Abstract
Fibromyalgia is a complex, heterogeneous disorder for which a multidisciplinary individualized approach is currently advocated. We executed a 1 week multidisciplinary fibromyalgia clinical program with 7 patients, based on our previous experience with our existing 1.5 day multidisciplinary fibromyalgia program that has demonstrated both short- and long-term benefits. The current expanded program was not designed as a clinical study, but rather as a clinical feasibility assessment and was multidisciplinary in nature, with cognitive behavioral therapy, activity pacing and graded exercise therapy as major components. We assessed changes in individual patients at 1 week and 3 months following the program utilizing validated self-report measures of pain, fatigue, and self-efficacy. All patients indicated at least small improvements in pain and physical symptoms both at 1 week and 3 months and all but one patient showed improvement in self-efficacy at 1 week and 3 months. Similar trends were observed for fatigue. Based on our early clinical experience, we conclude that the 1 week multidisciplinary fibromyalgia program is logistically feasible and has potential for clinical efficacy. Further research is needed and is planned to test the clinical efficacy of this program and compare it with other interventions.
Keywords: fibromyalgia, pain, fatigue, self-efficacy, self-management
Introduction
Fibromyalgia is a disorder characterized by widespread musculoskeletal pain, allodynia, fatigue, and difficulties with sleep, memory, and mood. The prevalence of this disorder is 2.0% overall, 3.4% for women and 0.5% for men, with prevalence increasing with age and reaching 7% in women aged 60 to 79 years (Wolfe, Ross, Anderson, Russell, & Hebert, 1995). The American College of Rheumatology (ACR) criteria for diagnosis of fibromyalgia include widespread musculoskeletal pain of at least 3 months duration and pain on palpation of at least 11 of 18 standard tender points (Wolfe, Smythe, Yunus, Bennett, Bombardier, Goldenberg, Tugwell, Campbell, Abeles, Clark, & et al., 1990).
Though the exact etiology of fibromyalgia is unknown, the existing recommendation for fibromyalgia is to utilize a multidisciplinary, individualized approach that combines medications, relaxation techniques, biofeedback, activity pacing, sleep hygiene, cognitive behavioral therapy, graded exercise, physical and occupational therapy, and appropriate complementary modalities (Hassett & Gevirtz, 2009). In support of this, several multimodality intervention programs have consistently demonstrated benefit (Bennett, 1996; Sim & Adams, 2002). The duration of these programs, however, was relatively long, ranging from 2.5 weeks to 6 months (Bailey, Starr, Alderson, & Moreland, 1999; Bennett, 1996; Burckhardt, Mannerkorpi, Hedenberg, & Bjelle, 1994; Cedraschi, Desmeules, Rapiti, Baumgartner, Cohen, Finckh, Allaz, & Vischer, 2004; King, Wessel, Bhambhani, Sholter, & Maksymowych, 2002; Lemstra & Olszynski, 2005; Mason, Goolkasian, & McCain, 1998; Wennemer, Borg-Stein, Gomba, Delaney, Rothmund, Barlow, Breeze, & Thompson, 2006).
At our institution, a tertiary referral center, over 60% of patients with fibromyalgia are out-of-state and a lengthy intervention program is not always feasible. Therefore, we developed a brief 1.5 day multidisciplinary fibromyalgia program in January 1999, which incorporates several aspects of a multidisciplinary approach with an emphasis on education and self-management of fibromyalgia. The program evolved over the years to stay current with the literature on clinical care for patients with fibromyalgia. We have reported both short-term (1 month) and long-term (6 and 12 month) benefits from this program (Luedtke, Thompson, Postier, Neubauer, Drach, & Newell, 2005; Oh, Stueve, Hoskin, Luedtke, Vincent, Moder, & Thompson, 2010; Pfeiffer, Thompson, Nelson, Tucker, Luedtke, Finnie, Sletten, & Postier, 2003; Worrel, Krahn, Sletten, & Pond, 2001). Although the brief fibromyalgia program is beneficial, patient remarks indicated that they would be open to a program that was slightly longer to delve more deeply into experiential aspects of self-management. Based on this feedback, we conducted a one-time 1 week multimodal, multidisciplinary fibromyalgia program, to assess its clinical and operational feasibility. In this paper we report limited patient outcome data collected utilizing validated self-report questionnaires.
