Abstract
Children are a vulnerable population in the operating room, and are particularly at risk of complications from unanticipated hemorrhage. The decision to prepare blood products prior to surgery varies depending on the personal experience of the clinician caring for the patient. We present the first application of a data visualization technique to study large datasets in the context of blood product transfusions at a tertiary pediatric hospital. The visual analytical interface allows real-time interaction with datasets from 230 000 procedure records. Clinicians can use the visual analytical interface to analyze blood product usage based on procedure- and patient-specific factors, and then use that information to guide policies for ordering blood products.
Keywords: clinical decision support, maximum surgical blood order schedule, pediatric, anesthesiology, visual analytics, transfusion
Background and significance
Timely ordering and administration of blood products during surgery can be critical and life saving for children; however, blood products are a limited resource worldwide. Clinicians traditionally use approaches such as a maximum surgical blood order schedule (MSBOS) to guide decisions to order blood products for a procedure.1 MSBOS policies are rare in pediatrics primarily because smaller volumes of blood products are required. In the USA, increased use of blood products has resulted in smaller blood product supply pools and more frequent shortages, with negative consequences.2 For instance, blood product shortages forced the cancelation of elective surgical cases in 12% of hospitals across the USA in 2001, and delay of availability of blood products has been linked to death.2–5 Thus clinicians must anticipate the need for blood products for children undergoing surgical procedures, so that they are available for patients at high risk of hemorrhage and to minimize unnecessary orders for blood products in low-risk procedures.6
Clinicians need data-driven decision support to identify children at high risk of surgery-induced hemorrhage and anticipate the potential need for transfusion. Electronic health records (EHRs) have been shown to provide a foundation for analytical models that reliably assess actual transfusion requirements.7–10 While a data-driven MSBOS tool has been described previously, that study excluded patients younger than 18 years old because of the wide variations in total volume of blood products transfused in pediatric patients.8
Visual analytical techniques can be used to rapidly analyze relationships between variables by dynamically interacting with all of the variables within the database.11–13 This approach has been used to evaluate business processes in order to gain new perspectives about big data sets.14 In this article, we describe the application of the visual analytical approach to providing dynamic, individualized, surgical blood product clinical decision support.
Materials and methods
The Institutional Review Board at The Children's Hospital of Philadelphia approved this study. The anesthesia information management system (AIMS) (CompuRecord; Phillips, Andover, Massachusetts, USA) data warehouse and the blood bank database (BB) (MEDITECH, Westwood, Massachusetts, USA) were queried for anesthesia and blood transfusion records dated from October 1, 2001 to December 31, 2010. This includes patients throughout the entire hospital, ranging from premature newborns to adults with congenital conditions and obstetric patients with high-risk fetuses. All of the records for patients satisfying the inclusion criteria were retrieved for analysis. Patients who received blood products during surgery were identified from the AIMS records, and then cross-referenced to the BB records to identify patients who had blood products prepared within 72 h of surgery. The exclusion criteria involved any patients in the study period who did not have a procedure documented in the AIMS database.
We retrieved each patient's age, weight, date of birth, service date, gender, procedure timestamps, American Society of Anesthesiologists (ASA) Physical Status,15 pre-defined surgical procedure categories, International Classification of Disease (ICD-9), Current Procedural Terminology (CPT-4) and case length in minutes. International Classification of Disease (ICD-9), Current Procedural Terminology (CPT-4) and case length in minutes. We categorized ICD-9 codes using the Clinical Classification Software developed by the Agency for Healthcare Research and Quality (AHRQ).16 The CPT-4 codes were referenced with cross-walks developed by the ASA.17
Patient subcategories were created for each of the demographic variables. The user interface allows selection of any range for the age or weight variables. We created predefined age category filters to fit the distribution of the study. The groups were defined as younger than 1 month, 1–6 months, 6 months to 1 year, 1–3 years, 3–10 years, and older than 10 years. Weight was treated as a continuous variable. The case length was calculated from the AIMS database timestamps denoting the patient arrival and departure times in the operating room.
