Abstract
Polycystic ovary syndrome (PCOS), a common female endocrinopathy, is a complex metabolic syndrome of enhanced weight gain. The goal of this pilot study was to evaluate metabolic differences between normal (n=10) and PCOS (n=10) women via breath carbon isotope ratio, urinary nitrogen and nuclear magnetic resonance (NMR)-determined serum metabolites. Breath carbon stable isotopes measured by cavity ring down spectroscopy (CRDS) indicated diminished (p<0.030) lipid use as a metabolic substrate during overnight fasting in PCOS compared to normal women. Accompanying urinary analyses showed a trending correlation (p<0.057) between overnight total nitrogen and circulating testosterone in PCOS women, alone. Serum analyzed by NMR spectroscopy following overnight, fast and at 2 h following an oral glucose tolerance test showed that a transient elevation in blood glucose levels decreased circulating levels of lipid, glucose and amino acid metabolic intermediates (acetone, 2-oxocaporate, 2-aminobutyrate, pyruvate, formate, and sarcosine) in PCOS women, whereas the 2 h glucose challenge led to increases in the same intermediates in normal women. These pilot data suggest that PCOS-related inflexibility in fasting-related switching between lipid and carbohydrate/protein utilization for carbon metabolism may contribute to enhanced weight gain.
Keywords: Cavity ring down, glucose elevation, lipid metabolism, NMR-metabolomics, polycystic ovary syndrome (PCOS)
Introduction
Polycystic ovary syndrome (PCOS) is a complex metabolic and reproductive health disorder in women comprising multiple gene variants and hyperandrogenic hormonal pathophysiology [1]. It appears to manifest at puberty with multiple phenotypes [2, 3] characterized by androgen excess, ovulatory dysfunction and polycystic ovaries [4]. PCOS affects 6-15% of women [5-8] and is associated with obesity, type 2 diabetes mellitus (T2DM) and cardiovascular disease [9, 10]. Thirty-eight to 88% of women with PCOS are overweight or obese [11, 12]. Increased adiposity enhances not only the incidence of PCOS [13], but also the severity of the phenotype [14, 15]. Such an adverse effect of adiposity on PCOS phenotypic expression may begin in adolescence [16, 17] and continues with age as PCOS women develop greater abdominal adiposity than normal women with comparable body mass index (BMI). Women with PCOS demonstrate a significant correlation between enhanced abdominal visceral fat and insulin resistance [18] together with abnormal adipose function, abnormal adipokine release and glucoregulation [19]. Subcutaneous abdominal adipocytes also are larger in PCOS women [20], indicating potential constraint on adipocyte differentiation and a reduced ability of this adipose depot to safely store fat [21, 22]. Such constrained lipid adipose storage in subcutaneous adipocytes can promote ectopic lipid accumulation and lipotoxicity, thereby diminishing insulin action [21, 22] and providing a mechanistic basis for positive correlations between adipocyte diameter and insulin resistance [20, 23]. Reduced utilization of lipid as an energy substrate (24-26) has the potential to enhance such metabolic dysfunction.
In this pilot study, we employed complementary methods (breath carbon stable isotope analysis, total urinary nitrogen (N) excretion, and nuclear magnetic resonance (NMR)-based metabolomics) to further our understanding of metabolic perturbations in PCOS women that may contribute to their propensity towards obesity. We used changes in the 13C enrichment in stable isotope ratio of carbon in exhaled breath CO2 (i.e. 13CO2/12CO2 or δ 13C in parts per mil, denoted as ‰) to monitor substrate utilization [25-29]. Multiple stable isotopes of carbon, nitrogen, oxygen and sulfur are naturally found in body tissues. During lipid synthesis, however, isotopic discrimination against 13C by pyruvate dehydrogenase results in preferential accumulation of isotopically light carbon (12C) in fat compared to carbon incorporation into synthesized carbohydrate or protein [30]. Because endogenous lipids are enriched with isotopically lighter carbon than carbohydrate or protein, fat oxidation results in a decrease in the breath δ13CO2 compared to breath during carbohydrate/protein oxidation [27, 28, 31]. Breath analysis allows fast, non-invasive monitoring of lipid oxidation. Breath δ values alone, however, do not distinguish contribution from carbon isotopes derived from carbohydrates vs. proteins. Therefore, to further support the conclusion made from fasting breath δ13CO2, urinary N excretion was measured, and untargeted NMR-metabolomics was used to investigate basic metabolic processes, an approach that allows unbiased identification and quantification of small molecules (less than 1000 Da), including metabolites, present in the circulation. We challenged both normal and PCOS women with a 2-hour oral glucose tolerance test (2hOGTT) to ascertain impairments in glucose utilization. This pilot study suggests that subtle perturbations in macronutrient metabolism (i.e. differences in switching between metabolism of lipid and carbohydrate/protein for energy metabolism) occur in women with PCOS, and points to metabolic dysregulation as a factor in the progressive obesity that commonly accompanies PCOS.
Material and Methods
Participants and Ethics Statement
Ethics Statement
This study was approved by the Health Sciences Institutional Review Board at the University of Wisconsin-Madison. Subjects signed informed consent forms, and the study was in compliance with privacy-act guidelines.
Participants
Healthy control women (n=10) and women with PCOS (n=10) were recruited by newspaper advertisement and flyers posted at the Reproductive Endocrine and Infertility clinic and gynecology practices associated with the Department of Obstetrics and Gynecology at the University of Wisconsin-Madison. A subset of subjects was used for the glucose tolerance study (normal: 8 fasted, 8 2hOGTT; and PCOS: 7 fasted, 7 2hOGTT), and their sera samples were used for NMR data collection. An initial telephone screening confirmed inclusion criteria: female, age 18-38 years, and ruled out exclusion criteria: current smoker; history of diabetes or heart disease; prior or current use of diabetes medications (within past year), antidepressants, beta blockers, antipsychotic, or weight loss medications; use of hormonal contraception within the prior three months; pregnancy or planning to become pregnant; or weight instability (change > 5% within the month prior to enrollment).