Description of Existing 1.5 day Multidisciplinary Fibromyalgia Program
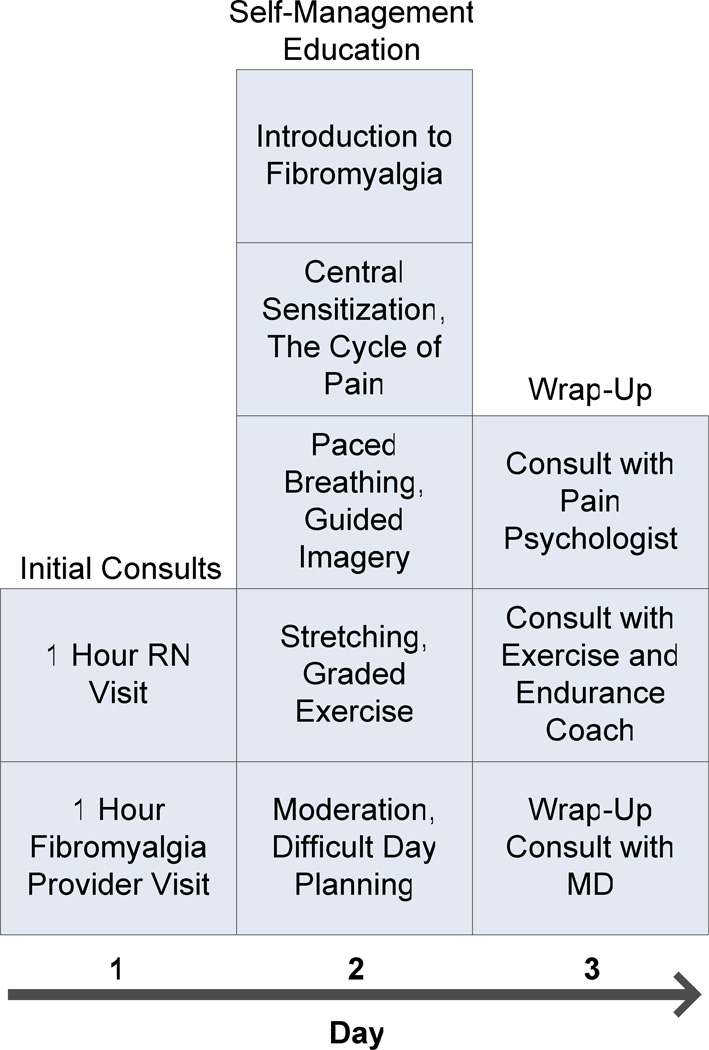
The existing 1.5 day Mayo Fibromyalgia Program (Figure 1) begins with appointments with a nurse and an internist with or without a nurse practitioner. The nurse interview is one hour long, during which a detailed history of symptoms pertaining to fibromyalgia (pain, fatigue, mood, sleep, and cognitive difficulties) and associated medical and psychiatric disorders is gathered, as is standard practice for a general medical assessment. The nurse then presents this information to an internist or nurse practitioner who sees the patient to confirm the diagnosis of fibromyalgia and review medications. During this evaluation, patients who have clinically significant sleep, mood, or joint disorders are referred to the appropriate sub specialists. The following day, patients spend 6–7 hours with a nurse with content expertise in fibromyalgia for interactive cognitive-behaviorally based sessions focused on understanding fibromyalgia, central sensitization, the cycle of chronic pain, and self-management activities. All patients then meet with an exercise and endurance coach to assess current health behavior, motivation to change, level of daily exercise and to establish a simple home exercise program. Physical and occupational therapists are available and are frequently added for consultation and treatment. The program is also staffed with physical medicine and rehabilitation specialists and a pain psychologist whose services are utilized as needed. The focus of the program is to provide evaluation of symptoms related to fibromyalgia, confirm the diagnosis, provide medication recommendations to the referring provider, and educate patients about strategies for self-management of their symptoms using a variety of interventions. Patients with fibromyalgia are referred to the program from primary care providers or sub specialists within Mayo Clinic and the Mayo Health System. The Mayo Health System is a network of clinics and hospitals located in 70 communities throughout Iowa, Minnesota, and Wisconsin that are staffed by community-based physicians who are supported by the tertiary specialized expertise and resources of Mayo Clinic.
Figure 1.
Program schedule for existing 1.5 day multidisciplinary fibromyalgia program.
Description of Modified 1 Week Multidisciplinary Fibromyalgia Program
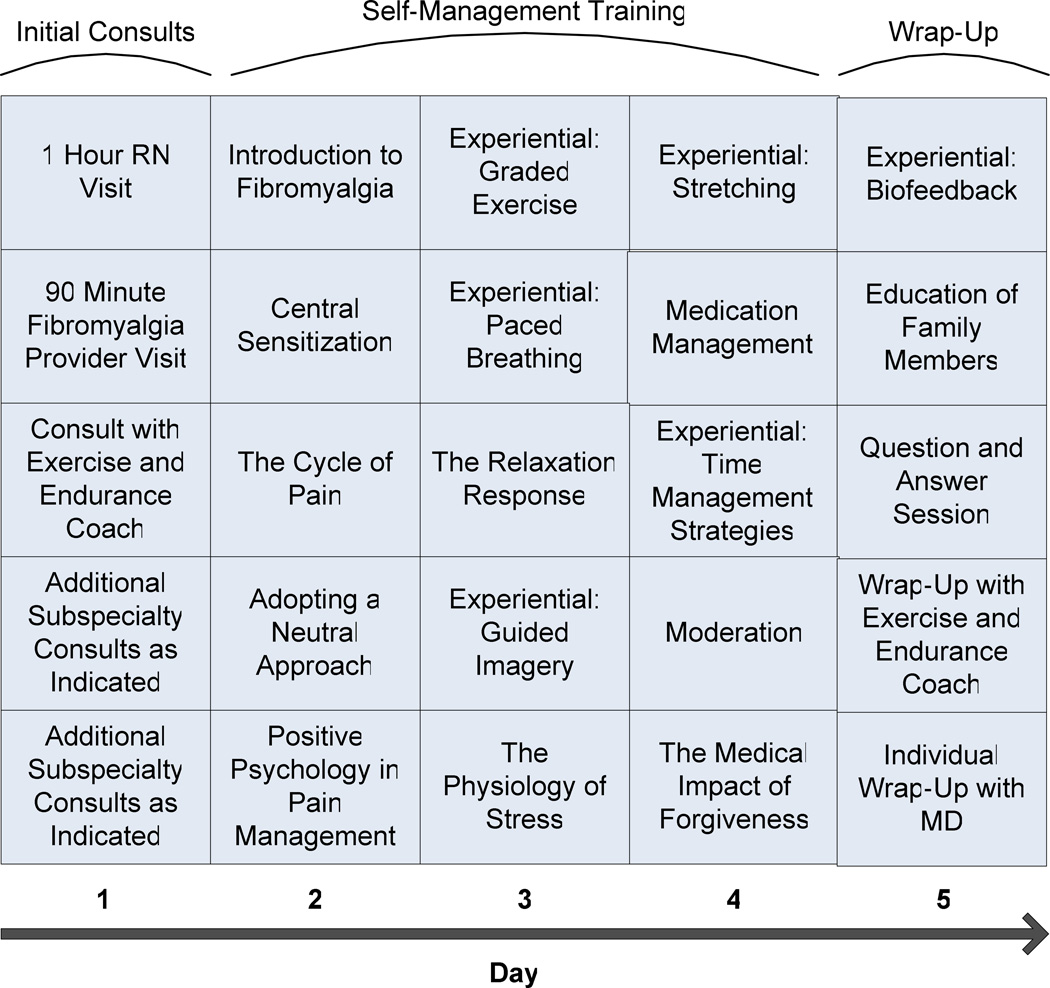
