Abstract
Background:
Para-dichlorobenzene (PDCB) is an active ingredient of mothballs, deodorizers and fumigants. Due to the easy availability of this chemical, there is a considerable risk for accidental or intentional toxic exposure. Recently, multiple cases of PDCB toxicity due to mothball ingestion were reported. PDCB toxicity can affect multiple organ systems including liver, kidneys, skin, lung and the central nervous system (CNS). CNS toxicity often results in leukoencephalopathy and heterogeneous neurological manifestations.
Objectives:
The objective of this study was to illustrate the clinical presentation, imaging findings, diagnosis and management of PDCB toxicity.
Methods:
We carried out a literature review of the pharmacological and toxicological properties of PDCB.
Conclusions:
PDCB and other aromatic hydrocarbons are capable of CNS tissue damage and in promoting functional neurological decline. While very little is currently known about prevalence of PDCB addiction, it cannot be ruled out that its illicit use among young people is under-recognized. The number of cases of PDCB toxicity might also rise due to the increasing industrial and domestic use of this chemical.
Keywords: Paradichlorobenzene, mothballs, neurotoxicity, aromatic hydrocarbons, leukoencephalopathy, demyelination
Introduction
Toxic ingestions are a frequent cause of hospital admissions worldwide. Common ingestions include illicit drugs, prescription medications and various household chemicals. Ingestion of mothballs has only recently been observed and typically appears to be accidental. However, toxicity secondary to pica of mothballs has been reported [Avila et al. 2006; Passov et al. 2011].
Mothballs are generally composed of either naphthalene or para-dichlorobenzene (PDCB). Both these substances are aromatic hydrocarbons which are volatile and lipid soluble. As PDCB is considered to be less toxic it has replaced naphthalene as the principal component in mothballs [Kumar et al. 2009]. PDCB toxicity affects the liver, skin, lungs, kidney, central and peripheral nervous system [Kumar et al. 2009; Hawkins et al. 1980]. Neurotoxicity related to PDCB is rare and can have varied clinical presentations mimicking different neurological disorders. This article presents a review of clinical features, imaging findings, assessment and management of neurotoxicity from PDCB.
Pharmacokinetics of PDCB
PDCB can be absorbed via ingestion, inhalation or dermal exposure. In mice the oral route was found to be more rapid than inhalation in a study conducted with several human volunteers who were exposed to PDCB through inhalation [Yoshida et al. 2002]. Absorption rate following inhalation route was found to be 46–67% [Hernandez et al. 2010]. In another study following inhalation exposure to PDCB for 10 days, researchers found that the concentration of the chemical compound peaked on day 6. The greatest concentration was found to be in adipose tissue, indicating the lipophilic nature of PDCB [Suhua et al. 2010]. Oral administration of PDCB in Fischer 344 rats for 28 days showed that the blood plasma concentration peaked on the third day. Decline in concentration after 3 days was hypothesized to be secondary to enzyme induction [Oikawa and Kawanishi, 1996].
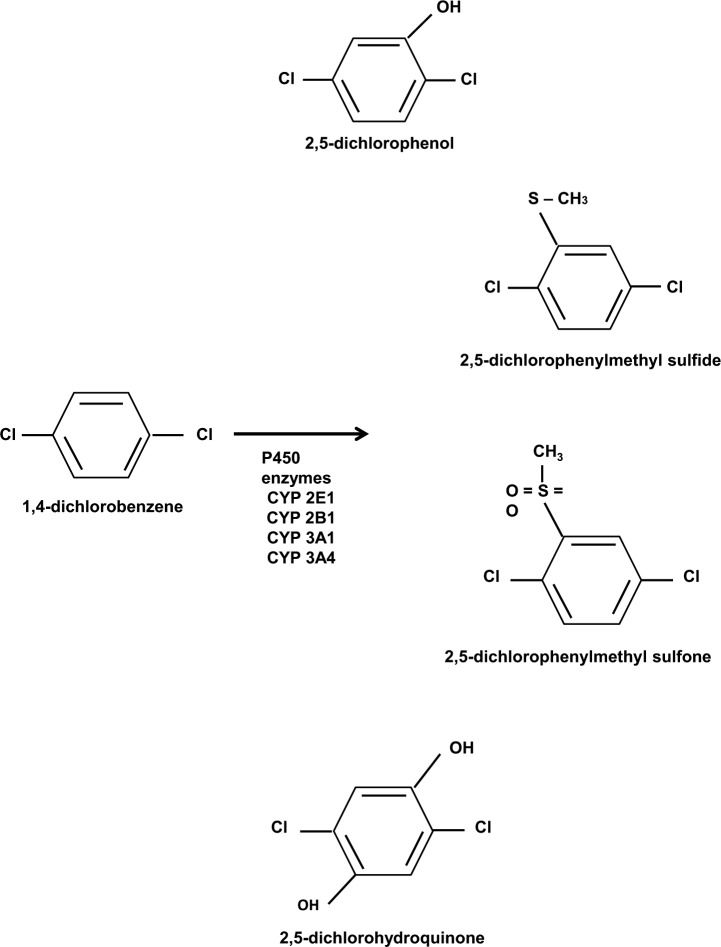
PDCB is metabolized in the liver by the cytochrome P450 system to a primary metabolite, 2,5-dichlorophenol (2,5-DCP) [Hawkins et al. 1980]. Other metabolites that have been detected in urine include 2,5-dichlorohydroquinone (2,5-DCHQ), 2,5-dichlorophenylmethyl sulfide, 2,5-dichlorophenylmethyl sulfone and mercapturic acid [Hernandez et al. 2010]. Several P450 enzymes are involved in the metabolism of PDCB including 2B1, 3A1 and 3A4, but the primary isoenzyme responsible for metabolism is CYP2E1 [Hawkins et al. 1980]. In an animal study, CYP2E1 induction via isoniazid increased the clearance rate and reduced the serum halflife of the compound. Urinary excretion of 2,5-DCP was increased in rats pretreated with isoniazid [Weintraub et al. 2000].
