Abstract
Background
The goal of our study was to determine the therapeutic effects of thymoquinone in a dose-dependent manner in a model of neuropathic pain following an experimentally applied spinal cord injury (SCI).
Methods
Fifty female adult Wistar albino rats weighing between 220 and 260 g were included in the study and were divided into 5 groups as follows: Group S (sham), Group C (control), Group T100 (100 mg/kg thymoquinone), Group T200 (200 mg/kg thymoquinone), and Group T400 (400 mg/kg thymoquinone). To begin the experiment, SCI was applied to all groups (with the exception of the sham group) following a mechanical and heat–cold test. Two weeks later, the mechanical and heat–cold tests were repeated, and a single normal saline dose was given to the sham and control groups, whereas 3 varying doses of thymoquinone were given to the other groups. The mechanical and heat–cold tests were repeated at 30, 60, 120, and 180 minutes after receiving thymoquinone. Finally, the animals were put to death via the removal of intracardiac blood. The levels of nitric oxide, total oxidant status, total antioxidant status, paraoxonase, malondialdehyde, tumor necrosis factor-α, and interleukin-1β were determined in all of the blood samples.
Results
The withdrawal threshold and withdrawal latency values recorded from the mechanical and heat–cold allodynia measurements for all 3 thymoquinone groups were higher than that of the control group at all time points (ie, 30, 60, 120, and 180 minutes). There were no differences in these results between the 3 thymoquinone groups. The paraoxonase and total antioxidant status serum levels of all 3 thymoquinone groups were higher than those of the control group, whereas total oxidant status, nitric oxide, malondialdehyde, interleuken-1β, and tumor necrosis factor-α levels were lower in the 3 thymoquinone groups than in the control group.
Conclusions
Thymoquinone is beneficial for decreasing experimental neuropathic pain following SCI. However, increasing the dose does not change the effect.
Key words: heat-cold allodynia, mechanical allodynia, neuropathic pain, thymoquinone
Introduction
Neuropathic pain affects millions of people worldwide.1 It is a chronic type of pain that worsens as it continues, and can range from mild to extreme pain levels. It can develop following illnesses of the peripheral or central nervous systems, and can also develop as a result of an injury. Despite the many experimental and animal studies on neuropathic pain, its pathophysiologic mechanisms have not been completely elucidated.
Pain that occurs due to a spinal cord injury (SCI) can diminish quality of life and hinder successful physical rehabilitation. Neuropathic pain is difficult to relieve. Many patients have failed to benefit from current medications, and therefore there is a need to develop new medications.2
Thymoquinone is extracted from the oil of Nigella sativa, and in recent studies it has been reported to be immunomodulatory,3 anti-inflammatory,4 antitumor,5 antidiabetic,6,7 and antinociceptive.8 However, the effects of thymoquinone have not yet been determined for neuropathic pain following SCI.
Generally, neuropathic pain is monitored with mechanical9,10 and heat–cold8,11 allodynia tests, although it can be detected via biochemical parameters as well.6
Our study was conducted to determine the dose-dependent therapeutic effects of PO thymoquinone in a model of neuropathic pain.
Methods
Animals
Following approval from our ethics committee, 50 female adult Wistar albino rats weighing between 220 and 260 g with normal motor activity were selected from the Dicle University Health Research Center. The animals were kept under standard laboratory conditions (ie, exposed to light from 12:00 pm to 12:00 am at a room temperature of 20°C–22°C) during the duration of the experiment (2 weeks). The rats were fed ad libitum. Their bladders were emptied twice a day via manual compression. During the experiment, none of the animals developed infections or paralysis of the back legs following cord trauma, and none displayed dragging behaviors.
Experimental protocol
The rats were equally divided into 5 random groups: Group S: Sham + normal saline (NS), Group C: SCI + NS, Group T100: SCI + thymoquinone 100 mg/kg/PO, Group T200: SCI + thymoquinone 200 mg/kg/PO, and Group T400: SCI + thymoquinone 400 mg/kg/PO.
