Abstract
OBJECTIVE
The aim of this article was to define risk factors for incidence of peripheral arterial disease (PAD) in a large cohort of patients with type 2 diabetes mellitus (T2DM), overall and within the context of differing glycemic control strategies.
RESEARCH DESIGN AND METHODS
The Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI 2D) randomized controlled trial assigned participants to insulin-sensitizing (IS) therapy versus insulin-providing (IP) therapy. A total of 1,479 participants with normal ankle-brachial index (ABI) at study entry were eligible for analysis. PAD outcomes included new ABI ≤0.9 with decrease at least 0.1 from baseline, lower extremity revascularization, or lower extremity amputation. Baseline risk factors within the overall cohort and time-varying risk factors within each assigned glycemic control arm were assessed using Cox proportional hazards models.
RESULTS
During an average 4.6 years of follow-up, 303 participants (20.5%) experienced an incident case of PAD. Age, sex, race, and baseline smoking status were all significantly associated with incident PAD in the BARI 2D cohort. Additional baseline risk factors included pulse pressure, HbA1c, and albumin-to-creatinine ratio (P < 0.05 for each). In stratified analyses of time-varying covariates, changes in BMI, LDL, HDL, systolic blood pressure, and pulse pressure were most predictive among IS patients, while change in HbA1c was most predictive among IP patients.
CONCLUSIONS
Among patients with T2DM, traditional cardiovascular risk factors were the main predictors of incident PAD cases. Stratified analyses showed different risk factors were predictive for patients treated with IS medications versus those treated with IP medications.
Introduction
Peripheral arterial disease (PAD) is a critical manifestation of atherosclerosis that is associated with increased risk of all-cause and cardiovascular mortality (1–3). Moreover, PAD increases the risk of functional limitation, leg revascularization, and amputation (4–6). PAD is especially common among patients with type 2 diabetes mellitus (T2DM), with a threefold increased risk compared with the general population (7). PAD also tends to progress faster and lead to worse outcomes in T2DM patients (8,9). Several risk factors for PAD have been established, including age, race, smoking, hypertension, and lipids (10–13). There is also evidence that biomarkers indicative of inflammation and/or coagulation, such as C-reactive protein (CRP), d-dimer, and fibrinogen, may be associated with increased PAD risk and/or worse outcomes (14–21).
Results from the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI 2D) trial suggest that T2DM patients treated with an insulin-sensitizing (IS) regimen experienced improvements in biomarker profiles not observed in those assigned to an insulin-providing (IP) regimen (22). While this did not result in a reduction in all-cause mortality, patients assigned to the IS strategy experienced significantly lower incidence of PAD than those assigned to the IP strategy (23). Identifying risk factors for incident PAD in this population may improve our understanding of how IS and IP medications affect the progression of atherosclerosis. Furthermore, few existing studies have explored biomarkers as risk factors for incident PAD in a population of T2DM patients. Therefore, our principal aim is to establish the associations between cardiovascular risk factors, including inflammatory biomarkers, and incidence of PAD in T2DM patients. As a secondary aim, we will determine whether the associations differ according to the assigned BARI 2D glycemic control strategy.
Research Design and Methods
BARI 2D was a randomized controlled trial designed to determine the optimal treatment strategy for patients with stable coronary artery disease (CAD) and T2DM (24). BARI 2D participants were randomly assigned via a 2 × 2 factorial design to prompt coronary revascularization with intensive medical therapy versus intensive medical therapy alone and simultaneously randomly assigned to either an IS glycemic control strategy or an IP strategy. All participants were treated medically to achieve targets of HbA1c <7.0% (53 mmol/mol), LDL cholesterol <100 mg/dL, and blood pressure ≤130/80 mmHg as well as given counseling for smoking cessation, weight loss, and exercise. BARI 2D was coordinated at the University of Pittsburgh and included 49 clinical sites throughout North America, South America, and Europe. Recruitment began in 2001 and continued until 2005; treatment continued until the 6-year visit or the last annual visit before 1 December 2008. The overall study cohort for BARI 2D consisted of 2,368 participants. The primary end point for BARI 2D was death from any cause, and the principal secondary end point was a composite of death, myocardial infarction, or stroke.
This article reports the results of post hoc analyses that examine associations between baseline and time-varying cardiovascular risk factors and PAD outcomes. As noted above, the BARI 2D study population is composed entirely of patients with CAD and T2DM, comprising a group at especially high risk for PAD and PAD-related lower extremity outcomes. While PAD was not a primary outcome of the BARI 2D trial, the ankle-brachial index (ABI) was measured at study entry and annually throughout follow-up, providing the necessary follow-up data to examine PAD incidence in this population.
Patient Selection
Of the 2,368 participants enrolled in the BARI 2D trial, only 1,479 participants with normal ABI (0.91–1.30) at study entry were eligible for analysis in this article. The range for normal ABI is chosen based on guidelines published in a 2003 American Diabetes Association consensus statement regarding PAD in diabetes (25). A total of 138 participants with missing ABI at baseline were excluded because we are unable to determine baseline PAD status for those participants. A total of 430 participants with ABI ≤0.90 at baseline were excluded because they already had the end point of interest pertinent to this study and thereby cannot be an incident case. Participants with ABI >1.30 (n = 182) or noncompressible arteries (n = 139) at baseline were excluded because the likely presence of medial arterial calcification in these patients renders future ABI measurement unreliable for diagnosis of PAD in these participants.