The excretion of 2,5-DCP is primarily mediated by the kidneys [Yoshida et al. 2002]. Human volunteers following PDCB inhalation excreted 4.8–16.2% of 2,5-DCP in urine within 10 hours of exposure [Hernandez et al. 2010].
The median lethal doses (LD50) of PDCB following acute oral toxicities in rats were found to be 3864 mg/kg and 3790 mg/kg in males and females, respectively [Cheong et al. 2006]. The LD50 for dermal exposure was greater than 6000 mg/kg, and lethal concentration on inhalation was found to be 6.0 mg/l [Gervais et al. 2010 Murray et al. 2010].
Mode of PDCB toxicity
The primary component of mothballs is PDCB (Figure 1). While PDCB is also utilized in deodorizer, fumigants and repellants, the most common use is in mothballs and in urinal deodorizer cakes. PDCB is rapidly absorbed via inhalation or ingestion [Hernandez et al. 2010]. It is thought that the lipophilic properties of PDCB result in its accumulation within the central nervous system (CNS), subsequently leading to demyelination and leukoencephalopathy. Due to its lipophilic nature it rapidly distributes in adipose tissue. Depletion of the adipose stores of PDCB is slow, and starvation increases the release of PDCB in blood stream due to mobilization of fat reserves in response to stress [Avila et al. 2006]. This might explain the sustained elevation of serum concentration even on cessation of exposure. This phenomenon is called coasting [Hawkins et al. 1980].
Figure 1.
Para-dichlorobenzene (PDCB) structure and metabolism. PDCB is metabolized in the liver by the cytochrome P450 system (CYP2E1, 2B1, 3A1 and 3A4) to 2,5-dichlorophenol, 2,5-dichlorohydroquinone, 2,5-dichlorophenylmethyl sulfide and 2,5-dichlorophenylmethyl sulfone.
The mechanism of action of PDCB and its toxicological properties in humans have not been studied well. However, multiple animal studies have been conducted to better understand its toxicological effects. In one study, mice exposed to high dose of mothballs showed decreased levels of reduced gluthathione and superoxide dismutase in kidneys and liver, respectively [Weintraub et al. 2000]. Alteration in levels of zinc, selenium and iron were also noted [Weintraub et al. 2000]. This study suggested oxidative damage and alteration of trace elements in different tissues as the cause of toxicity [Suhua et al. 2010]. Another study showed that some metabolites of PDCB – 2,5-DCHQ and 2,5-dichloro-p-benzoquinone (DCBQ) – can cause oxidative DNA damage in the presence of Cu(II) and nicotinamide adenine dinucleotide (NADH) [Oikawa and Kawanishi, 1996]. Chronic inhalation of PDCB in mice has also been shown to increase the incidence of liver cancer suggesting that it has carcinogenic properties [Aiso et al. 2005].
Yan and colleagues found that PDCB affects calcium homeostasis in neural tissue by inhibition of nicotinic acetylcholine receptor ion channels [Yan et al. 2008]. Subsequently PDCB mediated alteration in intracellular and extracellular calcium ion concentration in vitro might obstruct neuronal signaling leading to neuronal toxicity.
Clinical manifestations of PDCB toxicity
Toxicity due to mothball ingestion has been presented in several case reports. The most common manifestations include aplastic anemia, hepatic insufficiency, acute renal failure, pulmonary granulomatosis and neurotoxicity [Avila et al. 2006; Hernandez et al. 2010; Weintraub et al. 2000; Hsiao et al. 2009]. While these manifestations may occur after an acute ingestion, they may also occur after prolonged exposure due to slow depletion of PDCB from the adipose tissue [Avila et al. 2006; Cheong et al. 2006]. In general, there appears to be no clear clinical distinction between acute and chronic toxicity [NPCI, 2013]. Given its biochemical properties, it is conceivable that the absolute exposure of PDCB is more relevant than the duration of exposure.