All groups were subjected to mechanical and heat–cold tests before the start of the experiment. With the exception of the sham group, SCI was applied to all of the animals. Two weeks later, the mechanical and heat–cold tests were repeated, and the sham and control groups were given a single dose of NS, whereas the other groups were given the appropriate dose of thymoquinone (Sigma catalog-274666; Cas No. 490-91-5, in sterile packaging) in NS by nasogastric feeding tube. Doses of thymoquinone were chosen according to the study by Abdel-Fattah et al.8 The mechanical and heat–cold hypersensitivity tests were repeated at 30, 60, 120, and 180 minutes after receiving NS or thymoquinone. After all animals were anesthetized (using 80 mg/kg ketamine), they were put to death after 180 minutes by removal of intracardiac blood and perfusion of NS with 10% formaldehyde.
SCI
Anesthesia (80 mg/kg ketamine) was administered intraperitoneally to all rats in a manner that ensured the preservation of spontaneous breathing. Using interscapular distance as a reference, a 3 × 2 cm area on each animal’s back was shaved, and local antiseptic (povidone-iodine) was applied. Then, lidocaine was administered as local anesthetic, which passed the cutaneous and subcutaneous tissue at the T5–12 levels. While under anesthesia, the rats were placed in a facedown position and were monitored with a rectal heat probe to maintain a body temperature of 37°C. The paravertebral muscle fascia were pulled apart using lateral blunt dissection until the T6–7 lamina could be seen. A total laminectomy was carefully applied at a single level to prevent damage to the dura. A Yaşargil aneurysm clip (model FE 721 K; Aesculap AG and Co, Tuttlingen, Germany) for standard trauma was clipped around the dura and spinal cord between T6–7 for 1 minute with 63 g force.12 Then, the clip was removed and following hemostasis the incision area was closed with 3-0 silk sutures appropriate for anatomic layers. The rats were then placed in their cages.
Tests
Mechanical test
The withdrawal threshold measurement, which is the most common method used to measure mechanical allodynia, was taken with an electronic von Frey device (Model EVF3; Bioseb, Chaville, France). For the test, every rat was placed on a mesh floor inside a transparent plastic cage. The rats were kept in a silent environment at room temperature for 20 minutes, and then force (in grams) was applied to the rats through the plantar hind paw up-down method.13 The upper pressure limit was 15 g, and the point at which each rat pulled its paw back was recorded. This test determined the mechanical excitability threshold.
Heat (8)–cold (44) test
Flicking of the hindlimb or jumping as the withdrawal latency measurement, which is the most commonly used method to measure heat–cold allodynia using a hot–cold plate analgesia meter (Cold Hot Plate Test; Bioseb, Chaville, France), was measured. The plate can be heated to 65°C or cooled to −3°C (±0.1°C) in ambient room temperature (20oC–25oC). Each animal was placed in a Plexiglas cylinder (165 mm diameter × 25 mm height) with a drilled cover.11 A temperature of 55oC (±1oC) was used to determine heat hypersensitivity, and a cut-off time of 60 seconds was used to prevent tissue damage. A temperature of −3oC was chosen to determine cold hypersensitivity.14,15
Biochemical analysis
Blood samples were immediately centrifuged at 4000 rpm (4oC for 10 minutes), transferred into Eppendorf tubes (Relassay, Gaziantep, Turkey), and were kept at −70oC until the end of the study, which was completed in 3 months. Total antioxidant status (TAS) and total oxidant status (TOS) were measured by Erel’s methods,16,17 whereas malondialdehyde (MDA) content was measured using a spectrophotometer, as previously described.18 Serum paraoxonase (PON) levels were measured using a spectrophotometer with a modified Eckerson method. Initial rates of paraoxon hydrolysis (0.0-diethyl-0-p-nitrophenylphosphate; Sigma Chemical Co, London, United Kingdom) were determined by measuring liberated p-nitrophenol at 405 nm (37°C).19 Plasma nitric oxide (NO; Cayman Chemicals, Ann Arbor, Michigan), tumor necrosis factor-α (TNF-α; Bender MedSystems, Vienna, Austria), and interleukin-1β (IL-1β; Bender MedSystems, Vienna, Austria) levels were determined with an enzyme-linked immunosorbent assay method.