Definition of PAD and Related Lower Extremity Outcomes
The primary outcome reported in this article is a composite lower extremity outcome used in previous BARI 2D analyses (23). Patients were considered as having incident PAD or a lower extremity event if they experienced one or more of the following outcomes: decrease in ABI to abnormal level (ABI ≤0.90) and a change in ABI >0.10, lower extremity revascularization, or lower extremity amputation. Intermittent claudication was not evaluated as an outcome because the BARI 2D trial did not use a validated claudication questionnaire.
Assays of Biomarkers
The biomarker assays used in BARI 2D were previously reported by Sobel et al. (22). Plasminogen activator inhibitor-1 (PAI-1) activity, PAI-1 antigen, tissue plasminogen activator (tPA), and insulin were measured in the fibrinolysis core laboratory at the University of Vermont in samples obtained at baseline, 1 month, 3 months, 6 months, and every 6 months thereafter over 5 years of follow-up. PAI-1 activity was assessed using a modified chromogenic substrate enzymatic assay developed by Chmielewska and Wiman. PAI-1 antigen and tPA levels were determined with commercially available enzyme-linked immunoassay kits (Trinity Biotech Plc, Bray, Wicklow, Ireland). CRP, d-dimer, and fibrinogen were assayed at the same core laboratory as part of an ancillary study, with data through the first 24 months of follow-up. Fibrinogen was measured by the Claus method, and d-dimer was measured immunoturbidimetrically with STA-Liatest d-Dimer reagents (Diagnostica Stago, Parsippany, NJ) on an STA Compact (Roche Professional Diagnostics, Basel, Switzerland). HbA1c was assayed in whole blood samples in the BARI 2D biochemistry laboratory at the University of Minnesota or certified core laboratories in Brazil and Europe.
Statistical Methods
Baseline descriptive statistics are reported as means ± SDs for continuous variables with normal distributions; medians and interquartile ranges are presented for continuous but nonnormally distributed variables, and proportions are reported for categorical variables. The baseline distributions of all risk factors and biomarkers were compared across the assigned glycemic treatment arms using t tests, Wilcoxon rank-sum tests, and χ2 tests for continuous, skewed continuous, and categorical data, respectively.
Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and associated 95% CIs for the associations between each potential risk factor and composite PAD outcome. Time-to-event was calculated from the date of randomization to the first recorded PAD outcome; participants with no event were censored at their last study protocol follow-up visit. Most predictor variables were examined as continuous variables; a natural logarithm transformation was applied to those with skewed distributions and/or nonlinear associations with outcome. The first series of Cox models was constructed to assess the effects of each risk factor while adjusting for known PAD risk factors (age, sex, race, baseline smoking, and baseline ABI) that demonstrated significant (P < 0.05) univariate associations with PAD. To determine which of the baseline risk factors showed the strongest independent association with PAD outcomes when adjusting for other candidate variables, a multivariate model was constructed using forward selection with age, sex, race, baseline smoking, and baseline ABI forced to enter the model plus all candidate variables that met an entry threshold of P ≤ 0.10 also included in the final multivariate model. Interactions between assigned treatment and each risk factor were tested and found to be nonsignificant; therefore, we present one set of models for the baseline risk factors calculated using all 1,479 subjects eligible for inclusion.
A second series of Cox models was constructed to assess the effects of each risk factor assessed as a time-varying covariate, updating each value annually to be consistent with the availability of updated ABI measurements (also performed annually). These models were also adjusted for baseline values of known PAD risk factors (age, sex, race, baseline smoking, and baseline ABI). Since previous BARI 2D analyses have shown differential trends in several candidate variables as well as differences in the incidence of PAD outcomes between glycemic control arms during the trial, we tested for interactions between assigned glycemic control strategy and each of the time-varying risk factors; there were several significant interactions between candidate variables and assigned treatment, suggesting that stratified analyses are appropriate. Therefore, the models with time-varying covariates were constructed separately for each glycemic control arm.
For each Cox model involving baseline covariates, the proportional-hazards assumption was checked for each baseline covariate using Martingale residuals (26); none were found to violate the proportional-hazards assumption. Goodness-of-fit was assessed using the likelihood ratio test for each Cox model. SAS version 9.2 (SAS Institute, Cary, NC) was used for all statistical analyses. P values <0.10 are reported for informational purposes, but only P values <0.05 are considered statistically significant. No adjustment was made for multiple comparisons.