As PDCB toxicity can affect any part of nervous system, there are no signs or symptoms specific for PDCB-induced neurotoxicity. Clinical manifestations can include psychomotor retardation, cognitive decline, memory issues, dysarthria, ataxia, vision loss, peripheral neuropathy, stupor and coma. A summary of clinical presentation, magnetic resonance imaging (MRI) findings and clinical outcome of various case reports is presented in Table 1. All the reported cases mentioned some degree of cognitive decline and 57% of the cases reviewed had encephalopathy. Ataxia was reported in nearly half of the cases. Amongst the reviewed cases 21% had dysarthria and 7% had bradykinesia. Peripheral neuropathy and optic neuritis were each mentioned in one case report. All of these clinical manifestations can occur in individuals exclusively exposed to PDCB, or in concert with other neurotoxic substances. Other causes of leukoencephalopathy are listed in Tables 2 and 3. Therefore, a thorough history needs to be obtained from patients with a suspected exposure.
Table 1.
Case reports and case series of PDCB toxicity.
| Case reports | Age/sex | Probable reason for pica | Mode of exposure | Duration of exposure | Serum level | Clinical manifestations | EEG findings | MRI findings | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Avila et al. [2006] | 21 years, female | Postpartum pica | Oral ingestion | 7 months | 0.5 mcg/ml | Encephalopathy, nystagmus, ataxia, seizures | Status epilepticus | Diffuse leukoencephalopathy | Improvement of mental status at 2 months |
| Cheong et al. [2006] | 42 years, female | NS | Oral ingestion | 2 years | NS | Encephalopathy, tremor, rigidity, ataxia, bradyphrenia and catatonia | Diffuse slowing | Diffuse leukoencephalopathy and involvement of deep cerebellar nuclei bilaterally | Marked improvement of mental status at 6 months |
| Feuillet et al. [2006] | Case 1: 18 years, female | Case 1: iron deficiency anemia | Inhalation | Case 1 and Case 2: 4–6 months | NS | Case 1: encephalopathy, pyramidal signs, unsteady gait, urinary retention, ichthyosis | Normal | Normal | Case 1: complete recovery at 6 months |
| Case 2: twin sister | Case 2: NS | Case 2: pyramidal signs, unsteady gait, ichthyosis | Case 2: complete recovery in 3 months | ||||||
| Reygagne et al. [1992] | 16 years, female | NS | Inhalation | Unknown duration | NS | Encephalopathy, bilateral optic neuritis, ataxia | NS | Normal | NS |
| Miyai et al. [26] | 25 years, female | Neurosis | Inhalation | 6 years | NS | Dysarthria, moderate weakness in extremities, hyporeflexia, truncal ataxia | Normal | Normal | Marked improvement in 6 months |
| Weintraub et al. [2000] | 54 years, female | Depression | Inhalation initially, followed by oral ingestion | Sniffing since her teens, chewing for 6 years | NS | Progressive weakness in all extremities, peripheral neuropathy | NS | Diffuse leukoencephalopathy, lacunar infarcts of the midbrain and pons | NS |
| Murray et al. [2010] | 31 years, female | Depression | Oral ingestion | 2 months | NS | Encephalopathy | Generalized slowing | Diffuse leukoencephalopathy | Improvement at 7 months |
| Hernandez et al. [2010] | 44 years, male | Depression, iron deficiency anemia | Inhalation and oral ingestion | 4 months | 1.2 mcg/ml | Encephalopathy, hyperreflexia, cogwheel rigidity | Focal slowing in the temporal lobes | Subcortical white matter changes in anterior temporal lobes | Symptoms persisted for >4 months |
| Kumar et al. [2009] | 32 years, female | NS | Inhalation initially followed by ingestion | Mothball sniffing since childhood, ingestion since 2 years | 34 mcg/ml | Cognitive decline, dysarthria, limb spasticity, bradykinesia, distal limb weakness, hyperreflexia, ataxia | NS | Diffuse white matter changes | NS |
| Frank et al. [27] | 19 years, female | NS | Oral ingestion | 2.5 years | NS | Mental slowing, unsteady gait, upper extremity tremor and icthyosis | NS | NS | Symptoms resolved 4 months post exposure |
| Passov et al. [2011] | 32 year, female | NS | Inhalation | 5 years | NS | Amnesia, behavioral changes, dysarthria, generalized weakness, ataxia | NS | Hyperintensity on FLAIR MRI in the brain stem, cerebellar peduncles, perventricular white matter, corpus callosum, and internal capsule | Marked improvement at 8 month follow up |
| Buckmann et al. [28] | 40 years, Female | Depression, anxiety | Oral ingestion | NS | NS | Encephalopathy, ataxia, cogwheel rigidity | NS | Diffuse periventricular white matter disease | Discharged home in stable condition |
| Hession et al. [2014] | 38 years, female | Iron deficiency anemia, depression | Inhalation, and oral ingestion | 21 years | 18.5 mcg/ml | Encephalopathy, dysconjugate gaze, left lower extremity weakness, urinary incontinence | Diffuse slowing | Hyperintensities on FLAIR in bilateral corona radiata, anterior basis pons, cerebral peduncles | Improvement in cognition at 3 month follow up |
FLAIR, fluid attenuated inversion recovery; MRI, magnetic resonance imaging; PDCB, para-dichlorobenzene; NS, not specified.