Statistical analysis
Statistical analyses were performed using SPSS for Windows 13.0 (IBM-SPSS Inc, Armonk, New York). Biochemical values are presented as mean values (SD). Differences between groups were compared with the nonparametric Kruskal-Wallis test. A Mann- Whitney U test was used for binary comparisons. The Bonferonni correction was applied, and Ps < 0.01 were considered to be statistically significant.
Results
The withdrawal threshold and withdrawal latency values recorded from the mechanical and heat–cold allodynia measurements for all 3 thymoquinone groups were higher than that of the control group at all time points (ie, 30, 60, 120, and 180 minutes) (P < 0.01). There were no differences in these results between the 3 thymoquinone groups (ie, T100, T200, and T400) (Figures 1–3 and Table I).
Figure 1.
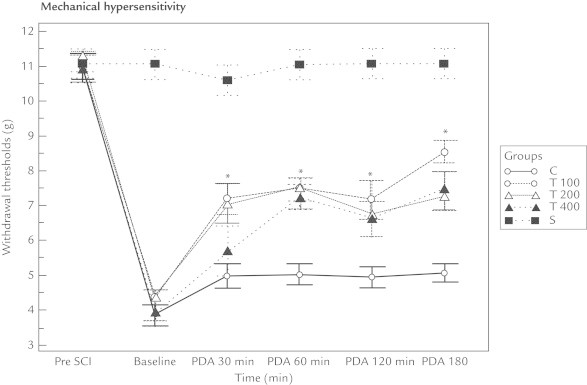
Thymoquinone affected the mechanical hypersensitivity of rats with neuropathic pain following spinal cord injury (SCI). Baseline = 2 weeks following SCI and just before giving the medication; PDA = postdrug administration; Pre SCI = values before the experiment. *Compared with the control group (Group C), the withdrawal thresholds of all the thymoquinone groups (T100, T200, and T400) increased. However, no difference was found between the thymoquinone groups.
Figure 2.
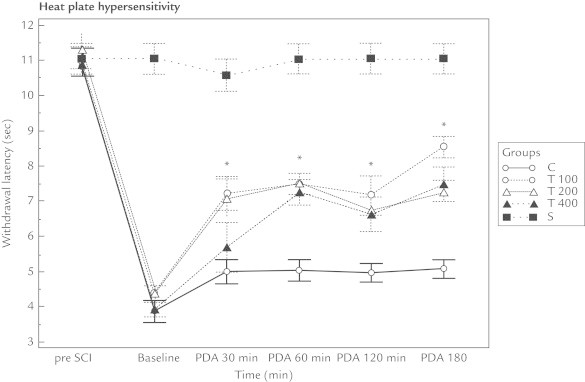
Thymoquinone affected the heat plate hypersensitivity of rats with neuropathic pain following spinal cord injury (SCI). Compared with the control group (Group C), the withdrawal latency of all the thymoquinone groups (T100, T200, and T400) increased. However, no difference was found between the thymoquinone groups. Baseline = 2 weeks following SCI and just before giving the medication; PDA = postdrug administration; Pre SCI = values before the experiment.
Figure 3.