Similar to the 1.5 day program, the 1 week multimodal multidisciplinary program (Figure 2) began with appointments with a nurse, an internist ± a nurse practitioner for assessment and services. Following this, patients met with the exercise and endurance coach to assess current health behavior, motivation to change, level of daily exercise and to establish goals for personal health and exercise. The following 3 days of the program (days 2–4) consisted of interactive cognitive-behaviorally based sessions focused on understanding fibromyalgia, central sensitization, the cycle of chronic pain, and self-management activities. All sessions were delivered by registered nurses with content expertise in fibromyalgia and lasted approximately 6 hours per day. Ten minute stretch breaks were provided every hour. Family members were encouraged to attend all components of the program and received instruction on how to help patients implement self-management strategies when they returned home. On the fifth day, patients concluded the program with a wrap-up visit with the internist who helped plan their individualized medication and self-management strategies. Family members also participated in a session specifically designed for them, teaching them how to offer support for improved functioning and quality of life rather than inadvertently fostering self-limiting behavior or further medical diagnostic tests or interventions. Patients left with a binder containing resources and materials presented in the program for reinforcement. All patients then received 3 prescheduled follow-up phone calls from a registered nurse at 2 weeks, 1 month, and 3 months after the program to help reinforce self-management concepts and answer related questions.
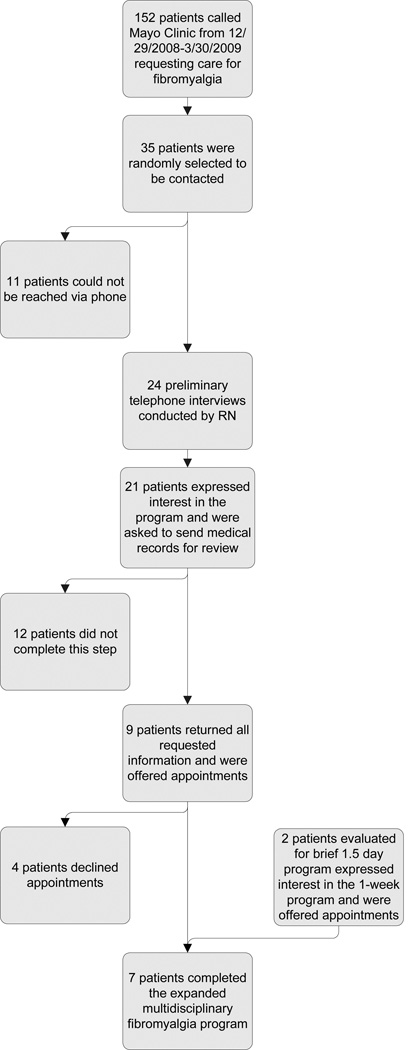
Figure 2.
Description of patient triage process.
Patient Triage
Given the limited number of providers in our clinic and the limited patient volume we were able to accommodate, we deliberately planned a small patient group for this expanded multidisciplinary fibromyalgia program. All patients had a previous diagnosis of fibromyalgia and met the 1990 ACR criteria for fibromyalgia (Wolfe et al., 1990). The detailed triage process is illustrated in Figure 3. Patients were identified from a group of patients who called Mayo Clinic requesting care for fibromyalgia. A registered nurse attempted to contact a subset of these patients, and despite multiple attempts not all patients could be reached. A preliminary telephone interview was conducted with the patients who could be reached, during which our approach to symptom self-management was discussed. Patients interested in such an approach were asked to provide authorization to facilitate the transfer and/or exchange of medical information with their primary care physician. All patients who completed these steps were offered appointments and a total of 7 patients were signed up for and completed the expanded multidisciplinary fibromyalgia program. Demographics, clinical characteristics, medical and psychiatric comorbidities, and medications of the participants are illustrated in Table 1. Approval to collect and publish our observations from this fibromyalgia program was obtained from the Mayo Clinic Institutional Review Board and all patients.