Table 2.
Potential causes of toxic leukoencephalopathy.
| Potential cause | Examples |
|---|---|
| Antineoplastic interventions | Bleomycin, bortezomib, carmustine, cisplatin, cranial irradiation, cyclophosphamide, cytarabine, doxorubicin, etoposide, fludarabine, 5-fluorouracil, interleukin-2, interferon-α, levamisole, methotrexate, thiotepa |
| Immunosuppressive drugs | Azathioprine, cyclosporine, dexamethasone, prednisolone, prednisone,rituximab, tacrolimus |
| Antimicrobial agents | Acyclovir, amphotericin B, hexachlorophene, levamisole, linezolid |
| Illicit drugs use | Cocaine, ethanol, inhaled heroin, intravenous heroin, 3,4-methylenedioxymethamphetamine, psilocybin, pyrolysate, toluene |
| Environmental toxins | Arsenic, carbon monoxide, carbon tetrachloride, ethylene glycol, methanol |
Table 3.
Potential causes of leukoencephalopathy.
| Potential cause | Examples |
|---|---|
| Genetic | Aminoacidurias (phenylketonuria, maple syrup urine disease), Krabbe’s disease, leukodystrophies (adrenoleukodystrophies, metachromatic leukodystrophy), lipid metabolism disorders (cerebrotendinous xanthomatosis), lysosomal disorders (Fabry’s disease, GM1 gangliosidosis, GM2 gangliosidosis), metal metabolism disorders (Menke’s kinky hair disease, molybdenum cofactor deficiency), mitochondrial cytopathies (Kearns–Sayre syndrome, Leigh’s disease, MELAS syndrome, MERFF syndrome) |
| Demyelinating disease | Acute disseminated encephalomyelitis, acute hemorrhagic encephalomyelitis, Baló’s disease, Marburg disease, multiple sclerosis, osmotic demyelination syndrome. |
| Infection | Acquired immunodeficiency syndrome dementia complex, cytomegalovirus encephalitis, Lyme encephalopathy, progressive multifocal leukoencephalopathy, progressive rubella panencephalitis, subacute sclerosing panencephalitis, varicella–zoster encephalitis |
| Metabolic disorder | Cobalamin deficiency, eclampsia, folate deficiency, high-altitude cerebral edema hypoxia, hypertensive encephalopathy |
| Vascular disorder | Binswanger’s disease, cerebral amyloid angiopathy, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy |
| Trauma | Diffuse axonal injury secondary to traumatic brain injury, hydrocephalus |
MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke; MERFF, myoclonic epilepsy with ragged red fibers.
The cases reported had variable time course for the manifestation of symptoms. Murray and colleagues reported a case of mothball induced encephalopathy in a 31 year old woman presenting with psychomotor retardation, flat affect and cognitive decline after 2 months of mothball ingestion [Murray et al. 2010]. Cheong and colleagues reported a case of 42 year old woman presenting with encephalopathy after mothball ingestion for 2 years [Cheong et al. 2006]. Passov and colleagues reported a case of PDCB-associated dementia in a 32 year old female with loss of memory, slurred speech, reduced oral intake and ataxia that only started after 5 years of mothball inhalation [Passov et al. 2011]. As seen in the different cases (Table 1), the duration of clinical manifestations varies, which may be due to mode of consumption, amount consumed and other factors including age and other comorbidities.
Similarly, the time until clinical improvement occurs after diagnosis of mothball toxicity varies greatly. In some cases complete recovery was not achieved for several months after stopping the exposure. In the case reported by Passov and colleagues, the patient had residual impairment in short-term memory, visuospatial and executive function 8 months after diagnosis [Passov et al. 2011]. However, complete resolution of symptoms is also reported after 3–6 months in three other cases [Feuiellet et al. 2006; Cheong et al. 2006; Frank et al. 1961]. It should be noted that, in these cases, time of recovery was not dependent on duration of exposure. It is not clear why in some cases the recovery is delayed compared with rapid recovery reported after halting the exposure. No clear association between serum concentration of PDCB and degree of neurotoxicity could be derived.
Multiple sclerosis disease progression and PDCB
Our group recently described the case of a 38 year old woman with an established diagnosis of relapsing-remitting multiple sclerosis (RRMS), who had been admitted with acute worsening of fatigue, worsening lower extremity weakness and new onset of an ichthyosiform rash that symmetrically involved her legs, arms and trunk (Table 1) [Hession et al. 2014]. The patient had an 11-year history of RRMS, white matter lesions on brain magnetic resonance (MR) images and her cerebrospinal fluid (CSF) was positive for oligoclonal bands (OCB). She met diagnostic criteria for RRMS [Polman et al. 2011]. The patient had received 31 doses of natalizumab after experiencing ongoing disease activity. On natalizumab therapy, there had been no more relapses but the patient had continued to accumulate neurological disability, which manifested itself primarily through cognitive decline, mood changes, fatigue, lower extremity weakness, and eventually an inability to ambulate.