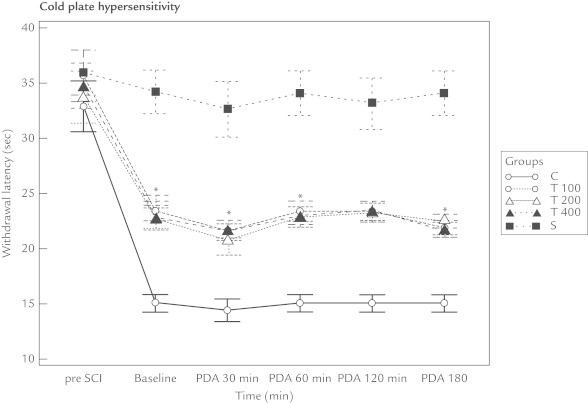
Thymoquinone affected the cold plate hypersensitivity of rats with neuropathic pain following spinal cord injury (SCI). Compared with the control group (Group C), the withdrawal latency of all the thymoquinone groups (T100, T200, and T400) increased. However, no difference was found between the thymoquinone groups. Baseline = 2 weeks following SCI and just before giving the medication; PDA = postdrug administration; Pre SCI = values before the experiment.
Table I.
Mean (SD) values of mechanical hypersensitivity, heat plate hypersensitivity, and cold plate hypersensitivity, by thymoquinone dose.
| Times | Parameter | S | C | T100 | T200 | T400 |
|---|---|---|---|---|---|---|
| Pre-SCI | CPH* | 35.95 (6.51) | 32.86 (7.13) | 35.65 (7.44) | 33.77 (7.48) | 34.71 (6.56) |
| HPH† | 11.05 (1.36) | 10.95 (1.30) | 10.95 (1.48) | 11.30 (1.62) | 10.89 (1.28) | |
| MH‡ | 15 | 15 | 15 | 15 | 15 | |
| Baseline | CPH | 34.33 (6.25) | 15.05 (2.44) | 23.38 (2.85) | 22.69 (2.92) | 22.74 (3.48) |
| HPH | 11.05 (1.36) | 3.86 (0.96) | 4.38 (0.68) | 4.37 (0.74) | 3.93 (0.67) | |
| MH | 15 | 2.67 (0.51) | 4.47 (1.25) | 4.80 (1.54) | 4.42 (1.25) | |
| PDA 30 min | CPH | 32.63 (7.90) | 14.40 (3.11) | 21.57 (2.08) | 20.75 (4.38) | 21.63 (2.88) |
| HPH | 10.58 (1.46) | 4.99 (1.08) | 7.22 (1.50) | 7.07 (1.78) | 5.70 (2.26) | |
| MH | 15 | 2.62 (0.51) | 6.79 (1.11) | 7.44 (1.46) | 6.49 (1.38) | |
| PDA 60 min | CPH | 34.05 (6.35) | 15.05 (2.44) | 23.38 (2.85) | 22.85 (2.92) | 22.93 (2.46) |
| HPH | 11.05 (1.36) | 5.03 (0.97) | 7.49 (0.97) | 7.53 (1.06) | 7.26 (1.12) | |
| MH | 15 | 2.61 (0.42) | 8.60 (1.25) | 8.43 (1.59) | 7.90 (2.10) | |
| PDA 120 min | CPH | 33.14 (7.36) | 15.05 (2.43) | 23.36 (2.86) | 23.23 (2.73) | 23.38 (2.85) |
| HPH | 11.05 (1.35) | 4.95 (0.83) | 7.17 (1.75) | 6.74 (1.97) | 6.62 (1.58) | |
| MH | 15 | 2.59 (0.39) | 8.75 (1.36) | 8.16 (1.43) | 8.61 (1.46) | |
| PDA 180 min | CPH | 34.05 (6.34) | 15.00 (2.46) | 21.88 (1.98) | 22.50 (2.02) | 21.74 (2.10) |
| HPH | 11.04 (1.34) | 5.06 (0.83) | 8.53 (0.97) | 7.24 (1.11) | 7.47 (1.60) | |
| MH | 15 | 2.72 (0.46) | 8.13 (1.72) | 7.96 (1.65) | 7.27 (1.46) |
Baseline = 2 weeks following SCI and just before giving the medication; C = control group; CPH = cold plate hypersensitivity; HPH = heat plate hypersensitivity; MH = mechanical hypersensitivity; PDA = postdrug administration; Pre-SCI = values before the experiment; S = sham group; T100 = thymoquinone 100 mg/kg/PO group; T200 = thymoquinone 200 mg/kg/PO group; T400 = thymoquinone 400 mg/kg/PO group.