Clinical Study Assays and Procedures
Subjects meeting the above criteria underwent a screening visit that included a medical history questionnaire and body composition assessment by bioelectrical impedance analysis (Tanita, TBF-310, Arlington Heights, IL). Of all subjects screened, only one woman with PCOS reported family history of T2DM. Subjects also had an additional screening visit for fasting tests including serum total testosterone, a 75-g oral glucose tolerance test, fasting insulin, lipid panel, a pelvic ultrasound, as well as serum prolactin, 17-hydroxyprogesterone, and, thyroid stimulating hormone (TSH) to exclude hyperprolactinemia (elevated prolactin), non-classic congenital adrenal hyperplasia (elevated 17-hydroxyprogesterone), or thyroid dysfunction (above or below normal TSH). Serum hormone and glucose concentrations were determined at the UW Madison Institute for Clinical and Translational Research-WNPRC Assay Services laboratory [32, 33], and within the single assay performed for each hormone, intra-assay coefficients of variation were: total testosterone enzymeimmunoassay with celite chromatography (16.0%), insulin radioimmunoassay (4.5%) and glucose oxidase assay (2.9%). Blood biochemical analytes [36] were performed at the National University Hospital Referral Laboratory, which is accredited by the College of American Pathologists. Serum total cholesterol, triglyceride, and high-density lipoprotein cholesterol (HDL-C) were measured using an automated autoanalyzer (ADVIA 2400; Bayer Diagnostics, New York). Low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald formula. Subjects with impaired fasting glucose (fasting glucose 100-125 mg/dL), diabetes mellitus (fasting glucose ≥126 mg/dL, 2 h glucose ≥200 mg/dL), triglycerides over 800 mg/dL, or LDL cholesterol over 180 mg/dL were excluded.
PCOS women were confirmed to have PCOS (by Rotterdam criteria [7] requiring hyperandrogenism (Ferriman-Gallwey score of ≥8) and intermittent/absent menstrual cycles (≤8 cycles in a year, or <26 d or >35 d in length) with polycystic ovaries (≥ 12, 2-9mm follicles in at least one ovary, and/or increased ovarian volume >10ml). Control normal subjects were healthy with regular menstrual cycles and without polycystic ovaries. Homeostasis model of assessment - insulin resistance (HOMA-IR; fasting glucose (mg/dl) x fasting insulin (μU/mL) / 405) [34], fasting glucose-to-insulin ratio (FGIR) [35] and 2-hour GIR were calculated as surrogate measures of insulin resistance. Control subjects were scheduled for the additional screening visit on day 2 or 3 of their menstrual cycle. Within 1-4 days after their 2nd screening visit, subjects spent 26 h in the Clinical Translational Research Center for assessment in the human respiratory chamber (room calorimeter). Meals consisted of 35% kcal as fat, 15% protein, and 50% carbohydrate. Total energy level was adjusted to meet individual energy needs while maintaining this macronutrient profile. Subjects were provided with a lead-in diet with a similar macronutrient profile for 3 days [36] prior to their 26 h stay to standardize their nutrient intake and reduce diet effect on breath and biofluid metabolite analysis. Caloric content of lead-in diet approximated energy balance. For all subjects, early follicular phase or anovulation was confirmed by ultrasound (no lead follicles >10 mm) and serum progesterone level <3 ng/mL at the 2nd screen visit and during the overnight stay.
Urinary Nitrogen Sample Collection and Analysis
Protein oxidation from urinary nitrogen was measured by collecting all urine during the respiratory chamber stay. Urine was acidified with citric acid to prevent volatilization of nitrogen compounds. Samples were pooled into sleeping hours (night) and waking hours (day) and frozen at -80°C until dilution for nitrogen analysis using a chemiluminescent nitrogen analyzer (Antek 900 series nitrogen analyzer) as previously described [37].
Serum and NMR Sample Preparation
Serum samples were collected at fasting and 2 h after glucose challenge from normal women (8 fasted, 8 2hOGTT) and women with PCOS (7 fasted, 7 2hOGTT) and immediately frozen at −80°C. At the time of NMR analysis, samples were thawed on ice and only mixed by inversion. 500 μl of serum was transferred to a new tube, and two volumes of methanol were added to denature and precipitate proteins. Samples were kept at -20°C for 20 min. The precipitate was removed by centrifuging for 5 min at 14,000 rpm in micro-centrifuge tubes. The supernatant was dried in a speed-vacuum centrifuge overnight. The dried samples were re-suspended with 600 μl 20 mM phosphate buffer in 99%-D2O (pH= 7.4). All NMR spectra were collected at 25°C with 1 mM DSS (4,4-dimethyl-4-silapentane-1-sulfonic acid, an agent used for internal referencing NMR chemical shifts and relative concentrations) and 0.1 mM NaF.
NMR Spectroscopy and Data Analysis
All NMR spectra were collected at the National Magnetic Resonance Facility at Madison (NMRFAM) on a Varian NMR System spectrometer operating at 800 MHz. The spectrometer was equipped with cryogenic single z-axis gradient probe. One-dimensional proton spectra with a total of 512 acquisitions were collected for each sample with the temperature regulated to 25°C. The water signal was suppressed by applying a weak saturation pulse prior to the beginning of the pulse sequence. Furthermore, a low power 8 ms spinlock pulse was used to suppress fast relaxing signals from large molecules such as proteins. Software used to process and analyze NMR spectra included vnmrJ 1.1D (Varian, Inc.) and NMRpipe (http://spin.niddk.nih.gov/bax/software/NMRPipe). Metabolites were identified and their relative concentrations to the internal standard (DSS) were determined using Chenomx NMR Suite 6.1 (http://www.chenomx.com) (Supplementary Table 1). The online database MMCD (http://mmcd.nmrfam.wisc.edu) was used to verify molecule identifications.
To compare the net effect of glucose perturbation on the means of metabolites (Supplementary Table 1) Equation 1, below, was used to calculate fold change between the fasted and 2hOGTT conditions:
A subset of NMR metabolites, each with greater than a one-fold difference (increase or decrease) between normal and PCOS, were analyzed using Student's t-test. Data trends for p <0.08 are reported. Heat map graphical representation for the subset of metabolites increasing or decreasing were used to show changes in the mean values for normal and PCOS women at fasting and 2hOGTT (Supplementary Fig. 2). The heat map was generated using MATLAB software. Numerical values and SEM for the heat map matrix are reported in Supplementary Table 2.
Breath Measurements
Normal and PCOS were fed a controlled diet designed to meet their energy needs in an in-patient setting. Breath samples were collected at wake-up, and immediately prior to and following breakfast, lunch, dinner, and a prescribed exercise regime (1400h - 1500h). Patients were asked to exhale into a 1-L gas tight Tedlar bag. Each breath sample was diluted to 1% CO2 by mixing with nitrogen gas in approximately a 2 to 5 ratio (26). Isotopic CO2 was measured from diluted breath samples using a Picarro isotopic CO2 cavity ring-down spectrometer (Picarro Inc., Sunnyvale, CA). The breath CO2 carbon stable isotope ratio (13CO2/12CO2) was normalized to Pee Dee Belemnite (PDB), the international standard, and expressed as a delta value (8) in parts per mil (‰) notation. This notation, simply translated, is ten times the percent difference in isotope ratio relative to the standard [27].