We were eventually able to determine that the patient had a long-standing history of chewing on toilet bowel deodorizing cakes that consist to 99.9% of PDCB. The patient was ingesting PDCB intentionally on a daily basis and she reported an anxiolytic effect. Her PDCB level was 1.1 mcg/ml, which is substantially higher than in a reference population without PDCB toxicity [Hill et al. 1995]. At the time her PDCB level was obtained, there were new or evolving MRI lesions involving the corona radiata and centrum semiovale bilaterally, as well as the cerebral peduncles. It was strongly felt that the patient’s clinical progression was entirely due to PDCB toxicity. It was considered highly relevant that this patient had been treated with natalizumab and that a dramatic neurological deterioration under this agent is unusual [Havrdova et al. 2009]. The patient tested negative for natalizumab anti-idiotypic antibodies and her serology for JC virus was repeatedly negative. Other infectious and noninfectious causes that could have resulted in a pseudo-exacerbation had also been ruled out.
Diagnosis of PDCB toxicity
Diagnosis of PDCB toxicity can be challenging due to overlap in clinical manifestations with various neurological disorders. The low number reported cases in the scientific and popular literature is very likely the result of underreporting by the patients, who are likely teenagers and young adults. Therefore, a thorough history of exposure or ingestion is the key and can aid in early diagnosis of PDCB toxicity. However, in most of the cases diagnosis is delayed due to an inaccurate history provided by patients or because neurological deficits are attributed to a pre-existing disorder. In suspected cases, family members should be interrogated.
Detection of toxicity is typically made by measuring urinary levels of 2,5-DCP or serum concentrations of PDCB [Hill et al. 1995]. Urinary 2,5-DCP can also be used to evaluate PDCB exposure. In a study of 1000 US adults without suspected PDCP exposure or toxicity, 98% had detectable 2.5-DCP in the urine where as 96% had detectable PDCB serum levels [Hill et al. 1995]. In this study, 2,5-DCP concentrations in blood and urine were not related to either age or gender. These data suggest that PDCB is a common environmental contaminant.
High serum/urine concentration of PDCB, along with history of abuse and signs/symptoms of toxicity, often guide the decision making. However, given that PDCB is very lipophilic, serum and urine concentration are not likely to reliably reflect tissue concentrations within the CNS. Ultimately, a fat biopsy may be required to establish a diagnosis in an individual from whom a history cannot be obtained and in whom PDCB exposure is strongly suspected. Nonsurgical techniques of obtaining fat samples by aspiration from the gluteal prominence have been reported by several investigators. Daum and colleagues [1978] modified this technique for the analysis of fat-soluble hydrocarbon residues [Daum et al. 1978]. Aspiration of fat from the lateral gluteal prominence is performed under local anesthetic, which makes this a potentially feasible procedure. Interestingly, the anesthetic xylocaine also serves as the vehicle into which the fat is dissolved by the shearing action of a 15 gauge needle. A total of 200–500 ml of fat can be obtained by this method for hydrocarbon residue analysis. The only reported complication has been the occurrence of mild hematomas at the site of the aspiration. The method avoids sutures and takes less than 10 minutes.
Given the biochemical properties of PDCB, one might also expect nerve conduction studies (NCS) to be abnormal in affected individuals with CNS manifestation. However, in at least one reported case with confirmed PDCB encephalopathy NCS of the left peroneal, tibial and sural nerves, including F-wave latencies were essentially normal [Hession et al. 2014].
Other laboratory tests that can be abnormal after PDCB exposure but are not specific or pathognomonic for this intoxication include elevated white blood cell counts (WBC), alanine aminotransferase (ALT) and a decrease in the AST/ALT ratio [Hsiao et al. 2009]. Further workup including urine toxicology, MRI and electroencephalography (EEG) is often required to investigate complications secondary to toxicity.
Neuroimaging
Imaging of the brain is standard of care in all cases of toxic leukoencephalopathy to investigate the changes secondary to toxicity. Imaging findings in toxic leukoencephalopathy secondary to PDCB consumption are nonspecific and can include involvement of periventricular and supraventricular white matter, internal capsule, corpus callosum and cerebellum (Table 2). In cases reported by Avila and colleagues and Cheong and colleagues, MR brain images showed diffuse leukoencephalopathy and involvement of the splenium [Avila et al. 2006; Cheong et al. 2006]. In some published cases, acute or subacute restricted diffusion in periventricular white matter can also be seen on MRI [Muilenburg and Johnson, 2006]. The etiopathogenesis of diffusion restriction is unclear, but these findings may be secondary to endothelial damage, intramyelinic edema or direct toxic demyelination [Reygagne et al. 1992]. Although these imaging findings may be present in some affected individuals, they are not specific or pathognomonic for PDCP toxicity. Similar findings can be seen with leukoencephalopathy secondary to other aromatic compounds (naphthalene), cranial irradiation, demyelinating disorders, and leukodystrophies (Tables 2 and 3). As illustrated by the clinical case above, neuroimages associated with PDCB toxicity can be difficult to discern from those seen in patients with an established diagnosis of multiple sclerosis (MS). Careful history and laboratory workup is needed to differentiate these disorders as the management will differ.