Figure 3.
Program schedule for modified 1 week multidisciplinary fibromyalgia program.
Table 1.
Summary of patient characteristics.
| Patient | Age | Sex | FIQ at Baseline |
Symptom Duration (yrs) |
Medications Related to Fibromyalgia Symptoms |
Related Medical Comorbidities |
Psychiatric Comorbidities |
|---|---|---|---|---|---|---|---|
| 1 | 65 | F | 47.2 | 20 | Citalopram, Ibuprofen, Acetaminophen-Aspirin-Caffeine | Osteoarthritis, Irritable bladder, Insomnia | Depression |
| 2 | 55 | F | 66.0 | 30 | Hydrocodone-Acetaminphen, Nortriptyline | OSA, HTN, IBS, Lower extremity parathesias, Cervical stenosis, Carpal tunnel syndrome | Depression Anxiety |
| 3 | 72 | M | 42.1 | 5 | Escitalopram, Pregabalin, Naltrexone, Trazodone | OSA, HTN | Depression |
| 4 | 63 | F | 40.0 | 21 | Cyclobenzaprine, Pregabalin, Diazepam | HTN, Insomnia | |
| 5 | 45 | F | 47.9 | Since childhood | Duloxetine, Hyoscyamine, Lorazepam, Oxycodone-Acetaminophen, Trazodone | CFS, IBS, Cervical disc disease, Osteoarthritis | Depression Anxiety |
| 6 | 51 | F | 68.8 | 10 | Zolpidem, Baclofen, Clonazepam, Acetaminophen-Propoxyphene, Fluoxetine | CFS, IBS, Irritable bladder, OSA, Insomnia, TMJ, Osteoarthritis, HTN, Chronic headache | Depression Anxiety |
| 7 | 38 | F | 61.3 | 4 | Acetaminophen-Butalbital-Caffeine, Gabapentin, Escitalopram, Trazodone | Chronic fatigue | Depression |
Abbreviations: OSA – obstructive sleep apnea, IBS – irritable bowel syndrome, CAD – coronary artery disease, HTN – hypertension, TMJ – temperomandibular joint dysfunction, CFS – chronic fatigue syndrome
Measures
Validated self-report questionnaires were used to assess changes in pain, fatigue, and self-efficacy. These questionnaires included the Multidimensional Fatigue Symptom Inventory – Short Form (MFSI-SF), Chronic Pain Self-efficacy Scale (CPSS), Short Form Health Survey (SF-36), and Fibromyalgia Impact Questionnaire (FIQ). Patients completed questionnaires at the baseline (day 1) and 1 week (day 5) while they were in the clinical program, and at 3 months after completion of the program by mail.
The Multidimensional Fatigue Symptom Inventory – Short Form (MFSI-SF) is a validated 30-item measure that assesses the multidimensional aspects of fatigue. It has five sub scales 1) General, 2) Emotional, 3) Physical, 4) Mental and 5) Vigor. Each subscale has 6 items and each item is rated on a 5 point scale depending on how true the statement was for the patient in the past week. The range for total fatigue score is −24 to 96 and higher scores indicate worse fatigue except for the vigor subscale where higher scores indicate less fatigue. Though the MFSI-SF is not a fibromyalgia-specific fatigue scale, it has been demonstrated to be a valid and reliable measure of fatigue in patients with cancer with an internal consistency 0.87–0.96 (Stein, Jacobsen, Blanchard, & Thors, 2004).
The Chronic Pain Self-efficacy Scale (CPSS) is a validated 22-item self-report questionnaire that assesses patients’ self-efficacy to cope with chronic pain. The CPSS has 3 sub scales: self-efficacy for pain management, self-efficacy for coping with symptoms, and self-efficacy for physical function. The range of self-efficacy score is 1 to 10, with higher scores indicating greater self-efficacy. The CPSS has demonstrated good internal consistency (0.87–0.90) in patients with chronic pain (Anderson, Dowds, Pelletz, Thomas Edwards, & Peeters-Asdourian, 1995).
The Short form Health Survey 36 (SF-36) is a validated 36-item survey that provides overall physical and mental health scores as well as an 8-scale profile of functional health and well-being. One previous paper reports that patients with fibromyalgia have poorer mental and physical health summary scores compared to the general population and compared to patients with other rheumatologic conditions (Hoffman & Dukes, 2008). Standardized scores range from 0 to 100, with higher scores indicating improved quality of life. The SF-36 has demonstrated an internal consistency of 0.79–0.90 in patients with chronic pain (Meyer-Rosberg, Burckhardt, Huizar, Kvarnstrom, Nordfors, & Kristofferson, 2001).
The Fibromyalgia Impact Questionnaire (FIQ) is a validated 21-item multidimensional self-report questionnaire that assesses intensity of pain, physical functioning, fatigue, morning tiredness, stiffness, depression, anxiety, job difficulty, and overall well-being over the previous week. Scores range from 0–100, with higher scores indicating more severe symptoms. The FIQ has demonstrated an internal consistency of 0.83 in patients with fibromyalgia (Bennett, 2005).