Statistical Methods
Breath δ13CO2 values at wake-up and urinary nitrogen data were compared using Student's t-test. Results are reported as mean ± SEM. Data were considered significantly different at p<0.05. Repeated measures analysis of covariance was used to compare the normal to PCOS women across all time points. Because waking values from three subjects (1 normal, 2 PCOS) were missing, the mean of waking and pre-breakfast delta values was used as the covariate for each woman to adjust for the observed initial differences between the two groups.
Results
Baseline, Endocrine and Metabolic Parameter Characteristics of Human Subjects
Normal and PCOS subjects were similar in age, height, weight, % body fat and baseline characteristics, as shown in Table 1. Serum values for fasting glucose, glucose after a 2hOGTT, HOMA-IR, fasting insulin, fasting glucose-to-insulin ratio (FGIR), 2-hour GIR, total cholesterol, triglycerides and HDL, obtained during a 24-h hospital stay, did not differ between normal and PCOS women. PCOS women were distinguished from normal women in terms of hirsutism (Ferriman-Gallwey score), intermittent or absent menstrual cycles and polycystic ovaries (Table 1). Circulating total testosterone levels in the PCOS group were ∼34% greater than in controls, but not significantly different.
Table 1.
Mean (± SEM) baseline parameters in 10 PCOS and 10 non-PCOS, normal women. Ferriman Gallwey score is shown as median (range). * P < 0.01, PCOS vs. normal women.
| Normal | PCOS | |
|---|---|---|
| Age (y) | 28.2 ± 1.6 | 28.0 ± 1.4 |
| Height (cm) | 165.7 ± 3.1 | 165.7 ± 2.5 |
| Weight (kg) | 69.4 ± 4.9 | 77.7 ± 1.6 |
| BMI (kg/m2) | 25.4 ±1.9 | 28.4 ± 2.3 |
| % Total body fat | 31.3 ± 2.9 | 34.6 ± 2.8 |
| Fasting glucose (mg/dL) | 79.3 ± 2.1 | 79.7 ± 1.7 |
| 2hOGTT glucose (mg/dL) | 75.1 ±3.8 | 81.5 ± 8.3 |
| Fasting insulin (μU/dL) | 9.7 ± 1.5 | 13.5 ± 2.3 |
| 2hOGTT insulin (μU/dL) | 41.7 ± 10.7 | 49.0 ± 12.4 |
| HOMA-IR | 1.9 ± 0.3 | 2.7 ± 0.5 |
| FGIR | 9.6 ± 1.1 | 7.9 ± 1.7 |
| 2hOGTT GIR | 8.5 ± 0.8 | 7.4 ± 1.6 |
| Total cholesterol (mg/dL) | 149.2 ± 7.5 | 174.3 ± 9.8 |
| HDL cholesterol (mg/dL) | 54.8 ± 7.8 | 52.7 ± 2.4 |
| Triglycerides (mg/dL) | 80.1 ± 22.7 | 91.4 ± 12.3 |
| Ferriman Gallwey Score * | 0.8 ± 0.5 | 11.7 ± 0.8 |
| Total testosterone (pg/mL) | 522.0 ± 84.5 | 699.5 ± 126.0 |
| Intermittent/absent menstrual cycles * | 0% | 100% |
| Polycystic ovaries * | 0% | 100% |
Breath Data Analyses Indicate Diminished Lipid Oxidation in PCOS Women
Breath δ13C values are plotted as a function of time during the inpatient stay (Fig. 1) and indicate decreased fasting and daytime lipid oxidation in PCOS compared to normal women. Mean δ13C at wake-up for normal subjects was -23.1 ± 0.3‰ (0600h) that increased to a peak of -20.6 ± 0.4‰ at 15:00h, an increase of 2.5‰. In contrast, the mean δ value for PCOS women on waking was -21.6 ± 0.3‰ with a 0.8‰ increase to a peak of -20.8 ± 0.3‰ by pre-lunch (12:00h) (Fig. 1). The mean δ values at wake-up (06:00h) were higher (p < 0.04) in PCOS compared to normal subjects, and after covariance adjustment for this initial difference in δ13C values, PCOS subjects had lower breath δ values (p < 0.02) across the day. This may indicate diminished switching between lipids and other carbon sources for energy metabolism during fasting and across the daytime hours when meals were consumed in PCOS subjects.
Fig. (1).
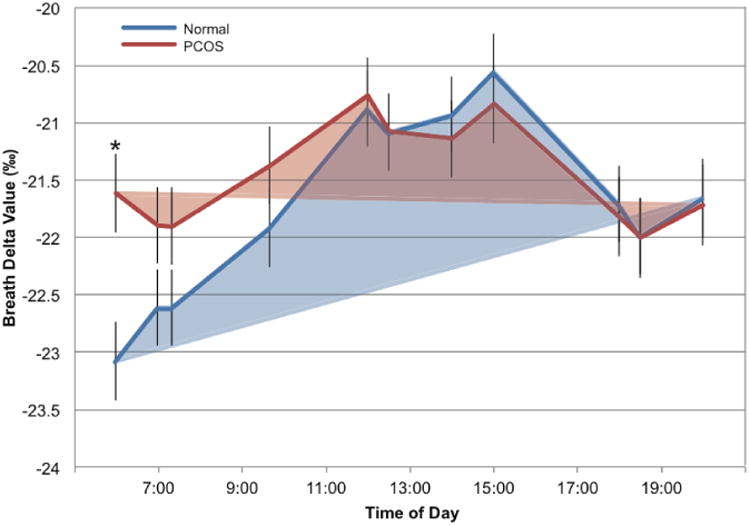
Normal and PCOS women were given a defined diet that was designed to meet their caloric needs (see methods). Breath samples were taken after an overnight fast (at wake-up, 0600h), before and after every meal and before and after an afternoon exercise bout. Breath δ13CO2values were determined using an isotopic CO2 cavity ring-down spectrometer. The observed means are shown as lines in blue for normal women and red for PCOS women. Morning-to-evening variation in breath delta values was analyzed by repeated measures analysis of covariance (ANCOVA) using the mean of the wake up and pre-breakfast delta values as the covariate.
Urinary nitrogen (N) analysis shows a trend towards increased amino acid metabolism in PCOS women
To initially explore the contribution of amino acid metabolism to the carbon content of breath δ values, we measured total urinary N during the 26-h inpatient stay. Overall, normalized nitrogen values were similar in normal and PCOS groups during both nighttime and daytime hours (Supplementary Table 3). Nighttime nitrogen values, however, trended toward a positive correlation (r2 = 0.38, p<0.057) with circulating testosterone levels in PCOS subjects. There were no other testosterone-related correlations involving urinary N or breath parameters.