Treatment
As of now there are no known proven effective antidotes for PDCB neurotoxicity. The goal of any potential intervention is reduction of exposure along with supportive care. Prevention of starvation by maintaining adequate diet through tube feeding in patients with decreased awareness and alertness prevents the mobilization of PDCB from the fat reserves [Avila et al. 2006]. The rationale behind this management strategy is to decrease the level of circulating toxin and hence reduce the transport of lipophilic PDCB from adipose tissue to lipid-rich CNS. There is no clear evidence even in animal studies whether this strategy would be useful in reducing the level of neurotoxicity. However, an argument could be made that increasing the mobilization of fat stores may stimulate the renal excretion of the toxin [Herron et al. 2002, Schweigert et al. 2002].
There are several strategies suggested to reduce serum and adipose tissue concentrations including hemodialysis, ketogenic diets, and administration of intravenous lipids [Polman et al. 2011]. As PDCB is primarily metabolized by CYP 2E1, P450 enzyme induction with ethanol, isoniazid and acetaminophen can enhance the rate of elimination [Flockhart et al. 2007, Kim et al. 2007]. In an animal study, isoniazid was shown to decrease the area under the curve of PDCB serum level, and increase its renal excretion [Hissink et al. 1997; Koop, 1992]. Possible interventions for patients with PDCB intoxication are listed in Figure 2.
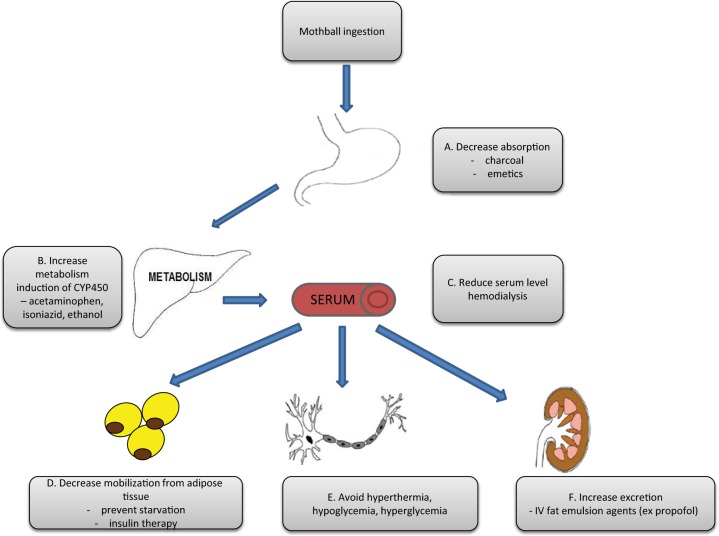
Figure 2.
Pharmacodynamics properties of para-dichlorobenzene (PDCB) and potential therapeutic interventions in individuals who have clinical manifestations from prolonged exposure. Potential therapeutic options are listed grey text boxes. (A) In cases of acute PDCB toxicity, the amount of absorbed toxin can be reduced by using either charcoal or emetics (e.g. Ipecac) [Gervais et al. 2010]. (B) The rate of metabolism of PDCB by CYP 2E1 enzymes can potentially be increased by various enzyme inducers including acetaminophen, isoniazid and ethanol [Flockhart et al. 2007, Kim et al. 2007]. (C) Serum level of the PDCB may be reduced by hemodialysis [Daum et al. 1978]. (D) The phenomenon of coasting due to mobilization of fat stores of PDCB following cessation of oral ingestion can be regulated by prevention of starvation (adequate diet or tube feeds) and use of hormones preventing lipolysis in adipose tissue through administration of insulin [Herron et al. 2002; Schweigert et al. 2002]. (E) Extent of neuronal damage could be limited by preventing hyperthermia, hyper/hypoglycemia. (F) Renal excretion of PDCB following acute ingestion can be increased by preventing its deposition in adipose tissue by using intra venous fat emulsion agents, including propofol [Avila et al. 2006; Hernandez et al. 2010].
Based on our experience in a single patient, acetaminophen reduced the serum level of PDCB significantly over 10 days (2 g, oral, daily). However, significant clinical improvement was not noticed at the end of the 10 day period. Subsequent clinical follow up at 3 months did show improvement in cognitive functions. To our knowledge no other case using acetaminophen as a therapeutic option has been reported. Further studies are needed to evaluate possible therapeutic options.
One must exercise caution in considering these potential therapies as the low case numbers provide insufficient evidence to support any of them. Therapy must be considered after weighing the risks and benefits on individual case basis.