The blood product data included the following categories: packed red blood cells, fresh-frozen plasma, platelets, cryoprecipitate, fresh whole blood, and reconstituted blood. Reconstituted blood consists of a 1:1 mixture of packed red blood cells and fresh-frozen plasma.18 In order to facilitate comparison of transfusions across various groups, we calculated the aggregated volume of blood products transfused divided by the patient's weight. The aggregate measurement consisted of the sum of volumes for packed red blood cells, reconstituted blood, and fresh whole blood. The resulting measure of volume/mass (mL/kg) of blood product transfused is used routinely in clinical practice.
The aforementioned databases were combined, cleansed, preprocessed, and reduced to a dimensional model using a visual analytical software tool (Qlikview; QlikTech, Radnor, Pennsylvania, USA).19 A graphical user interface in Qlikview was designed to allow the exploration and identification of trends, patterns, correlations, and data distributions, and the detection of outliers of the study variables through the use of color, interactive navigation, and graphical zooming.
Each numerical variable was evaluated independently using frequency analysis histograms, which were generated for each numerical variable (y-axis, frequency; x-axis, variable). Bar charts were used to display categorical data and nominal, ordinal, and interval variables, while scatter plots were used to explore data relationships and types. Finally, we took advantage of the associative in-memory engine represented by graphical data filters to query the entire dataset, which allowed us to explore every variable and its association with every other data point anywhere in the entire schema in real time.
Descriptive statistics (mean, SD, median, minimum, maximum, percentage of total) of all blood product types were developed into the model for analysis of the selected procedures. Outlying values in the upper/lower 1% in each variable were systematically flagged for individual chart review to assess data accuracy.
Results
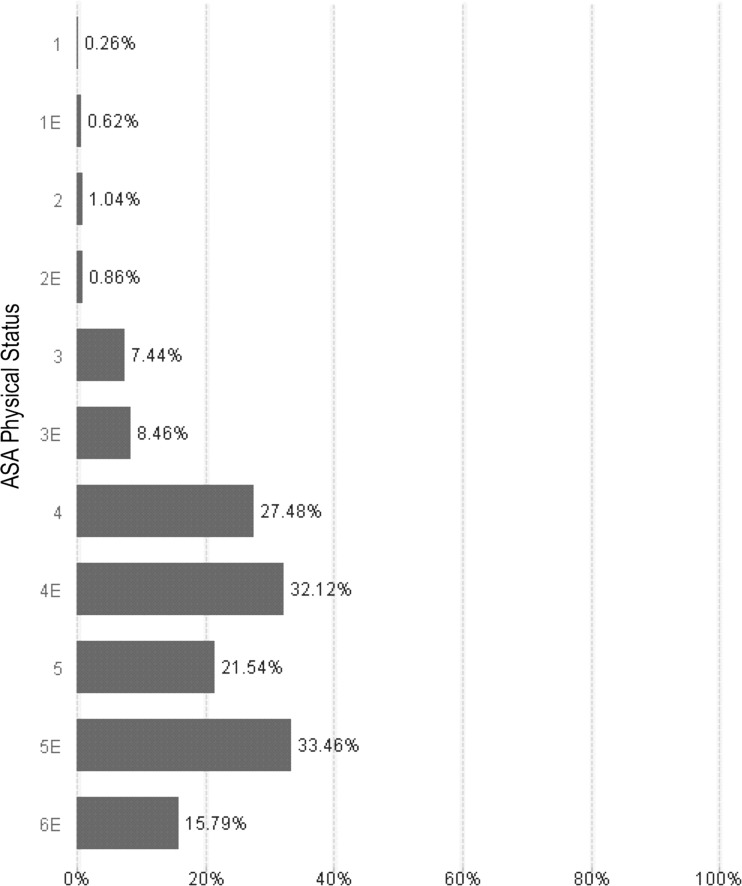
We retrieved 231 073 surgical procedure records from the AIMS database; a total of 3478 different types of surgical procedures were performed during the study period. The records were cross-referenced to the BB database to retrieve all associated data about blood products prepared for each patient. The databases were linked with a composite key consisting of each patient's medical record number and service date. A total of 8156 procedures required blood transfusions as identified by the administration of any blood product component. Our patient population age ranged from 0 days to 67 years, although the majority of the patients were younger than 18 years of age. The age distribution of patients who received blood transfusions is categorized in table 1. The visual interface allowed evaluation of relationships between variables, such as transfusion events and ASA physical status. Figure 1 shows the ASA physical status profile and associated transfusion rates for our patient population. Other filters such as weight, age, and procedure code can be applied using the analytical tool and are reflected on the graphs and histograms.