Outcome Summary
Patient outcome measures over time are presented for each individual using longitudinal line graphs. Exploratory hypothesis testing comparing pre and post-treatment scores were performed using Wilcoxon signed – rank tests and are reported along with median and ranges. Tests were performed using SAS (version 9.2, SAS Institute INC, Cary, NC) and were evaluated at an alpha level of 0.05.
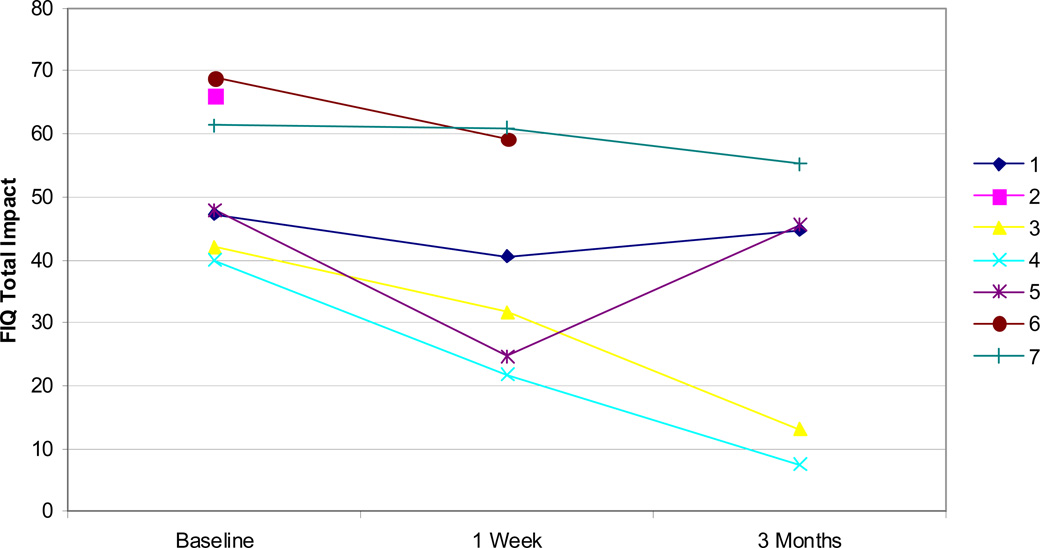
Six patients returned all questionnaires at 1 week and 5 patients returned all questionnaires at 3 months. As shown in Figure 4, all patients indicated at least a small improvement in fibromyalgia symptoms as measured by FIQ at both 1 week and at 3 months.
Figure 4.
FIQ total scores for all patients at baseline, 1 week, and 3 months. Lower scores indicate improved symptoms.
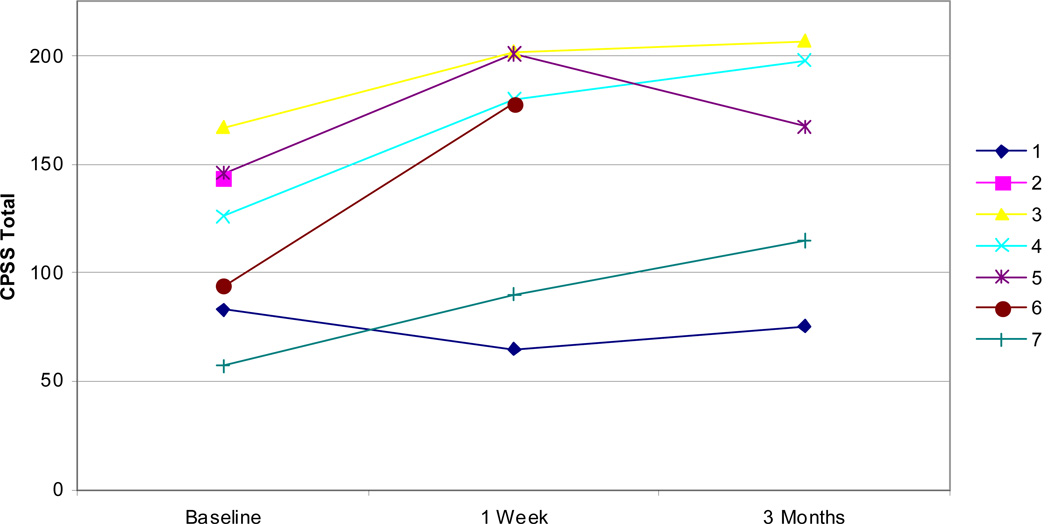
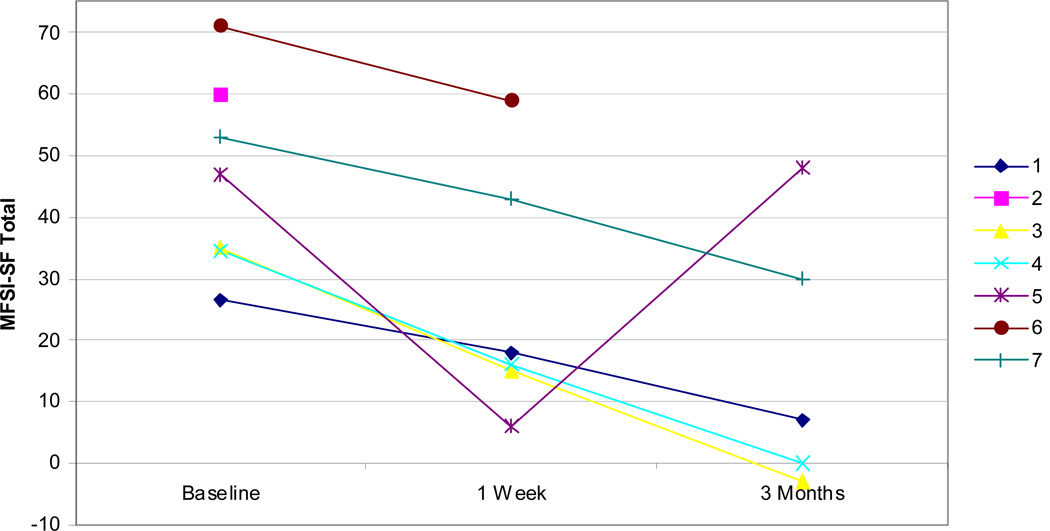