Conclusion
Here, we provide an in-depth review of the neurological manifestations of PDCB toxicity. While PDCB is a very common environmental contaminant, population-based studies show detectable tissue levels in individuals without a history of intentional ingestions. In addition, PDCB appears to be used as a recreational agent, as it is readily available, cheap and legal. While very little is currently known about prevalence of PDCB addiction, it cannot be ruled out that its illicit use among young people is under-recognized. For instance, one cross-sectional study found that 20.4% of children attending a middle school in rural Mississippi had used inhalants for recreational use [Muilenburg and Johnson, 2006]. A substantial proportion of surveyed students reported they had used inhalants on 10 or more occasions. PDCP is often used as an inhalant [Feuillet et al. 2006] and it is conceivable that it is one agent that was utilized in this study. The number of cases of PDCB toxicity might also rise due to the increasing industrial and domestic use of this chemical. In 2007, the California Environmental Protection Agency concluded that PDCB, due to its attendant capacity for atmospheric transport, has been detected everywhere around the globe, including remote polar areas. Importantly, bioaccumulation in sediments and food crops has been shown to occur and has led to contamination of aquatic species that are used for food consumption and is present in the human and animal food chain [Newhart, 2007].
Early identification of PDCB toxicity will likely be the most beneficial step for affected individuals, and the illustration of common clinical presentations and imaging findings in this review may help in the diagnosis of some cases. Cessation of exposure to the chemical and some experimental therapies may prove to be beneficial for some of these patients. However, further studies are required to establish meaningful outcomes in individuals who have clinical manifestations from prolonged PDCB exposure.
Finally, PDCB and other aromatic hydrocarbons clearly have the ability to cause CNS tissue damage and a substantial decline in neurological function. One recent case report of a patient with MS who progressed rapidly despite natalizumab therapy illustrates that PDCB has the capacity to promote noninflammatory acceleration of disease in a progressive fashion. This observation is intriguing, as factors that lead to a change from a relapsing-remitting to a progressive clinical phenotype are currently incompletely understood. While we do not postulate that PDCB is the causative agent in all cases, or even in the majority of cases, the role of this agent in the initiation and perpetuation of MS disease activity should be further investigated.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Divyanshu Dubey, Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Vibhash D. Sharma, Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, Texas, USA
Steven E. Pass, Department of Pharmacy Practice, Texas Tech University Health Sciences Center School of Pharmacy, Dallas, TX, USA
Anshudha Sawhney, Netaji Subhash Chandra Bose Medical College, Jabalpur, India.
Olaf Stüve, Neurology Section, VA North Texas Health Care System, Medical Service, 4500 South Lancaster Road, Dallas, TX 75216, USA.
References
- Aiso S., Takeuchi T., Arito H., Nagano K., Yamamoto S., et al. (2005) Carcinogenicity and chronic toxicity in mice and rats exposed by inhalation to para-dichlorobenzene for two years. J Vet Med Sci 67: 1019–1029 [DOI] [PubMed] [Google Scholar]
- Avila E., Schraeder P., Belliappa A., Faro S. (2006) Pica with paradichlorobenzene mothball ingestion associated with toxic leukoencephalopathy. J Neuroimaging 16: 78–81 [DOI] [PubMed] [Google Scholar]
- Buckman F. (2013) Paradichlorobenzene (toxin)-induced leucoencephalopathy. BMJ Case Rep 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong R., Wilson R., Cortese I., Newman-Toker D. (2006) Mothball withdrawal encephalopathy: case report and review of paradichlorobenzene neurotoxicity. Subst Abuse 27: 63–67 [DOI] [PubMed] [Google Scholar]
- Daum S., Knittle J., Roseman K., Rom W., Holstein E. (1978) A simple technique for fat biopsy of PBB-exposed individuals. Environ Health Perspect 23: 183–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillet L., Mallet S., Spadari M. (2006) Twin girls with neurocutaneous symptoms caused by mothball intoxication. N Engl J Med 355: 423–424 [DOI] [PubMed] [Google Scholar]
- Flockhart DA. (2007) Drug interactions: cytochrome P450 drug interaction table. Indiana University School of Medicine; Available from: http://medicine.iupui.edu/clinpharm/ddis/table.aspx (accessed September 2013). [Google Scholar]
- Frank S., Cohen HJ. (1961) Fixed drug eruption due to paradichlorobenzene. N Y State J Med 61: 4079 [Google Scholar]
- Gervias J., Luukinen B., Buhl K., Stone D. (2010) Paradichlorobenzene technical fact sheet; National Pesticide Information Center, Oregon State University Extension Services; Available from: http://npic.orst.edu/factsheets/PDBtech.pdf (accessed 31 January 2014). [Google Scholar]