Table 1 .
Distribution of patients receiving blood transfusions in each age group
| Age group | No of procedures | No of patients transfused | Percentage transfused | Volume required (mL/kg) | Transfusion* | No transfusion* | ||
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | Length (min) | Weight (kg) | Length (min) | |||||
| 1 month or younger | 6532 | 1556 | 23.8 | 36.23 (47.64) | 2.98 (0.73) | 159.55 (75.37) | 3.31 (2.92) | 75.25 (61.19) |
| 1–6 months | 12 291 | 1915 | 15.6 | 21.84 (25.60) | 4.76 (1.54) | 139.01 (81.44) | 5.11 (2.15) | 65.72 (55.40) |
| 6 months to 1 year | 15 769 | 842 | 5.3 | 30.39 (31.74) | 7.42 (1.64) | 175.7 (90.60) | 8.62 (6.94) | 45.67 (51.77) |
| 1–3 years | 46 506 | 985 | 2.1 | 22.2 (28.13) | 11.4 (2.47) | 181.39 (99.90) | 11.96 (6.55) | 31.92 (46.14) |
| 3–10 years | 86 785 | 1150 | 1.3 | 20.75 (27.56) | 19.68 (8.31) | 201.3 (134.00) | 22.52 (9.09) | 37.84 (48.00) |
| 10 years or older | 63 062 | 1706 | 2.7 | 12.92 (15.90) | 53.58 (19.10) | 271.06 (164.00) | 57.33 (21.79) | 65.5 (71.00) |
| All age groups | 230 945 | 8154 | 3.5 | 23.5 (31.70) | 17.8 (21.30) | 188.30 (123.10) | 27.8 (22.70) | 46.9 (57.80) |
Values are mean (SD).
*Patients were divided into two groups based on administration of any blood product (Transfusion) or no blood product administration during surgery (No transfusion). The patient weight and surgery length is shown for each subgroup.
Figure 1.
The American Society of Anesthesiologists (ASA) physical status categories describe patients as: (1) healthy (n1=63 315; n1E=4521); (2) mild systemic disease (n2=89 524; n2E=4430); (3) severe systemic disease (n3=56 459; n3E=3805); (4) severe systemic disease that is a constant threat to life (n4=6306; n4E=1943); (5) moribund, not expected to survive without surgery (n5=65; n5E=263); (6) declared brain dead, organ donor (n6=15; n6E=19)15. The ‘E’ modification indicates emergency surgery. We used the ASA physical status as a primary filter to identify patients who received blood transfusions within each ASA group. The incidence and proportion of patients receiving blood transfusions at each classification increases with severity of disease and emergency surgery.
Data validation
Two reviewers validated the accuracy of the database records during the data exploration phase and reviewed the following patient variables: age, weight, case length, ASA physical status, blood product categories, procedure category, and ICD-9 and CPT-4 codes. Manual chart review was performed to investigate the outliers that were identified during analysis of the transfusion histograms. For example, figure 2 depicts the frequency histogram of the ages (in days) of patients who received blood products. The ages of 79 patients were identified as >60 years; 77 patients were >100 years old. The correct ages of the outliers were determined by comparing each patient's date of birth with the date of the procedure. When applicable, the analytical database was updated with the correct information, although the original data were preserved for further reference. For each variable, >0.1% of the records were associated with possible data entry errors or missing values. Records that were identified as erroneous or could not be corrected were excluded from the analytical database; 84 records were excluded in this fashion.