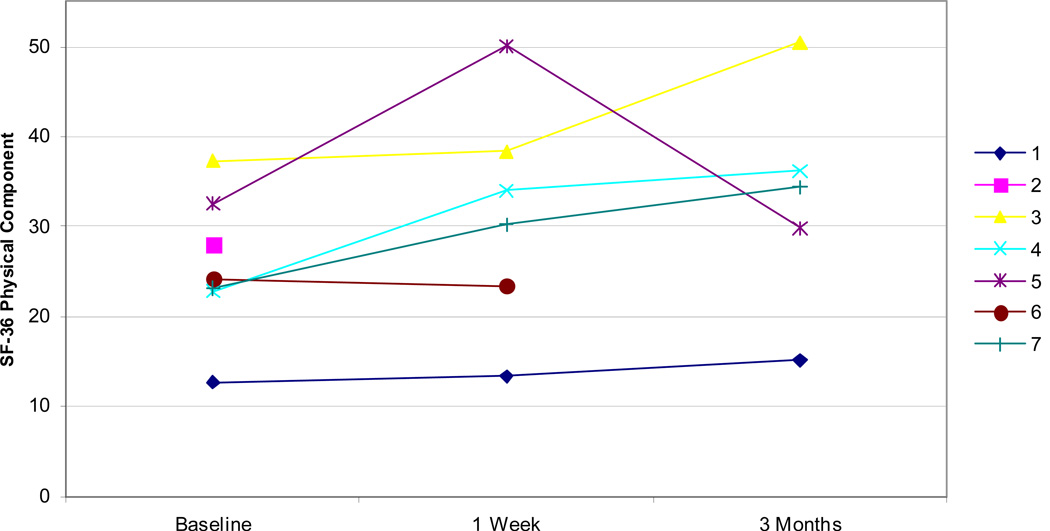
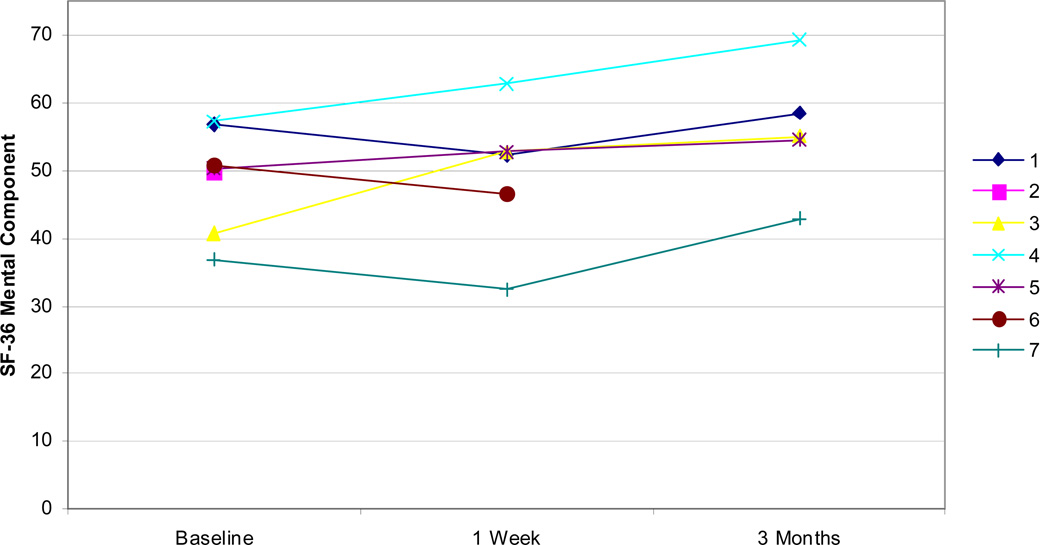
Examining other measures, all but one patient showed improvements in self-efficacy as measured by the CPSS both at 1 week and 3 months (Figure 5). Fatigue, as measured by the MFSI-SF, was improved in all patients at 1 week and all but one patient at 3 months (Figure 6). Improvements were similarly noted on SF-36 physical component scores at 1 week and 3 months in all but one patient (Figure 7). Patients did not demonstrate clear improvement on the SF-36 mental component at 1 week, but all patients reported improvement at 3 months (Figure 8).
Figure 5.
CPSS total scores for all patients at baseline, 1 week, and 3 months. Higher scores indicate increased self-efficacy.
Figure 6.
MFSI-SF total scores for all patients at baseline, 1 week, and 3 months. Lower scores indicate improved symptoms of fatigue.