Analysis of NMR-metabolomics Data from Fasting Serum Implicates Altered Lipid Oxidation, Amino Acid and Glucose Metabolism in PCOS Women
Representatives of overnight fasted serum NMR spectra from a normal and a PCOS woman with a subset of metabolites labeled are shown in Supplementary (Fig. 1). A total of 43 metabolites were identified in both normal and PCOS serum samples, and their relative concentrations (mM) to the internal standard (DSS) are reported in Supplementary Data Table 1. We investigated differences between fasting NMR-determined metabolite concentrations in PCOS vs. normal groups. The PCOS group showed increased serum levels for all identified metabolites (with the exception of citrate and glycerol) relative to normal group (Fig. 2). Concentrations of 8 metabolites were found to be ≥1-fold higher in PCOS than in control serum. These metabolites are involved in amino acid (2-oxocaproate, 2-aminobutyrate, homoserine, sarcosine) and glucose (glucose, pyruvate, acetoacetate) metabolism. Two additional metabolites exhibited statistical trends for increase in PCOS: sarcosine (p = 0.060), an amino acid intermediate, and acetoacetate (p = 0.079), a lipid metabolism pathway intermediate. Lactate, fructose, acetate, and threonine were increased by 0.7-0.8 fold in PCOS, but only acetate showed a trend in significance (p = 0.06) between PCOS and normal subjects at fasting.
Fig. (2).
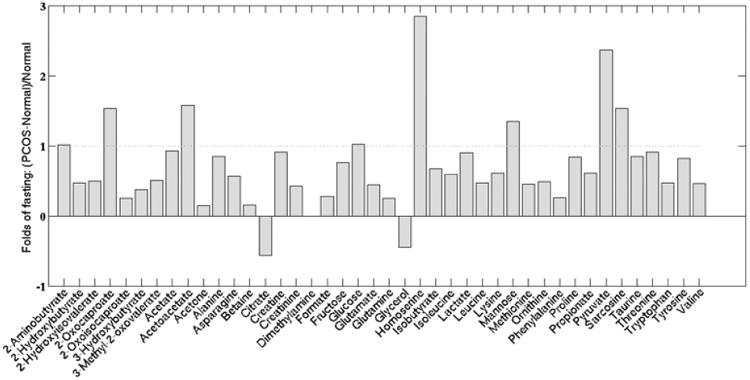
Fold changes in fasted serum metabolite levels in PCOS as compared to normal subjects. The dotted line indicates a 1-fold (2×) increase. All metabolites, with the exception of citrate and glycerol, were increased in PCOS compared to normal women.
NMR-metabolomics Data Show Metabolic Impairment of Glycolysis and Amino Acid Regulation in PCOS Women During Transiently Glucose Challenged Condition
To examine the effect of glucose metabolism in PCOS, we challenged subjects with a 2-h oral glucose tolerance test (2hOGTT) after an overnight fast and measured their serum metabolites before and 2 h after glucose administration. The transient glucose elevation changed the concentrations of NMR-measured serum metabolites in both groups of women, but at the 2hOGTT sampling time, little evidence remained of elevated glucose concentrations in circulation. PCOS subjects showed different responses in metabolite concentration for a subset of 12 metabolites compared to normal subjects. The complete fold change analyses of 2hOGTT samples, shown in (Fig. 3), identified 12 metabolites (≥1-fold difference to serum values in normal women; indicated with *) that changed in opposing directions between the two groups of women. Comparison of magnitude of fold change between fasting and 2hOGTT conditions for each group is shown as a heat map in (Supplementary Fig. 2). The glucose elevation-related changes in metabolite concentrations implicate molecules involved in energy substrate utilization: lipid/amino acid metabolic pathways (2-aminobutyrate, 2-oxocaporate, acetate, acetone, formate, and sarcosine) and glycolysis pathways (fructose, glucose, lactate, pyruvate, alanine, and taurine). The oral glucose challenge generally was associated with increases in serum concentrations of these 12 metabolites in normal subjects but with decreases in PCOS subjects, with the exceptions of fructose and glucose, which decreased in normal subjects but increased in PCOS subjects. PCOS subjects exhibited a >1-fold higher fasting concentration of 2-aminobutyrate, 2-oxocaporate, pyruvate, and sarcosine than normal women. At 2hOGTT, the serum concentrations of these 10 of 12 metabolites decreased in PCOS subjects, while the corresponding concentrations in normal subjects for formate, 2-oxocaporate, 2-aminobutyrate, and pyruvate increased resulting in a lesser net effect of changes in metabolites levels between the two groups (Supplementary Table 2). Sarcosine (p = 0.06), 2-amino butyrate (p = 0.055), alanine (p = 0.04), formate (p = 0.04), acetoacetate (p = 0.079) and acetate (p = 0.06) decreased, while glucose (p = 0.03) and fructose (p = 0.03) increased in PCOS subjects.
Fig. (3).
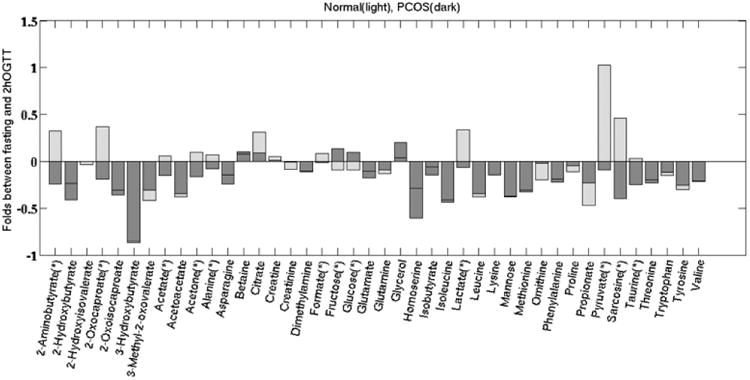
Fold changes between the fasting and 2hOGTT conditions for the normal and PCOS subjects. Metabolites labeled with * changed oppositely between the normal and PCOS groups: with exception of glucose and fructose, these metabolites increased from fasting to 2hOGTT in the normal subjects but decreased from fasted to 2hOGTT in the PCOS subjects. Those metabolites that showed trends toward significant increase are: acetoacetate (p = 0.079), acetate (p = 0.06), 2-amino butyrate (p = 0.055), and sarcosine (p = 0.06).
Discussion
This pilot study focuses on metabolic markers in breath and serum that may contribute to PCOS metabolic dysfunction. Our preliminary examination of exhaled breath, urinary nitrogen and NMR-based metabolomics of serum metabolites suggests subtle, but distinct, differences in lipid and glucose/amino acid metabolism between normal women and those with PCOS following an overnight fast and after an oral glucose challenge (transient elevation in circulating glucose concentrations).