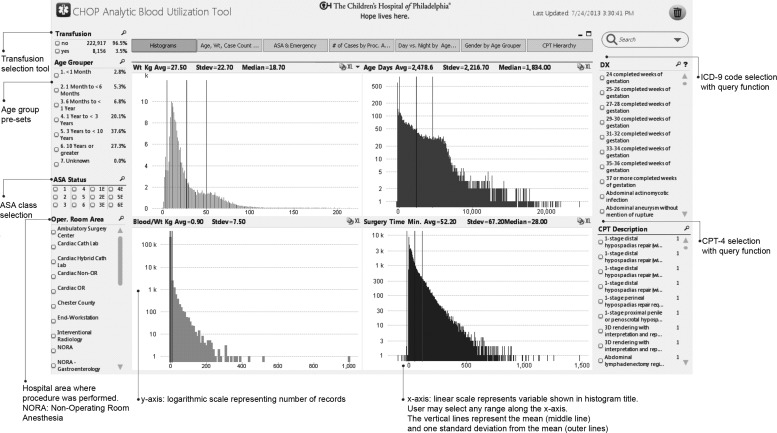
Figure 2.
The user interface allows selection of any number of filters. The ‘Transfusion’ section denotes patients who received any blood product or patients who did not receive any blood products during surgery. The ‘Age Grouper’ allows selection of predefined age categories. The ‘ASA Status’ allows selection of any American Society of Anesthesiologists (ASA) classification. The ‘Oper. Room Area’ allows selection of procedures based on the location within the hospital. On the right side of the screen, the ‘DX’ code represents the International Classification of Disease (ICD)-9 codes and the ‘CPT (Current Procedural Terminology) Description’ allows selection of specific procedures. Both the DX and CPT sections allow free-text search. The histograms displayed represent data distribution of individual variables. The vertical lines represent the average (red) and 1SD (gray). The top left histogram represents weight, top right is age in days, bottom left is the total blood product volume transfused in mL divided by the patient's weight (mL/kg), and the bottom right represents the surgery time (in min). The histograms update automatically when filters are applied.
Clinical use cases
After developing and validating the analytical dataset, we developed the user interface shown in figure 2. The broad procedure category filters were applied to evaluate the distribution of blood product transfusions within each age category. An example display shown in table 2 gives the surgery categories associated with the highest number of cases requiring blood products for the 0–1 month age group.
Table 2.
Top 15 procedure categories associated with blood product transfusion for patients ≤1 month of age
| Procedure | Volume required (mL/kg) | Percentage of patients transfused | No of procedures with transfusion | Total number of procedures |
|---|---|---|---|---|
| Cardiac (cardiopulmonary bypass) | 38.32 | 82 | 1011 | 1237 |
| Intracranial (excluding shunts) | 58.23 | 65 | 11 | 17 |
| Fetal surgery | 7.14 | 50 | 1 | 2 |
| Neonatal emergency (<1 month; premature; <45 weeks post-conceptual age) | 52.88 | 30 | 17 | 57 |
| Necrotizing enterocolitis | 38.36 | 29 | 5 | 17 |
| Premature infant—non-emergency (<45 weeks post-conceptual age) | 93.7 | 27 | 4 | 15 |
| Cardiac catheterization | 20.59 | 27 | 134 | 504 |
| Cardiac (no cardiopulmonary bypass) | 24.58 | 23 | 181 | 802 |
| Neonatal bowel obstruction | 14.63 | 20 | 3 | 15 |
| Intrathoracic/non-cardiac (intracavitary) | 20.73 | 16 | 24 | 152 |
| CT scan* | 65.43 | 14 | 3 | 21 |
| Craniofacial | 20 | 14 | 1 | 7 |
| Thoracic (superficial) | 14.29 | 14 | 1 | 7 |
| Gastroschisis | 14.33 | 12 | 5 | 41 |
| Plastic/reconstructive | 18 | 12 | 2 | 17 |
The list is ranked by the percentage of each procedure group associated with blood product transfusions. The source of the procedure category is data entry at the AIMS user interface. This display allowed us to identify data entry errors, such as cardiac procedures with cardiopulmonary bypass, that are not associated with blood transfusions. Chart review revealed that these procedures were mislabeled as cardiopulmonary bypass procedures.