Figure 7.
SF-36 physical component scores for all patients at baseline, 1 week, and 3 months. Higher scores indicate improved quality of life.
Figure 8.
SF-36 mental component total scores for all patients at baseline, 1 week, and 3 months.
Compared to baseline, significant differences were noted for both the FIQ total impact (p=0.03) and MFSI-SF total score (p=0.03) with median improvements of 10 points (range: 0.4 to 23.2) and 15.3 points (range: 8.5 to 41), respectively, at 1 week. At 3 months, none of the scores were significantly different from baseline, but there was a trend for FIQ total impact (p=0.06) with a median improvement of 6.2 points (range: 2.4 to 32.5) and SF-36 mental component score (p=0.06) with a median improvement of 6.1 points (range 1.6 to 14.4).
Table 1 describes summary of individual patient characteristics including medications. Only minor medication changes were instituted during this clinical program as our clinical experience suggests that taper of opioids and other pain medications are best instituted alongside, or following, longer periods of intensive physical and psychological rehabilitation. As such, we only made minor medication adjustments while patients were with us in an attempt to demonstrate to patients that self-management strategies could help them lower their use of pain medications. Patients were educated to institute formal tapers of opioids and related medications as indicated with the help of their primary care physicians.
Discussion
Our early experience with the expanded 1 week multimodal multidisciplinary fibromyalgia program appears promising and provides preliminary support that an expanded multidisciplinary fibromyalgia program is logistically feasible. In our previous paper we reported that the majority of our patients are younger than 60, employed, and come from out of state (Oh et al., 2010). Patients who travel long distances for specialty care are more willing to participate in shorter compared to lengthier treatment programs. We also observed wide individual variability in outcome (Oh et al., 2010) as reported by others (van Koulil, van Lankveld, Kraaimaat, van Helmond, Vedder, van Hoorn, Donders, de Jong, Haverman, Korff, van Riel, Cats, & Evers, 2010) and it was apparent that some of our patients needed different tailored treatment. It was also important to provide these patients with a pragmatic treatment that was both patient-friendly and high quality. Our program provided evaluation, confirmation, and validation of the diagnosis of fibromyalgia, which in our experience is a very important step for patients to move forward with self-management targeted to symptom improvement. Our 1 week program is unique in its comprehensiveness, brevity, and ease of administration. It could be considered for patients with higher symptomatology and for patients who desire a more comprehensive treatment than our existing 1.5 day program. It is not clear at this time which patients would benefit more from the 1 week vs 1.5 day program.
Bennett has suggested that a clinically meaningful change in FIQ impact score is 14% of baseline or larger (Bennett, Bushmakin, Cappelleri, Zlateva, & Sadosky, 2009). By this criterion, 5 of the 6 patients had clinically meaningful improvements at 1 week (improvements of 0.7%, 14.1%, 14.2%, 24.5%, 45.6%, and 48.4%). By the same criterion, at 3 months, 2 out of 5 patients had clinically significant improvements, with improvements of 69.1% and 81.4% relative to baseline, while the other 3 patients saw improvements of only 5.1%, 5.3%, and 10.1% at this time point.
Though a major limitation of this clinical report is the small patient number, this was not designed as a clinical trial and we acknowledge that we are unable to draw any conclusions on clinical efficacy. Furthermore, we had no control group and are unable to comment on how these symptoms might naturally change over time. However, there is a national paucity of clinical care for patients with fibromyalgia and we feel that dissemination of our clinical experience can help other clinical practices structure similar programs. Another limitation of our assessment strategy is that we did not measure patients’ adherence to the self-management strategies. However, the MFSI-SF, CPSS, SF-36, and FIQ are validated and commonly used questionnaires (Stein et al., 2004; Anderson et al., 1995; Meyer-Rosberg et al., 2001; Bennett, 2005). Therefore, utilizing these provided insight into the changes reported by individual patients.
In conclusion, a 1 week multimodal, multidisciplinary fibromyalgia program focused on self-management was logistically feasible and demonstrated potential for clinical efficacy. Further research is warranted to definitively demonstrate the clinical efficacy of a 1 week program. A vigorous design including randomization to a 1 week versus longer program with a larger, homogenous sample and a priori outcome of function is needed. Additional research to demonstrate this multifaceted intervention (i.e. comparatory arms of pharmacologic only interventions) would also be beneficial.
Acknowledgements
This research was supported in part by the Center for Translational Science Activities (CTSA) at Mayo Clinic. This center is funded in part by a grant from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) (RR024150, principal investigator, Robert A. Rizza, MD). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of CTSA, NCRR, or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this manuscript have been presented as a poster at the Musculoskeletal Disorders and Chronic Pain Conference, Los Angeles, California, February 10–12, 2011.