Breath is the waste product of cellular respiration and macronutrient oxidation, and the breath stable isotopes of carbon may be used to assess macronutrient oxidation [38]. We analyzed the changes in breath stable isotope δ 13CO2 values, which provide important information on the macro-nutrient source of oxidized carbon [30, 38, 39]. Daily variation in breath δ13CO2 values correlates with daily variation in macronutrient oxidation (more negative breath values are indicative of increased lipid relative to glucose/amino acid utilization) in non-human species [29]. Normally, as glycogen supplies are depleted during nighttime fasting, and protein turnover remains relatively constant, cellular respiration shows increasing reliance on lipid utilization throughout the night. Thus, as expected, normal women in this study exhibited breath δ values lower in the fasted morning sample compared to the fed state, consistent with increased overnight fasting lipid oxidation [27, 29]. In contrast, women with PCOS exhibited less negative δ13CO2 breath values (or less abundant with 13C) on waking, suggesting diminished switching to lipid oxidation. Moreover, in PCOS women, overall changes in breath δ13CO2 values during daytime hours were decreased compared to controls, suggesting an overall constraint on the variety of carbon sources contributing to daily energy metabolism. PCOS women thus appear to exhibit reduced transitioning between lipids and carbohydrate/amino acids, since diminished use of lipid as an energy substrate will diminish inclusion of the heavier carbon isotope in their breath samples (i.e. higher δ13CO2).
The positive correlational trend between circulating testosterone and nighttime nitrogen excretion suggests that PCOS women may, unlike normal controls, utilize amino acids as a preferential carbon source for energy metabolism during fasting. These urinary nitrogen results are consistent with our NMR-metabolomic outcomes implicating altered amino acid and glucose metabolic utilization during fasting in PCOS women, and support our metabolic inflexibility hypothesis, illustrated in (Fig. 4), by which diminished utilization of lipid as an energy substrate may lead to increased fat accumulation in both adipose stores and ectopic sites, such as liver and skeletal muscle [40]. Accompanying hyperandrogenic constraint on adipogenesis [41] may accelerate such lipid accumulation into lipotoxicity–driven hepatic/systemic insulin resistance and cardio-metabolic disease [42, 43].
Fig. (4).
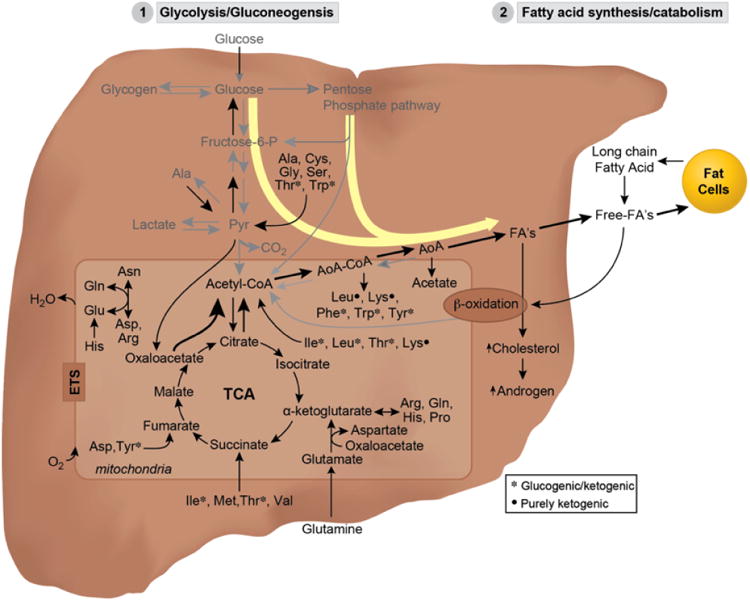
Diagrammatic representation of proposed metabolic pathways switching in PCOS suggested by NMR and supported by breath and urine data analysis. Simplified metabolic pathways and TCA cycle showing enhanced diversion of glucose metabolism through alternative pathways (i.e. glycolysis and pentose phosphate pathways) into fatty acid synthesis including increased amino acid utilization by the cell which may explain the observed increased fat accumulation and increased nitrogen excretion at fasting in PCOS women. Major metabolites identified during 2hOGTT metabolic perturbation are: formate, acetone, 2-oxocaproate, 2-aminobutyrate, sarcosine, pyruvate, which are intermediates of major metabolic processes listed in the diagram. These pathways are summarized as: 1) glycolysis/gluconeogenesis where is centered on glucose metabolism (via pyruvate), 2) fatty acid metabolism (via acetone, 2-oxocaproate, 2-aminobutyrate, sarcosine intermediates), centered on lipid metabolism as source of energy through free fatty acids, as a precursor of cholesterol biosynthesis, androgen biosynthesis or fat deposition in fat cells. 3) Redox homeostasis/detoxification (via 2-aminobutyrate, sarcosine), which is coupled with removal of free radical formation during metabolic stress and coupled to trans-sulfuration.
Overnight fasting NMR data are consistent with predominant utilization of ketogenic derived amino acids and glucose as energy substrates in PCOS women, i.e., ∼1-fold increases in serum concentrations of 2-aminobutyrate, 2-oxocaporate, acetate, acetone, formate and sarcosine compared to normal women. A transient glucose elevation additionally implicates increased intermediate metabolites in glycolysis and/or glucogenic amino acid pathways: lactate, pyruvate, alanine, and taurine in PCOS women in contrast to decreases in these same metabolites in normal women receiving the same glucose challenge (2hOGTT). The latter difference may reflect relatively impaired glucose oxidation in PCOS compared to normal women, but there is no evidence of insulin resistance in the PCOS women in our study that might explain the related diminution of glycolysis [44].
Alanine and pyruvate, however, are part of a reversible transamination reaction in liver, named alanine-amino-transferase (ALT) activity, in which alanine can be either converted to or made from pyruvate in this reaction by either donating or accepting an amine group as part of urea cycle and amino acid metabolism. ALT is also found in plasma and is thought to be associated with diurnal variations. Although NMR-metabolites reported are from a wide range of BMI (Table 1), the higher levels of pyruvate/lactate/acetate in PCOS subjects compared to normal subjects suggest a lesser fat oxidation in PCOS women at fasting (Fig. 2). Increased levels of 2-amino butyrate (a secondary metabolite) and 2-oxo-caproate (an amino acid intermediate) may also provide additional substrates for the ALT pathway consistent with our hypothesis of increased amino acid metabolism as a source of alternative energy metabolism in PCOS (Fig. 4).
To further understand the role of glucose metabolism in PCOS, we transiently challenged both groups with oral glucose stimulation. Our NMR analysis of serum samples from the 2hOGTT experiment indicates an interesting inverse effect of glucose on only a subset of metabolites in PCOS vs. normal women. Those metabolites in PCOS women that showed inverse responses to glucose are initially reported at higher than normal levels at fasting. Although upon stimulation with glucose, there is a decrease in pyruvate, alanine, lactate, and acetate levels, while those of fructose and glucose increase, levels of all these metabolites still remain elevated compared to normal subjects, suggesting possible impairments in glucose and fat metabolism.