*Further examination revealed CT scans associated with blood transfusions. These charts were flagged for review and noted that CT scan consisted of one of the procedures performed during the anesthetic.
AIMS, anesthesia information management system.
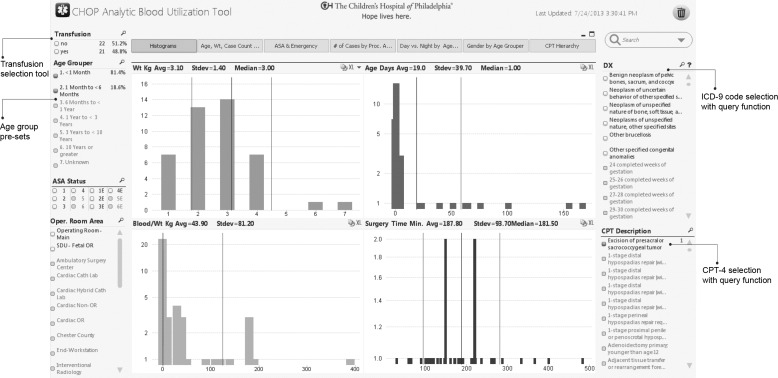
The interface allows clinicians preparing for a procedure to rapidly determine the historical frequency of blood product utilization during specific procedures, and then customize the transfusion data by age, weight, ASA physical status, and comorbidities. For example, an anesthesiologist preparing for a nephrectomy in a 1–3-year-old patient can rapidly determine that the historical frequency of blood product utilization was 13.4%, and the mean (SD) blood product requirement was 11 (3.3) mL/kg packed red blood cells. A use-case scenario based on neonatal excision of a sacrococcygeal teratoma is displayed in figure 3.
Figure 3.
A real use case based on a clinical inquiry for transfusion requirements for neonates and infants younger than 6 months undergoing resection of a sacrococcygeal teratoma. This condition is usually present at birth and typically requires large transfusion volumes. The initial Current Procedural Terminology (CPT) filter retrieved 43 patients (age 0 days to 6 months), of which 21 received blood transfusions. The mean (SD) weight for the patients in this selection was 3.1 (1.4) kg, age was 19 (39.7) days, and transfusion requirement was 43.9 (81.20) mL/kg. For reference, the circulating blood volume in a term newborn is ∼90 mL/kg. ICD, International Classification of Disease.
Discussion
In this study, we demonstrated the first reported use of a visual analytical interactive tool for evaluating comprehensive transfusion practice in a pediatric hospital. EHR database analysis to develop an MSBOS has been previously applied in an adult surgical setting.7 Although that study evaluated blood product use by type of surgical procedure and intraoperative transfusion requirements, it excluded patients under 18 years of age. Pediatric patients were excluded because of variable blood product requirements, which in children are routinely determined in volume per body mass (mL/kg). Keung et al20 described transfusion requirements in a pediatric cohort, but limited the evaluation to packed red blood cells used in the perioperative period. Both of these studies focused their analysis on the absolute number of packed red blood cell units used, and omitted other blood product components.
There are several limitations to our study related to the inherent data quality issues that can arise when using administrative data as well as data collected in the process of providing clinical care.