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- Anderson KO, Dowds BN, Pelletz RE, Thomas Edwards W, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63(1):77–83. doi: 10.1016/0304-3959(95)00021-J. [DOI] [PubMed] [Google Scholar]
- Bailey A, Starr L, Alderson M, Moreland J. A comparative evaluation of a fibromyalgia rehabilitation program. Arthritis Care & Research. 1999;12(5):336–340. doi: 10.1002/1529-0131(199910)12:5<336::aid-art5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Bennett RM. Multidisciplinary group programs to treat fibromyalgia patients. Rheumatic Diseases Clinics of North America. 1996;22(2):351–367. doi: 10.1016/s0889-857x(05)70276-3. [DOI] [PubMed] [Google Scholar]
- Bennett RM. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23(5) Suppl 39:S154–S162. [PubMed] [Google Scholar]
- Bennett RM, Bushmakin AG, Cappelleri JC, Zlateva G, Sadosky AB. Minimal clinically important difference in the fibromyalgia impact questionnaire. Journal of Rheumatology. 2009;36(6):1304–1311. doi: 10.3899/jrheum.081090. [DOI] [PubMed] [Google Scholar]
- Burckhardt CS, Mannerkorpi K, Hedenberg L, Bjelle A. A randomized, controlled clinical trial of education and physical training for women with fibromyalgia. Journal of Rheumatology. 1994;21(4):714–720. [PubMed] [Google Scholar]
- Cedraschi C, Desmeules J, Rapiti E, Baumgartner E, Cohen P, Finckh A, Allaz AF, Vischer TL. Fibromyalgia: a randomised, controlled trial of a treatment programme based on self management. Annals of the Rheumatic Diseases. 2004;63(3):290–296. doi: 10.1136/ard.2002.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett AL, Gevirtz RN. Nonpharmacologic treatment for fibromyalgia: patient education, cognitive-behavioral therapy, relaxation techniques, and complementary and alternative medicine. Rheumatic Diseases Clinics of North America. 2009;35(2):393–407. doi: 10.1016/j.rdc.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DL, Dukes EM. The health status burden of people with fibromyalgia: a review of studies that assessed health status with the SF-36 or the SF-12. Int J Clin Pract. 2008;62(1):115–126. doi: 10.1111/j.1742-1241.2007.01638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Wessel J, Bhambhani Y, Sholter D, Maksymowych W. Predictors of success of intervention programs for persons with fibromyalgia. Journal of Rheumatology. 2002;29(5):1034–1040. [PubMed] [Google Scholar]
- Lemstra M, Olszynski WP. The effectiveness of multidisciplinary rehabilitation in the treatment of fibromyalgia: a randomized controlled trial. Clinical Journal of Pain. 2005;21(2):166–174. doi: 10.1097/00002508-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Luedtke CA, Thompson JM, Postier JA, Neubauer BL, Drach S, Newell L. A description of a brief multidisciplinary treatment program for fibromyalgia. Pain Management Nursing. 2005;6(2):76–80. doi: 10.1016/j.pmn.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Mason LW, Goolkasian P, McCain GA. Evaluation of multimodal treatment program for fibromyalgia. Journal of Behavioral Medicine. 1998;21(2):163–178. doi: 10.1023/a:1018727924432. [DOI] [PubMed] [Google Scholar]
- Meyer-Rosberg K, Burckhardt CS, Huizar K, Kvarnstrom A, Nordfors LO, Kristofferson A. A comparison of the SF-36 and Nottingham Health Profile in patients with chronic neuropathic pain. European Journal of Pain: Ejp. 2001;5(4):391–403. doi: 10.1053/eujp.2001.0260. [DOI] [PubMed] [Google Scholar]
- Oh TH, Stueve MH, Hoskin TL, Luedtke CA, Vincent A, Moder KG, Thompson JM. Brief interdisciplinary treatment program for fibromyalgia: six to twelve months outcome. American Journal of Physical Medicine & Rehabilitation. 2010;89(2):115–124. doi: 10.1097/PHM.0b013e3181c9d817. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Thompson JM, Nelson A, Tucker S, Luedtke C, Finnie S, Sletten C, Postier J. Effects of a 1.5-day multidisciplinary outpatient treatment program for fibromyalgia: a pilot study. American Journal of Physical Medicine & Rehabilitation. 2003;82(3):186–191. doi: 10.1097/01.PHM.0000046625.72055.35. [DOI] [PubMed] [Google Scholar]
- Sim J, Adams N. Systematic review of randomized controlled trials of nonpharmacological interventions for fibromyalgia. Clinical Journal of Pain. 2002;18(5):324–336. doi: 10.1097/00002508-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. Journal of Pain & Symptom Management. 2004;27(1):14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Koulil S, van Lankveld W, Kraaimaat FW, van Helmond T, Vedder A, van Hoorn H, Donders R, de Jong AJL, Haverman JF, Korff K-J, van Riel PLCM, Cats HA, Evers AWM. Tailored cognitive-behavioral therapy and exercise training for high-risk patients with fibromyalgia. Arthritis Care & Research. 2010;62(10):1377–1385. doi: 10.1002/acr.20268. [DOI] [PubMed] [Google Scholar]
- Wennemer HK, Borg-Stein J, Gomba L, Delaney B, Rothmund A, Barlow D, Breeze G, Thompson A. Functionally oriented rehabilitation program for patients with fibromyalgia: preliminary results. American Journal of Physical Medicine & Rehabilitation. 2006;85(8):659–666. doi: 10.1097/01.phm.0000228677.46845.b2. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis & Rheumatism. 1995;38(1):19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia: Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Worrel LM, Krahn LE, Sletten CD, Pond GR. Treating fibromyalgia with a brief interdisciplinary program: initial outcomes and predictors of response. Mayo Clinic Proceedings. 2001;76(4):384–390. doi: 10.4065/76.4.384. [DOI] [PubMed] [Google Scholar]