Thymoquinone affected the CPH of rats with neuropathic pain following SCI. Compared with Group C, the withdrawal latency of all the thymoquinone groups increased. However, no difference was found between the thymoquinone groups.
Thymoquinone affected the HPH of rats with neuropathic pain following SCI. Compared with Group C, the withdrawal latency (seconds) of all the thymoquinone groups increased. However, no difference was found between the thymoquinone groups.
Thymoquinone affected the MH of rats with neuropathic pain following SCI. Compared with Group C, the withdrawal thresholds (in grams) of all the thymoquinone groups increased. However, no difference was found between the thymoquinone groups.
Serum PON and TAS levels in the thymoquinone groups were higher than those of the control group, whereas serum TOS, NO, MDA, IL-1β, and TNF-α levels were lower than those of the control group (P < 0.01) (Table II).
Table II.
Levels of biochemical markers in the serum of experimental animals, by dose of thymoquinone.
| Marker | S |
C |
T100 |
T200 |
T400 |
P |
|---|---|---|---|---|---|---|
| Mean (SD) | ||||||
| PON | 30.67 (4.49) | 25.99 (5.68) | 78.63 (1.80)*,† | 80.15 (3.48)*,† | 112.76 (17.98)*,†,‡ | <0.01 |
| TAS | 0.44 (0.06) | 0.04 (0.03)* | 2.25 (0.46)*,† | 2.88 (0.36)*,† | 2.10 (0.42)*,† | <0.01 |
| TOS | 22.77 (4.86) | 34.27 (1.29) | 1.31 (1.02)*,† | 3.41 (1.61)*,†,‡ | 1.94 (1.30)*,† | <0.01 |
| IL-1β | 1.22 (0.53) | 2.48 (1.17)* | 1.76 (1.38)*,† | 1.82 (1.41)* | 1.91 (1.43)* | <0.01 |
| TNF-α | 0.23 (0.41) | 3.57 (3.62)* | 0.89 (1.45)*,† | 0.45 (0.83)† | 0.25 (0.47)‡ | <0.01 |
| NO | 0.17 (0.04) | 0.24 (0.06)* | 0.16 (0.25)† | 0.19 (0.08)†,‡ | 0.19 (0.06)*,†,‡ | <0.01 |
| MDA | 20.37 (4.54) | 37.20 (8.92)* | 19.40 (2.49)† | 22.20 (2.55)† | 20.89 (2.63)† | <0.01 |
C = control group; IL-1β = interleukin-1 β; MDA = malondialdehyde; NO = nitric oxide; PON = serum paraoxonase; S = sham group; T100 = thymoquinone 100 mg/kg/PO group; T200 = thymoquinone 200 mg/kg/PO group; T400 = thymoquinone 400 mg/kg/PO group; TAS = total antioxidant status; TNF-α = tumor necrosis factor α; TOS = total oxidant status.
Different from S group (P < 0.01).
Different from C group (P < 0.01).
Different from T100 group (P<0.01).