First, this visual analytical tool draws from a clinical data warehouse, which incorporates data from several sources that require subject-matter expertise to interpret. The process to identify procedures relied on CPT-4 codes to establish subgroups of procedures. The CPT-4 codes were organized into subgroups with hierarchical crosswalk tools such as the ASA 2012 Crosswalk and the AHRQ Clinical Classification Software Tool.16 17 However, the procedure codes reflect the reported billing data, which may not reflect the comprehensive details found in the medical history and procedure notes.
Second, manual data entry errors in the anesthesia record (eg, volume and type of blood product being transfused) were unavoidable. These values were cross-checked against the BB, which is the gold standard for transfusion records. These data are validated at the time of data entry by two-person verification during blood product labeling, release from blood bank, and administration to the patient. There is a very low likelihood of error in the number of units administered, and a higher chance of error for the volume issued versus the volume transfused. The latter error is not clinically important because the pre-procedure orders are mostly based on units of blood rather than a specific volume or fraction of a unit. Despite the potential for data entry errors regarding blood volumes administered, the database can identify procedures where blood product administration occurred. Our ultimate goal is to identify procedures where we can anticipate the need to order blood products, and, if so, how many units are indicated.
The concept of applying decision support to blood product ordering is well described. The MSBOS approach, originally developed by Friedman, defined a list of surgical procedures with specific recommendations for blood product allocation.1 This approach has been applied in various settings, ranging from subspecialty specific to generalized recommendations.8 21 However, application of MSBOS in pediatric hospitals is not widespread, and is typically limited to subsets of procedures that routinely use blood transfusions, such as cardiovascular procedures. To date, there are no reports of data-driven MSBOS systems for pediatric populations. Our approach offers the advantage of dynamic, patient-specific decision support. In addition, the visual analytical tool enables the enterprise-scale evaluation of transfusion practice, and can assist in the development of institutional MSBOS policies and guidelines.7 8 22
In summary, the visual analytical interface we designed enables clinicians to perform rapid analysis of the historical transfusion practices across procedures and age groups. This is the first reported use of an interactive visual analytical tool specifically designed to study perioperative transfusion practice in a large surgical cohort in a free-standing children's hospital. Clinicians are able to navigate the database in real time to gain understanding of actual practice to guide decisions for similar patients. Future work will determine if accessing these data can improve blood product allocation by ensuring that high-risk patients have blood products available while reducing unnecessary blood tests for low-risk patients. Integration of this data visualization tool with the EHR will allow near-real-time evaluation of evolving transfusion trends in the institution as a quality outcome metric.
It is our goal to use this platform to enhance blood use management and standardize transfusion practices at our institution. As a part of this process, we plan to evaluate the tool in the development of policies for blood product recommendations with expert panels at our institution. Furthermore, any changes in practice will be evaluated by monitoring transfusion practice and blood product availability prior to the procedure.
Footnotes
Contributors: The following contributed to conception and design, acquisition of data or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published: JAG, LA, AFS, EEL, CPB, DC, WRE, DF, DAS-P, MAR. AFJ contributed to conception and design, analysis and interpretation of data and revising the article for important intellectual content. JAG is guarantor for the integrity of the work, from inception to published article.
Funding: This work was supported by the McCabe Foundation, which played no role in the study design, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the paper for publication.
Competing interests: None.
Ethics approval: Institutional Review Board at the Children's Hospital of Philadelphia.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The database contains information that could potentially be used to identify patients. As such, we are not making the current database accessible to the public. The cross-walks we used for this database are accessible via the Agency for Healthcare Quality Improvement and will not be duplicated here.