- Havrdova E., Galetta S., Hutchinson M., Stefoski D., Bates D., Polan C., et al. (2009) Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 8: 254–260 [DOI] [PubMed] [Google Scholar]
- Hawkins D., Chasseaud L., Woodhouse R., Cresswell D. (1980) The distribution excretion and biotransformation of p-dichloro[14C]benzene in rats after repeated inhalation, oral and subcutaneous doses. Xenobiotica 10: 81–95 [DOI] [PubMed] [Google Scholar]
- Hernandez S., Wiener S., Smith S. (2010) Case files of the New York City poison control center: paradichlorobenzene-induced leukoencephalopathy. J Med Toxicol 6: 217–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron RE., Fagan JB. (2002) Lipophil-mediated reduction of toxicants in humans: an evaluation of an ayurvedic detoxification procedure. Altern Ther Health Med. 8: 40–51 [PubMed] [Google Scholar]
- Hession R., Sharma V., Spiegel D., Tat C., Hwang D., Dieppa M., et al. (2014) Multiple sclerosis disease progression and para-dichlorobenzene – a tale of mothballs and toilet cleaner. JAMA Neurology, in press. [DOI] [PubMed] [Google Scholar]
- Hill R., Jr., Ashley D., Head S., Needham L., Pirkle J. (1995) p-Dichlorobenzene exposure among 1,000 adults in the United States. Arch Environ Health 50: 277–280 [DOI] [PubMed] [Google Scholar]
- Hissink A., Dunnewijk R., van Ommen B., van Bladeren P. (1997) Kinetics and metabolism of 1,4-dichlorobenzene in male Wistar rats: no evidence for quinone metabolites. Chem Biol Interact 103: 17–33 [DOI] [PubMed] [Google Scholar]
- Hsiao P., Lin Y., Shih T., Chiung Y. (2009) Effects of occupational exposure to 1,4-dichlorobenzene on hematologic, kidney, and liver functions. Int Arch Occup Environ Health 82: 1077–1085 [DOI] [PubMed] [Google Scholar]
- Kim SN., Seo JY., Jung da W., Lee MY., Jung YS., Kim YC. (2007) Induction of hepatic CYP2E1 by a subtoxic dose of acetaminophen in rats: increase in dichloromethane metabolism and carboxyhemoglobin elevation. Drug Metab Dispos 35:1754–8 Epub 2007 Jul 9. [DOI] [PubMed] [Google Scholar]
- Koop D. (1992) Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J 6: 724–730 [DOI] [PubMed] [Google Scholar]
- Kumar N., Dale L., Wijdicks E. (2009) Mothball mayhem: relapsing toxic leukoencephalopathy due to p-dichlorobenzene neurotoxicity. Ann Intern Med 150: 362–363 [DOI] [PubMed] [Google Scholar]
- Miyai I., Hirono N., Fujita M., Kameyama M. (1988) Reversible ataxia following chronic exposure to paradichlorobenzene. J Neurol Neurosurg Psychiatry 51: 453–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muilenburg J., Johnson W. (2006) Inhalant use and risky behavior correlates in a sample of rural middle school students. Subst Abuse 27: 21–25 [DOI] [PubMed] [Google Scholar]
- Murray S., Dwight-Johnson M., Levy M. (2010) Mothball induced encephalopathy presenting as depression: it’s all in the history. Gen Hosp Psychiatry 32: 341 e347–349 [DOI] [PubMed] [Google Scholar]
- Newhart K. (2007) Environmental fate of paradichlorobenzene. Sacramento, CA: California Environmental Protection Agency, California Department of Pesticide Regulation; Available from: http://www.cdpr.ca.gov/docs/emon/pubs/fatememo/paradichlorobenzene.pdf (accessed 21 January 2014). [Google Scholar]
- Oikawa S., Kawanishi S. (1996) Copper-mediated DNA damage by metabolites of p-dichlorobenzene. Carcinogenesis 17: 2733–2739 [DOI] [PubMed] [Google Scholar]
- Passov V., Milliner E., Rundell J. (2011) Partially reversible dementia associated with the use of para-dichlorobenzene in the case of pica. Psychosomatics 52: 283–285 [DOI] [PubMed] [Google Scholar]
- Polman C., Reingold S., Banwell B., Clanet M., Cohen J., Filippi M., et al. (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69: 292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reygagne A., Garnier R., Chataigner D., Echenne B., Efthymiou M. (1992) [Encephalopathy caused by repeated voluntary inhalation of paradichlorobenzene]. Presse Med 21: 267. [PubMed] [Google Scholar]
- Schweigert FJ., Luppertz M., Stobo WT. (2002) Fasting and lactation effect fat-soluble vitamin A and E levels in blood and their distribution in tissue of grey seals (Halichoerus grypus). Comp Biochem Physiol A Mol Integr Physiol. 131: 901–908 [DOI] [PubMed] [Google Scholar]
- Suhua W., Rongzhu L., Changqing Y., Guangwei X., Fangan H., et al. (2010) Lipid peroxidation and changes of trace elements in mice treated with paradichlorobenzene. Biol Trace Elem Res 136: 320–336 [DOI] [PubMed] [Google Scholar]
- Weintraub E., Gandhi D., Robinson C. (2000) Medical complications due to mothball abuse. South Med J 93: 427–429 [PubMed] [Google Scholar]
- Yoshida T., Andoh K., Fukuhara M. (2002) Urinary 2,5-dichlorophenol as biological index for p-dichlorobenzene exposure in the general population. Arch Environ Contam Toxicol 43: 481–485 [DOI] [PubMed] [Google Scholar]
- Yan RM., Chiung YM., Pan CY., Liu JH., Liu PS. (2008) Effects of dichlorobenzene on acetylcholine receptors in human neuroblastoma SH-SY5Y cells. Toxicology 20: 28–35 [DOI] [PubMed] [Google Scholar]