Discussion
Patients experiencing neuropathic pain often seek relief from physicians, who provide many different medications and treatment options.20 Recently, it has been suggested that thymoquinone provides immunomodulatory, anti-inflammatory, and antinociceptive effects.3,4,8
Oral administration of N. sativa produces a suppressive effect on nociceptive responses and the antinociceptive effect of N. sativa oil is partly attributable to thymoquinone, 1 of its components. It is assumed that the supraspinal opioid systems are involved in the antinociceptive effect of thymoquinone.8
Thymoquinone, when administered orally and intraperitoneally, has positive analgesic effects that cannot be reversed by opioid antagonists.4 Thymoquinone has been shown to increase the pain threshold in experimental diabetic neuropathic therapy.6 A study by Bashir et al21 reported that thymoquinone has analgesic/antinociceptive effects. In our study, we determined that thymoquinone had analgesic/antinociceptive effects on central pain following SCI. Previous studies have determined the effects of thymoquinone via mechanical allodynia, heat–cold allodynia, or biochemical analyses. In our work, we used all 3 forms of analysis, which strengthens our finding that thymoquinone has an antinociceptive effect. However, we did not observe a dose-dependent antinociceptive effect of thymoquinone between 100, 200, and 400 mg/kg. Therefore, our results indicate that the lowest dose of thymoquinone (ie, 100 mg/kg) is sufficient.
Abdel-Fattah et al8 used doses of thymoquinone between 50 and 400 mg/kg/PO to treat peripheral neuropathy. In our work, which aimed to alleviate neuropathic pain following SCI, we used between 100 and 400 mg/kg thymoquinone administered orally. This dose is well below the oral LD50 for thymoquinone (794.3 mg/kg).22
TNF-α and IL-1β increase inflammatory cytokines seen in SCI, and are related to cell death and degree of inflammation.23 TNF-α is an important mediator in neuropathic pain, whereas IL-1β also plays an important role in pain modulation. Chvatal et al. and Çelik et al.24,25 have reported that the tissue involved in spinal cord trauma has higher levels of TNF-α and IL-1β than does uninjured tissue. Other experimental studies have reported that thymoquinone reduces proinflammatory cytokines (TNF-α, interleuken-6, and IL-1β) in blood and tissues, and protects tissue by reducing inflammation in the blood.26–28 Similar to the literature, we showed that all 3 groups given thymoquinone (T 100, T 200, and T 400) had lower levels of TNF-α and IL-1β than did the control group (Table I).
In an organism, oxidant and antioxidant systems are held in a tight balance. When positive oxygen capacity is disturbed, leukocytes, inflammatory mediators, and free oxygen radicals are created, which can cause damage at the cellular level.29 PON, an antioxidant bioscavenger, is responsible for hydrolyzing lipid peroxides, and also plays a major role in the antioxidant system.30 Previous studies have shown that SCI causes a decrease in the level of PON.31 In our study, serum PON levels in the thymoquinone groups were higher than those of the control group and the PON levels of the T400 group were significantly higher than those of the T100 group. PON levels of the T400 group were higher than those of the T200 group, but this result is not statistically significant (Table II).
Kanter et al32 reported that as oxidative stress decreases in rats in a traumatic SCI model, neuron count and morphology normalize. TAS is a collection of enzymatic and nonenzymatic antioxidants, whereas TOS is a collection of oxidants. In the body, TAS works as a basic defense mechanism against the development of oxidative stress. MDA is a marker of oxidative stress, whereas NO is a molecule that is characteristic of both oxidants and antioxidants. If inducible NO synthase leads to the creation of oxidants in excess, it can cause an increase in oxidative stress.25,33,34
In our study, although TAS increased in the thymoquinone groups, TOS, NO, and MDA decreased, indicating that thymoquinone reduces cell damage due to oxidative stress (Table II).
We did not study the biochemical parameters of the spinal cord tissue, which is a limitation of our study. Also, the significance of some of the biomarker findings after a single dose is unclear. This is especially important because the purported mechanism for the primary pharmacodynamics outcome in this model is not clearly identified.