A diminished ability to switch to lipid from carbohydrate/protein usage in periods of fasting has been reported by others and appears to be a key feature of metabolic inflexibility associated with bed rest [45, 46], aging [47, 48], obesity [49], insulin resistance [50], T2DM [51, 52] and PCOS [53]. In hyper- compared to normo-androgenic PCOS women, Di Sarra and colleagues [53] recently reported a greater degree of metabolic inflexibility in the former PCOS women in terms of diminished increase in respiratory quotient (RQ) values during a hyperinsulinemic-euglycemic clamp [53]. These RQ findings suggest diminished switching of substrate metabolism from predominant lipid oxidation at low (overnight fasted) insulin levels to predominant glucose utilization at high (clamp) insulin levels [53] in only hyperandrogenic PCOS phenotypes, a finding consistent with our finding of a positive association between increased nighttime (fasting) utilization of amino acids as an energy substrate and circulating testosterone levels in subjects with PCOS. Such PCOS associations between testosterone and diminished utilization of lipid or glucose as an energy substrate are contrary to expectations from normal women [54] and men [55], in whom testosterone enhances lipid oxidation and protein synthesis. In men, testosterone acting through the androgen receptor [56] diminishes utilization of amino acid as an energy substrate and increases fat-free mass [57-59]. Increased reliance on glucose and a corresponding decrease in fatty acid oxidation in skeletal muscle [60, 61] has also been found in PCOS women, and associated with decreased oxidative metabolism [62] in tandem with decreased mitochondrial respiration [63]. In the latter PCOS-associated metabolic inflexibility, diminished gene expression regulating fatty acid oxidation and lipid metabolism, combined with increased expression of glycolytic enzymes [64, 65], may result in reduced glycogen synthesis coincident with increased reliance on glucose, rather than lipid, as an energy source [61, 66, 67]. An additional recent study, however, also employing changes in RQ values during a hyperinsulinemic-euglycemic clamp, found evidence of metabolic inflexibility in overweight and obese women that was not influenced by the presence of PCOS [68].
4. Conclusions
In conclusion, our pilot study suggests that women with PCOS may exhibit a diminished ability to switch during overnight fasting and daily metabolism from glucose/amino acid to lipid oxidation. It is unclear, however, whether such a metabolic inflexibility is a fundamental, intrinsic PCOS trait, is subsequent to progressive PCOS pathology that may have developmental origins [69], or is dependent on extent chronic androgen excess [53]. Reduced utilization of lipid stores by PCOS women could contribute to obesity and increased rates of metabolic disorders, particularly T2DM.
Supplementary Material
Acknowledgments
This research was supported by NIH grants R01 DC009018 (F.A.P., PI), UL1TR000427 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, Wisconsin Institute of Discovery Grant (WID-135A039), RC4 EY021357 (to F.A.P.), 8P41 GM103399, which funds the National Magnetic Resonance Facility at Madison, and funding from the Department of Obstetrics and Gynecology, University of Wisconsin-Madison.
Footnotes
Conflict Of Interest: The authors have no financial conflict of interest. Authors FAP, DB, HE, MC, WPP, MT are cofounders of Isomark LLC.
References
- 1.Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Molecular Cellul Endocrinol. 2013;373(1-2):29–38. doi: 10.1016/j.mce.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewens KG, Stewart DR, Ankener W, Urbanek M, McAllister JM, Chen C, et al. Family-based analysis of candidate genes for polycystic ovary syndrome. J Clin Endocr Metab. 2010;95(5):2306–15. doi: 10.1210/jc.2009-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–31. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metabol. 2006;91(1):48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 5.Diamanti-Kandarakis E. Current aspects of antiandrogen therapy in women. Curr Pharm Des. 1999;5(9):707–23. [PubMed] [Google Scholar]
- 6.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metabol. 2000;85(7):2434–8. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 7.Azziz R. PCOS: A diagnostic challenge. Reprod Biomed Online. 2004;8(6):644–8. doi: 10.1016/s1472-6483(10)61644-6. [DOI] [PubMed] [Google Scholar]
- 8.Balen A. The pathophysiology of polycystic ovary syndrome: Trying to understand PCOS and its endocrinology. Best Pract Res Clin Obstet Gynaecol. 2004;18(5):685–706. doi: 10.1016/j.bpobgyn.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metabol. 2010;95(5):2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 10.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. United States: 2012 American Society for Reproductive Medicine. Published by Elsevier Inc; 2012. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group Fertility And Sterility 97; pp. 28–38 e25. [DOI] [PubMed] [Google Scholar]
- 11.Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Human Reprod. 1995;10(8):2107–11. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- 12.Legro RS. The genetics of obesity. Lessons for polycystic ovary syndrome. Ann New York Acad Scienc. 2000;900:193–202. doi: 10.1111/j.1749-6632.2000.tb06230.x. [DOI] [PubMed] [Google Scholar]
- 13.Teede HJ, Joham AE, Paul E, Moran LJ, Loxton D, Jolley D, et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: Results of an observational study in young women. Obesity. 2013;21:1526–1532. doi: 10.1002/oby.20213. [DOI] [PubMed] [Google Scholar]
- 14.Barber TM, McCarthy MI, Wass JAH, Franks S. Obesity and polycystic ovary syndrome. Clinical Endocrinol. 2006;65:137–45. doi: 10.1111/j.1365-2265.2006.02587.x. [DOI] [PubMed] [Google Scholar]
- 15.Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obesity Rev. 2013;14(2):95–109. doi: 10.1111/j.1467-789X.2012.01053.x. [DOI] [PubMed] [Google Scholar]
