759–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myers RP, Leung Y, Shaheen AA, et al. Validation of ICD-9-CM/ICD-10 coding algorithms for the identification of patients with acetaminophen overdose and hepatotoxicity using administrative data. BMC Health Serv Res 2007;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sims SA, Snow LA, Porucznik CA. Surveillance of methadone-related adverse drug events using multiple public health data sources. J Biomed Inform 2007;40:382–9 [DOI] [PubMed] [Google Scholar]
- 48.Wysowski DK, Swartz L, Borders-Hemphill BV, et al. Use of parenteral iron products and serious anaphylactic-type reactions. Am J Hematol 2010;85:650–4 [DOI] [PubMed] [Google Scholar]
- 49.Burgess CL, Holman CDAJ, Satti AG. Adverse drug reactions in older Australians, 1981–2002. Med J Aust 2005;182:267–70 [DOI] [PubMed] [Google Scholar]
- 50.Hauck K, Zhao X. How dangerous is a day in hospital? A model of adverse events and length of stay for medical inpatients. Med Care 2011;49:1068–75 [DOI] [PubMed] [Google Scholar]
- 51.Hodgkinson MR, Dirnbauer NJ, Larmour I. Identification of adverse drug reactions using the ICD-10 Australian modification clinical coding surveillance. J Pharm Pract Res 2009;39:19–23 [Google Scholar]
- 52.Jackson TJ, Michel JL, Roberts RF, et al. A classification of hospital-acquired diagnoses for use with routine hospital data. Med J Aust 2009;191:544–8 [DOI] [PubMed] [Google Scholar]
- 53.Malpass A, Helps SC, Sexton EJ, et al. A classification for adverse drug events. J Qual Clin Pract 1999;19:23–6 [DOI] [PubMed] [Google Scholar]
- 54.Runciman WB, Roughead EE, Semple SJ, et al. Adverse drug events and medication errors in Australia. Int J Qual Health Care 2003;15:i49–59 [DOI] [PubMed] [Google Scholar]
- 55.Sood S, Howell J, Sundarajan V, et al. Epidemiology of paracetamol overdose in Victoria over 7 years. J Gastroenterol Hepatol 2010;25(Suppl. 3):A34 [Google Scholar]
- 56.Zhang M, D'Arcy C, Holman J, et al. Comorbidity and repeat admission to hospital for adverse drug reactions in older adults: retrospective cohort study. BMJ 2009;338:2752–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang M, Holman CDAJ, Preen DB, et al. Repeat adverse drug reactions causing hospitalization in older Australians: a population-based longitudinal study 1980–2003. Br J Clin Pharmacol 2007;63:163–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang S-H, Lee S, Koo H-K, et al. Evaluation of a computer-based adverse drug-event monitor. Am J Health-Syst Pharm 2008;65:2265–72 [DOI] [PubMed] [Google Scholar]
- 59.Rhee CW, Kang DY, Park SY, et al. Concomitant use of diuretics and risk of digoxin intoxication among elderly heart failure patients. Pharmacoepidemiol Drug Saf 2010;19:S267 [Google Scholar]
- 60.Treeprasertsuk S, Huntrakul J, Ridtitid W, et al. The predictors of complications in patients with drug-induced liver injury caused by antimicrobial agents. Aliment Pharmacol Ther 2010;31:1200–7 [DOI] [PubMed] [Google Scholar]
- 61.Australian Commission on Safety and Quality in Healthcare. Classification of Hospital Acquired Diagonses. 2012; 4. http://www.safetyandquality.gov.au/our-work/information-strategy/health-information-standards/ (accessed 6 Jan 2013).
- 62.Bergman U, Mejyr S, Odar-Cederlo I, et al. Pharmacovigilance and patient safety—a plea for regional pharmacovigilance centres. Basic Clin Pharmacol Toxicol 2009;105(Suppl. 1):3019371263 [Google Scholar]
- 63.Budnitz DS, Lovegrove MC, Shehab N, et al. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011;365:2002–12 [DOI] [PubMed] [Google Scholar]
- 64.Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med Dec 2001;38:666–71 [DOI] [PubMed] [Google Scholar]
- 65.Zed PJ, Abu-Laban RB, Balen RM, et al. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. CMAJ 2008;178:1563–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parternship for Patients. http://partnershipforpatients.cms.gov/about-the-partnership/aboutthepartnershipforpatients.html (accessed 29 Apr 2013).
- 67.Accreditation Canada. Required Orgnanizational Practice. 2011. http://www.accreditation.ca/uploadedFiles/ROP%20Handbook%20EN.pdf (accessed 20 Jun 2011).
- 68.Improvement IfH. 5 Million Lives Campaign. Getting Started, Kit: Prevent Adverse Drug Events (Medication Reconciliation) How-to Guide. Protective 5 Million Lives from harm Cambridge, MA: Institute for Healthcare Improvement, 2008 [Google Scholar]
- 69.Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices. Arch Intern Med 2012;172:1057–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Velentgas P, Dreyer N, Nourjah P, et al., eds. Developing a protocol for observational comparative effectiveness research: a user's guide. AHRQ Publication No. 12(13)-EHC099. 2013. http://www.effectivehealthcare.ahrq.gov/Methods-OCER.cfm [PubMed]
- 71.Schneeweiss S, Gagne JJ, Glynn RJ, et al. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther 2011;90:777–90 [DOI] [PubMed] [Google Scholar]
- 72.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356:1255–9 [DOI] [PubMed] [Google Scholar]
- 73.Classen D, Pestotnik S, Evans S, et al. Computerized surveillance of adverse drug events in hospital patients. JAMA 1991;266:2847–51 [PubMed] [Google Scholar]
- 74.J Jha AK, Kuperman GJ, Teich JM, et al. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc 1998;5:305–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raschke R, Gollihare B, Wunderlich T, et al. A computer alert system to prevent injury from adverse drug events. JAMA 1998;280:1317–20 [DOI] [PubMed] [Google Scholar]
- 76.VHA. Monitoring adverse drug events: finding the needle in haystack. Irving, Texas: VHA Research Series, 2002 [Google Scholar]
- 77.Stausberg J. ICD-10-GM-Kodes für unerwünschte Arzneimittelereignisse (UAE). 2012. http://www.ekmed.de/routinedaten/main4.php (accessed 11 Dec 2012).
- 78.Schneeweiss S, Hasford J, Gottler M, et al. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. Eur J Clin Pharmacol 2002;58:285–91 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.