Conclusions
Thymoquinone was beneficial for decreasing experimental neuropathic pain following SCI, but better effect was not noticed with higher doses of this drug. Due to the positive results of our study, there is a need for additional studies evaluating the use of thymoquinone for clinical use.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
The authors thank the Dicle University Coordination Committee of Scientific Research Projects for providing scientific and financial support. Dr. Çelik helped write the manuscript. Dr. Göçmez helped conduct the study. Drs. Karaman and Tufek helped design the study, conduct the study, and write the manuscript. Drs. Kamaşak and Guzel helped analyze the data. Drs. Kaplan, Akıl, and Uzar helped design the study.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Backonja M.M., Serra J. Pharmacologic management part 2: lesser-studied neuropathic pain diseases. Pain Med. 2004;5(Suppl 1):S48–S59. doi: 10.1111/j.1526-4637.2004.04021.x. [DOI] [PubMed] [Google Scholar]
- 2.Jensen T.S. Anticonvulsants in neuropathic pain: rationale and clinical evidence. Eur J Pain. 2002;6(Suppl A):61–68. doi: 10.1053/eujp.2001.0324. [DOI] [PubMed] [Google Scholar]
- 3.Chakravarty N. Inhibition of histamine release from mast cells by nigellone. Ann Allergy. 1993;70:237–242. [PubMed] [Google Scholar]
- 4.Ghannadi A., Hajhashemi V., Jafarabadi H. An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J Med Food. 2005;8:488–493. doi: 10.1089/jmf.2005.8.488. [DOI] [PubMed] [Google Scholar]
- 5.Badary O.A., Gamal El-Din A.M. Inhibitory effects of thymoquinone against 20-methycholanthreneinduced fibrosarcoma tumorigenesis. Cancer Detect Prev. 2001;25:362–368. [PubMed] [Google Scholar]
- 6.Ansari M.A., Ahmad S.J., Khanum R., Akhtar M. pharmacological investigation of protective effects of Nigella sativa oil in experimental diabetic neuropathy in rats. Indian J Pharm Educ Res. 2009;43:166–176. [Google Scholar]
- 7.Abdelmeguid N.E., Fakhoury R., Kamal S.M. Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic β-cells of streptozotocin-induced diabetic rats. J Diabetes. 2010;2:256–266. doi: 10.1111/j.1753-0407.2010.00091.x. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Fattah A.M., Matsumoto K., Watanabe H. Antinociceptive effects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur J Pharmacol. 2000;400:87–89. doi: 10.1016/s0014-2999(00)00340-x. [DOI] [PubMed] [Google Scholar]
- 9.Yamama Y., Nishikawa K., Funao T., Mori T., Asada A. Intrathecal gabapentin and clonidine synergistically inhibit allodynia in spinal nerve-ligated rats. Life Sci. 2010;87:565–571. doi: 10.1016/j.lfs.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Jang Y., Kim E.S., Park S.S. The suppressive effects of oxcarbazepine on mechanical and cold allodynia in a rat model of neuropathic pain. Anesth Analg. 2005;101:800–806. doi: 10.1213/01.ane.0000167283.80463.d7. [DOI] [PubMed] [Google Scholar]
- 11.Yalcin I., Charlet A., Freund-Mercier M.J. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. J Pain. 2009;10:767–773. doi: 10.1016/j.jpain.2009.01.325. [DOI] [PubMed] [Google Scholar]
- 12.Hama A., Sagen J. Behavioral characterization and effect of clinical drugs in a rat model of pain following spinal cord compression. Brain Res. 2007;1185:117–128. doi: 10.1016/j.brainres.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Chaplan S.R., Bach F.W., Pogrel J.W. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 14.Bennett G.J., Xie Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 15.Woolfe G., McDonald A.D. The evaluation of the analgesic action of pethidine hydrochloride (Demerol) J Pharmacol Exp Ther. 1944;80:300–307. [Google Scholar]