- 16.Trottier A, Battista MC, Geller DH, Moreau B, Carpentier AC, Simoneau-Roy J, et al. Adipose tissue insulin resistance in peripubertal girls with first-degree family history of polycystic ovary syndrome. Fertility Sterility. 2012;98(6):1627–34. doi: 10.1016/j.fertnstert.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maliqueo M, Galgani JE, Perez-Bravo F, Echiburu B, de Guevara AL, Crisosto N, et al. Relationship of serum adipocyte-derived proteins with insulin sensitivity and reproductive features in pre-pubertal and pubertal daughters of polycystic ovary syndrome women. Eur J Obstet Gynecol Reproduct Biol. 2012;161(1):56–61. doi: 10.1016/j.ejogrb.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Barber TM, Golding SJ, Alvey C, Wass JA, Karpe F, Franks S, et al. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J Clin Endocrinol Metabol. 2008;93(3):999–1004. doi: 10.1210/jc.2007-2117. [DOI] [PubMed] [Google Scholar]
- 19.Barber TM, Franks S. Adipocyte biology in polycystic ovary syndrome. Molecul Cellul Endocrinol. 2013;373(1-2):68–76. doi: 10.1016/j.mce.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Oden A, Holm G, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metabol. 2011;96(2):E304–11. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 21.de Zegher F, Lopez-Bermejo A, Ibanez L. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metabol TEM. 2009;20(9):418–23. doi: 10.1016/j.tem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochimica Et Biophysica Acta. 2010;1801(3):338–49. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diabet Rep. 2005;5(1):70–5. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 24.Gautier JF, Pirnay F, Jandrain B, Lacroix M, Mosora F, Scheen AJ, et al. Endogenous substrate oxidation during exercise and variations in breath 13CO2/12CO2. J Appl Physiol. 1993;74(1):133–8. doi: 10.1152/jappl.1993.74.1.133. [DOI] [PubMed] [Google Scholar]
- 25.Hatch KA, Sacksteder KA, Treichel IW, Cook ME, Porter WP. Early detection of catabolic state via change in 13C/12C ratios of blood proteins. BBRC. 1995;212(3):719–26. doi: 10.1006/bbrc.1995.2030. [DOI] [PubMed] [Google Scholar]
- 26.Butz DE, Cook ME, Eghbalnia HR, Assadi-Porter F, Porter WP. Changes in the natural abundance of 13CO2/12CO2in breath due to lipopolysacchride-induced acute phase response. Rapid Commun Mass Spectromet. 2009;23(23):3729–35. doi: 10.1002/rcm.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoeller DA, Brown C, Nakamura K, Nakagawa A, Mazzeo RS, Brooks GA, et al. Influence of metabolic fuel on the 13C/12C ratio of breath CO2. Biomed Mass Spectrom. 1984;11(11):557–61. doi: 10.1002/bms.1200111103. [DOI] [PubMed] [Google Scholar]
- 28.Gautier JF, Pirnay F, Jandrain B, Lacroix M, Mosora F, Scheen AJ, et al. Endogenous substrate oxidation during exercise and variations in breath 13CO2/12CO2. J Appl Physiol. 1993;74(1):133–8. doi: 10.1152/jappl.1993.74.1.133. [DOI] [PubMed] [Google Scholar]
- 29.McCue MD, Sivan O, McWilliams SR, Pinshow B. Tracking the oxidative kinetics of carbohydrates, amino acids and fatty acids in the house sparrow using exhaled 13CO2. J Experiment Biol. 2010;213(5):782–9. doi: 10.1242/jeb.039842. [DOI] [PubMed] [Google Scholar]
- 30.DeNiro MJ, Epstein S. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science. 1977;197(4300):261–3. doi: 10.1126/science.327543. [DOI] [PubMed] [Google Scholar]
- 31.Trimmer JK, Casazza GA, Horning MA, Brooks GA. Recovery of (13)CO2during rest and exercise after [1-(13)C]acetate, [2-(13)C]acetate, and NaH(13)CO3 infusions. Amer J Physiol Endocrinol Metabol. 2001;281(4):E683–92. doi: 10.1152/ajpendo.2001.281.4.E683. [DOI] [PubMed] [Google Scholar]
- 32.Phy J, Foong S, Session D, Thornhill A, Tummon I, Dumesic D. Transvaginal ultrasound detection of multifollicular ovaries in non-hirsute ovulatory women. Ultrasound Obstet Gynecol. 2004;23(2):183–7. doi: 10.1002/uog.954. [DOI] [PubMed] [Google Scholar]
- 33.Dumesic DA, Lesnick TG, Abbott DH. Increased adiposity enhances intrafollicular estradiol levels in normoandrogenic ovulatory women receiving gonadotropin-releasing hormone analog/recombinant human follicle-stimulating hormone therapy for in vitro fertilization. J Clin Endocrinol Metabol. 2007;92(4):1438–41. doi: 10.1210/jc.2006-2161. [DOI] [PubMed] [Google Scholar]
- 34.Matthews DR, Rudenski AS, Burnett MA, Darling P, Turner RC. The half-life of endogenous insulin and C-peptide in man assessed by somatostatin suppression. Clin Endocrinol. 1985;23(1):71–9. doi: 10.1111/j.1365-2265.1985.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 35.Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metabol. 1998;83(8):2694–8. doi: 10.1210/jcem.83.8.5054. [DOI] [PubMed] [Google Scholar]
- 36.Schutz Y, Flatt JP, Jequier E. Failure of dietary fat intake to promote fat oxidation: a factor favoring the development of obesity. Amer J Clin Nutrit. 1989;50(2):307–14. doi: 10.1093/ajcn/50.2.307. [DOI] [PubMed] [Google Scholar]
- 37.Radom-Aizik S, Zaldivar F, Jr, Oliver S, Galassetti P, Cooper DM. Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. J Appl Physiol. 2010;109:252–61. doi: 10.1152/japplphysiol.01291.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatch KA, Pinshow B, Speakman JR. The analysis of 13C/12C ratios in exhaled CO2: Its advantages and potential application to field research to infer diet, changes in diet over time, and substrate metabolism in birds. Integrat Comparat Biol. 2002;42(1):21–33. doi: 10.1093/icb/42.1.21. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Schoeller DA, Winkler FJ, Schmidt HL. Geographical variations in the carbon isotope composition of the diet and hair in contemporary man. Biomed Mass Spectrom. 1982;9(9):390–4. doi: 10.1002/bms.1200090906. [DOI] [PubMed] [Google Scholar]
- 40.Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Amer J Physiol Endocrinol Metabol. 2008;295(5):E1009, 17. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78(9):920–6. doi: 10.1016/j.steroids.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. New Engl J Med. 2010;363(14):1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 43.Bellanger S, Battista MC, Fink GD, Baillargeon JP. Saturated fatty acid exposure induces androgen overproduction in bovine adrenal cells. Steroids. 2012;77(4):347–53. doi: 10.1016/j.steroids.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golay A, Defronzo RA, Thorin D, Jequier E, Felber JP. Glucose disposal in obese non-diabetic and diabetic type II patients. A study by indirect calorimetry and euglycemic insulin clamp. Diabet Metabol. 1988;14(4):443–51. [PubMed] [Google Scholar]