References
- 1.Friedman BA. An analysis of surgical blood use in United States hospitals with application to the maximum surgical blood order schedule. Transfusion 1979;19:268–78 [DOI] [PubMed] [Google Scholar]
- 2.Sullivan MT, Cotten R, Read EJ, et al. Blood collection and transfusion in the United States in 2001. Transfusion 2007;47:385–94 [DOI] [PubMed] [Google Scholar]
- 3.Ramamoorthy C, Haberkern CM, Bhananker SM, et al. Anesthesia-related cardiac arrest in children with heart disease. Anesth Analg 2010;110:1376–82 [DOI] [PubMed] [Google Scholar]
- 4.Morray JP, Geiduschek JM, Ramamoorthy C, et al. Anesthesia-related cardiac arrest in children: initial findings of the Pediatric Perioperative Cardiac Arrest (POCA) Registry. Anesthesiology 2000;93:6–14 [DOI] [PubMed] [Google Scholar]
- 5.Bhananker SM, Ramamoorthy C, Geiduschek JM, et al. Anesthesia-related cardiac arrest in children: update from the pediatric perioperative cardiac arrest registry. Anesth Analg 2007;105:344–50 [DOI] [PubMed] [Google Scholar]
- 6.Waters JH, Ness PM. Patient blood management: a growing challenge and opportunity. Transfusion 2011;51:902–3 [DOI] [PubMed] [Google Scholar]
- 7.Dexter F, Ledolter J, Davis E, et al. Systematic criteria for type and screen based on procedure's probability of erythrocyte transfusion. Anesthesiology 2012;116:768–78 [DOI] [PubMed] [Google Scholar]
- 8.Frank SM, Rothschild JA, Masear CG, et al. Optimizing preoperative blood ordering with data acquired from an anesthesia information management system. Anesthesiology 2013;118:1286–97 [DOI] [PubMed] [Google Scholar]
- 9.Muravchick S. Anesthesia information management systems. Curr Opin Anaesthesiol 2009;22:764–8 [DOI] [PubMed] [Google Scholar]
- 10.Hurrell MJ, Monk TG, Nicol A, et al. Implementation of a standards-based anaesthesia record compliant with the health level 7 (HL7) clinical document architecture (CDA). J Clin Monit Comput 2012;26:295–304 [DOI] [PubMed] [Google Scholar]
- 11.Jacobs A. The pathologies of big data. Commun ACM 2009;52:36–44 [Google Scholar]
- 12.Tsolakidis A, Sgouropoulou C, Papageorgiou E. Using Visual Representation for Decision Support in Institutional Research Evaluation, 2012
- 13.Zhang Q, Segall R, Cao M. Visual analytics and interactive technologies. IGI Global Snippet, 2011 [Google Scholar]
- 14.Doloc-Mihu A. Interactive Visualization Tool for Analysis of Large Image Databases.
- 15.ASA Physical Status Classification System. http://www.asahq.org/For-Members/Clinical-Information/ASA-Physical-Status-Classification-System.aspx (accessed 15 Feb 2013)
- 16.Clinical Classification Software. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp (accessed 28 Jul 2013)
- 17.American Society of Anesthesiologists ASO. 2013 CROSSWALK® book: a guide for surgery/anesthesia CPT® codes. American Society of nesthesiologists
- 18.Stricker PA, Fiadjoe JE, Davis AR, et al. Reconstituted blood reduces blood donor exposures in children undergoing craniofacial reconstruction surgery. Pediatr Anesth 2010;21:54–61 [DOI] [PubMed] [Google Scholar]
- 19.MacLennan J, Tang Z, Crivat B. Data mining with Microsoft SQL server 2008. John Wiley & Sons, 2011 [Google Scholar]
- 20.Keung CY, Smith KR, Savoia HF, et al. An audit of transfusion of red blood cell units in pediatric anesthesia. Pediatr Anesth 2009;19:320–8 [DOI] [PubMed] [Google Scholar]
- 21.Foley C, Mould T, Kennedy J, et al. A study of blood cross--matching requirements for surgery in gynecological oncology: Improved efficiency and cost saving. Int J Gynecol Cancer 2003;13:889–93 [DOI] [PubMed] [Google Scholar]
- 22.Voak D, Napier J, Boulton F. Guidelines for implementation of a maximum surgical blood order schedule. Clin Lab Haematol 1990;12:321–7 [PubMed] [Google Scholar]