- 16.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Esterbauer H., Cheeseman K.H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hidroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 19.Eckerson H.W., Romson J., Wyte C., La Du B.N. The human serum paraoxonase polymorphism: identification of phenotypes by their response to salts. Am J Hum Genet. 1993;35:214–227. [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain A.M., Afshan G. Use of anticonvulsants drugs for neuropathic painful conditions. J Pak Med Assoc. 2008;58:690–696. [PubMed] [Google Scholar]
- 21.Bashir M.U., Qureshi H.J. Analgesic effect of Nigella sativa seeds extract on experimentally induced pain in albino mice. J Coll Physicians Surg Pak. 2010;20:464–467. [PubMed] [Google Scholar]
- 22.Al-Ali A., Alkhawajah A.A., Randhawa M.A., Shaikh N.A. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J Ayub Med Coll Abbottabad. 2008;20:25–27. [PubMed] [Google Scholar]
- 23.Pineau I., Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 24.Chvatal S.A., Kim Y.T., Bratt-Leal A.M., Lee H., Bellamkonda R.V. Spatial distribution and acute anti-inflammatory effects of methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials. 2008;29:1967–1975. doi: 10.1016/j.biomaterials.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Celik F., Göçmez C., Kamaşak K. The comparison of neuroprotective effects of intrathecal dexmedetomidine and metilprednisolone in spinal cord injury. Int J Surg. 2013;11:414–418. doi: 10.1016/j.ijsu.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Isik F., Tunali Akbay T., Yarat A. Protective effects of black cumin (Nigella sativa) oil on TNBS-induced experimental colitis in rats. Dig Dis Sci. 2010;56:721–730. doi: 10.1007/s10620-010-1333-z. [DOI] [PubMed] [Google Scholar]
- 27.Tekeoglu I., Dogan A., Demiralp L. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2006;20:869–871. doi: 10.1002/ptr.1964. [DOI] [PubMed] [Google Scholar]
- 28.Vaillancourt F., Silva P., Shi Q. Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J Cell Biochem. 2011;112:107–117. doi: 10.1002/jcb.22884. [DOI] [PubMed] [Google Scholar]
- 29.Aldemir O., Celebi H., Cevik C., Duzgun E. The effects of propofol or halothane on free radical production after tourniquet induced ischaemia-reperfusion injury during knee arthroplasty. Acta Anaesthesiol Scand. 2001;45:1221–1225. doi: 10.1034/j.1399-6576.2001.451008.x. [DOI] [PubMed] [Google Scholar]
- 30.Liang B., Li Y.H., Kong H. Serum paraoxonase, arylesterase activities and oxidative status in patients with insomnia. Eur Rev Med Pharmacol Sci. 2013;17:2517–2522. [PubMed] [Google Scholar]
- 31.Topsakal C., Kilic N., Ozveren F. Effects of prostaglandin E1, melatonin, and oxytetracycline on lipid peroxidation, antioxidant defense system, paraoxonase (PON1) activities, and homocysteine levels in an animal model of spinal cord injury. Spine. 2003;28:1643–1652. doi: 10.1097/01.BRS.0000083163.03910.B1. [DOI] [PubMed] [Google Scholar]
- 32.Kanter M., Coskun O., Kalayci M. Neuroprotective effects of Nigella sativa on experimental spinal cord injury in rats. Hum Exp Toxicol. 2006;25:127–133. doi: 10.1191/0960327106ht608oa. [DOI] [PubMed] [Google Scholar]
- 33.Taysi S., Uslu C., Akcay F., Sutbeyaz M.Y. Levels of malondialdehyde and nitric oxide in plasma of patients with laryngeal cancer. Surg Today. 2003;33:651–654. doi: 10.1007/s00595-002-2562-3. [DOI] [PubMed] [Google Scholar]
- 34.Taysi S., Cikman O., Kaya A. Increased oxidant stress and decreased antioxidant status in erythrocytes of rats fed with Zn-deficient diet. Biol Trace Elem Res. 2008;123:161–167. doi: 10.1007/s12011-008-8095-x. [DOI] [PubMed] [Google Scholar]