- 45.Acheson KJ, Decombaz J, Piguet-Welsch C, Montigon F, Decarli B, Bartholdi I, et al. Energy, protein, and substrate metabolism in simulated microgravity. Amer J Physiol. 1995;269(2 Pt 2):R252–60. doi: 10.1152/ajpregu.1995.269.2.R252. [DOI] [PubMed] [Google Scholar]
- 46.Ritz P, Acheson KJ, Gachon P, Vico L, Bernard JJ, Alexandre C, et al. Energy and substrate metabolism during a 42-day bed-rest in a head-down tilt position in humans. Eur J Appl Physiol Occupation Physiol. 1998;78(4):308–14. doi: 10.1007/s004210050425. [DOI] [PubMed] [Google Scholar]
- 47.Beyer RE, Fattore JE. The influence of age and endurance exercise on the myoglobin concentration of skeletal muscle of the rat. J Gerontol. 1984;39(5):525–30. doi: 10.1093/geronj/39.5.525. [DOI] [PubMed] [Google Scholar]
- 48.Bonadonna RC, Groop LC, Simonson DC, DeFronzo RA. Free fatty acid and glucose metabolism in human aging: evidence for operation of the Randle cycle. Amer J Physiol. 1994;266(3 Pt 1):E501–9. doi: 10.1152/ajpendo.1994.266.3.E501. [DOI] [PubMed] [Google Scholar]
- 49.Goodpaster BH, Wolfe RR, Kelley DE. Effects of obesity on substrate utilization during exercise. Obesit Res. 2002;10(7):575–84. doi: 10.1038/oby.2002.78. [DOI] [PubMed] [Google Scholar]
- 50.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes. 2000;49(5):677–83. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 51.Kang J, Kelley DE, Robertson RJ, Goss FL, Suminski RR, Utter AC, et al. Substrate utilization and glucose turnover during exercise of varying intensities in individuals with NIDDM. Med Sci Sports Exerc. 1999;31(1):82–9. doi: 10.1097/00005768-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, et al. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes. 2007;56(3):720–7. doi: 10.2337/db06-0521. [DOI] [PubMed] [Google Scholar]
- 53.Di Sarra D, Tosi F, Bonin C, Fiers T, Kaufman JM, Signori C, et al. Metabolic inflexibility is a feature of women with polycystic ovary syndrome and is associated with both insulin resistance and hyperandrogenism. J Clin Endocrinol Metabol. 2013;98(6):2581–8. doi: 10.1210/jc.2013-1161. [DOI] [PubMed] [Google Scholar]
- 54.Salehzadeh F, Rune A, Osler M, Al-Khalili L. Testosterone or 17{beta}-estradiol exposure reveals sex-specific effects on glucose and lipid metabolism in human myotubes. J Endocrinol. 2011;210(2):219–29. doi: 10.1530/JOE-10-0497. [DOI] [PubMed] [Google Scholar]
- 55.Frederiksen L, Hojlund K, Hougaard DM, Brixen K, Andersen M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age. 2012;34(1):145–56. doi: 10.1007/s11357-011-9213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zachmann M, Zagalak M, Vollmin JA, Gitzelmann RP, Prader A. Influence of testosterone on urinary 15N-balance in normal subjects and patients with testicular feminization. Clin Chim Acta; Int J Clin Chem. 1977;77(2):147–57. doi: 10.1016/0009-8981(77)90022-5. [DOI] [PubMed] [Google Scholar]
- 57.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, et al. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metabol. 1997;82(2):407–13. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 58.Fryburg DA, Weltman A, Jahn LA, Weltman JY, Samojlik E, Hintz RL, et al. Short-term modulation of the androgen milieu alters pulsatile, but not exercise- or growth hormone (GH)-releasing hormone-stimulated GH secretion in healthy men: Impact of gonadal steroid and GH secretory changes on metabolic outcomes. J Clin Endocrinol Metabol. 1997;82(11):3710–9. doi: 10.1210/jcem.82.11.4379. [DOI] [PubMed] [Google Scholar]
- 59.Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metabol. 1998;83(6):1886–92. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- 60.Baldwin KM, Herrick RE, McCue SA. Substrate oxidation capacity in rodent skeletal muscle: effects of exposure to zero gravity. J Appl Physiol. 1993;75(6):2466–70. doi: 10.1152/jappl.1993.75.6.2466. [DOI] [PubMed] [Google Scholar]
- 61.Grichko VP, Heywood-Cooksey A, Kidd KR, Fitts RH. Substrate profile in rat soleus muscle fibers after hindlimb unloading and fatigue. J Appl Physiol. 2000;88(2):473–8. doi: 10.1152/jappl.2000.88.2.473. [DOI] [PubMed] [Google Scholar]
- 62.Ohira Y, Yasui W, Kariya F, Wakatsuki T, Nakamura K, Asakura T, et al. Metabolic adaptation of skeletal muscles to gravitational unloading. Acta Astronautica. 1994;33:113–7. doi: 10.1016/0094-5765(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 63.Yajid F, Mercier JG, Mercier BM, Dubouchaud H, Prefaut C. Effects of 4 wk of hindlimb suspension on skeletal muscle mitochondrial respiration in rats. J Appl Physiol. 1998;84(2):479–85. doi: 10.1152/jappl.1998.84.2.479. [DOI] [PubMed] [Google Scholar]
- 64.Stein T, Schluter M, Galante A, Soteropoulos P, Tolias P, Grindeland R, et al. Energy metabolism pathways in rat muscle under conditions of simulated microgravity. J Nutrit Biochem. 2002;13(8):471. doi: 10.1016/s0955-2863(02)00195-x. [DOI] [PubMed] [Google Scholar]
- 65.Wittwer M, Fluck M, Hoppeler H, Muller S, Desplanches D, Billeter R. Prolonged unloading of rat soleus muscle causes distinct adaptations of the gene profile. FASEB J. 2002;16(8):884–6. doi: 10.1096/fj.01-0792fje. [DOI] [PubMed] [Google Scholar]
- 66.Henriksen EJ, Tischler ME. Glucose uptake in rat soleus: Effect of acute unloading and subsequent reloading. J Appl Physiol. 1988;64(4):1428–32. doi: 10.1152/jappl.1988.64.4.1428. [DOI] [PubMed] [Google Scholar]
- 67.Langfort J, Zernicka E, Mayet-Sornay MH, Dubaniewicz A, Desplanches D. Effects of acute and chronic hindlimb suspension on sensitivity and responsiveness to insulin in the rat soleus muscle. Biochem Cell Biol = Biochimie Et Biologie Cellulaire. 1997;75(1):41–4. [PubMed] [Google Scholar]
- 68.Adamska A, Karczewska-Kupczewska M, Nikolajuk A, Otziomek E, Gorska M, Kowalska I, et al. Normal metabolic flexibility despite insulin resistance women with polycystic ovary syndrome. Endocrin J. 2013;60(9):1107–13. doi: 10.1507/endocrj.ej13-0115. [DOI] [PubMed] [Google Scholar]
- 69.Huang FP, Farquhar CF, Mabbott NA, Bruce ME, MacPherson GG. Migrating intestinal dendritic cells transport PrP(Sc) from the gut. J Gen Virol. 2001;83:267–71. doi: 10.1099/0022-1317-83-1-267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