Results
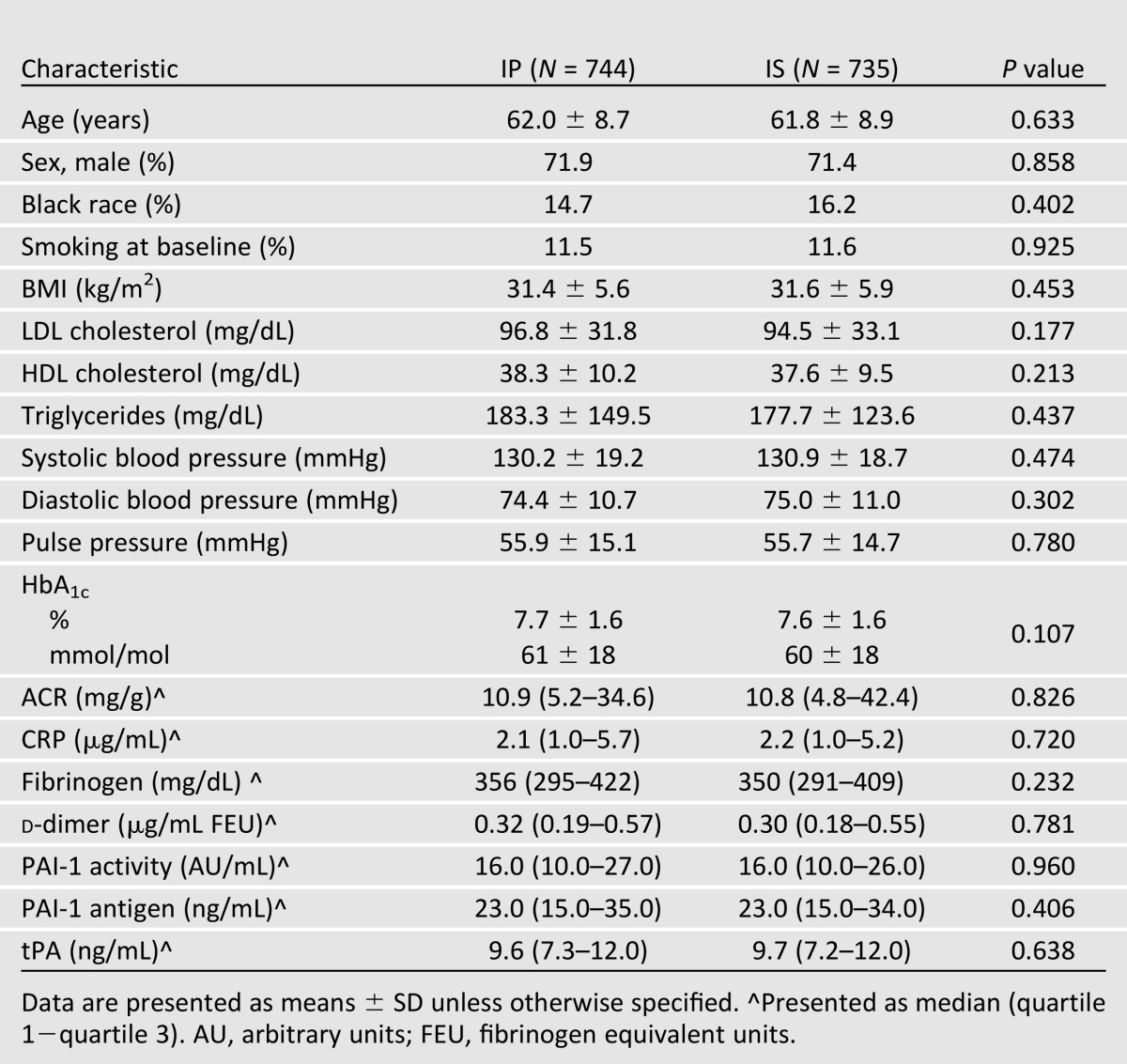
Of 2,368 overall participants in the BARI 2D trial, 1,479 participants met the inclusion criteria for this article's analysis (Supplementary Fig. 1). The baseline characteristics of those included in the primary analysis are presented in Table 1. Participants included in our analytic sample were 61.9 ± 8.0 years of age, 72% male, 15% identified as black race, and 12% were current smokers. Baseline distributions of BMI, lipids, blood pressure, HbA1c, albumin-to-creatinine ratio (ACR), and biomarkers of interest were similar between the assigned glycemic treatment groups; there were no significant differences in major demographic or clinical characteristics.
Table 1.
Baseline characteristics of participants available for PAD analysis
Three hundred three participants (20.5%) experienced one or more of the PAD-related outcomes, including new low ABI (n = 290), lower extremity revascularization (n = 25), and lower extremity amputation (n = 13) over an average 4.6 years of follow-up. Table 2 displays the associations between baseline risk factors and incidence of the composite PAD outcome when adjusting for age, sex, race, and baseline smoking status (each of which was significantly associated with the composite outcome in a multivariate model; see Supplementary Table 1). Baseline HbA1c was significantly associated with the incidence of PAD outcomes (HR 1.17; P < 0.01). Baseline pulse pressure and log-transformed ACR were also significantly associated (P < 0.05 for each) with PAD outcomes when adjusting for age, sex, race, baseline smoking, and baseline ABI.
Table 2.
Associations^ between baseline risk factors and incidence of PAD-related lower extremity outcomes
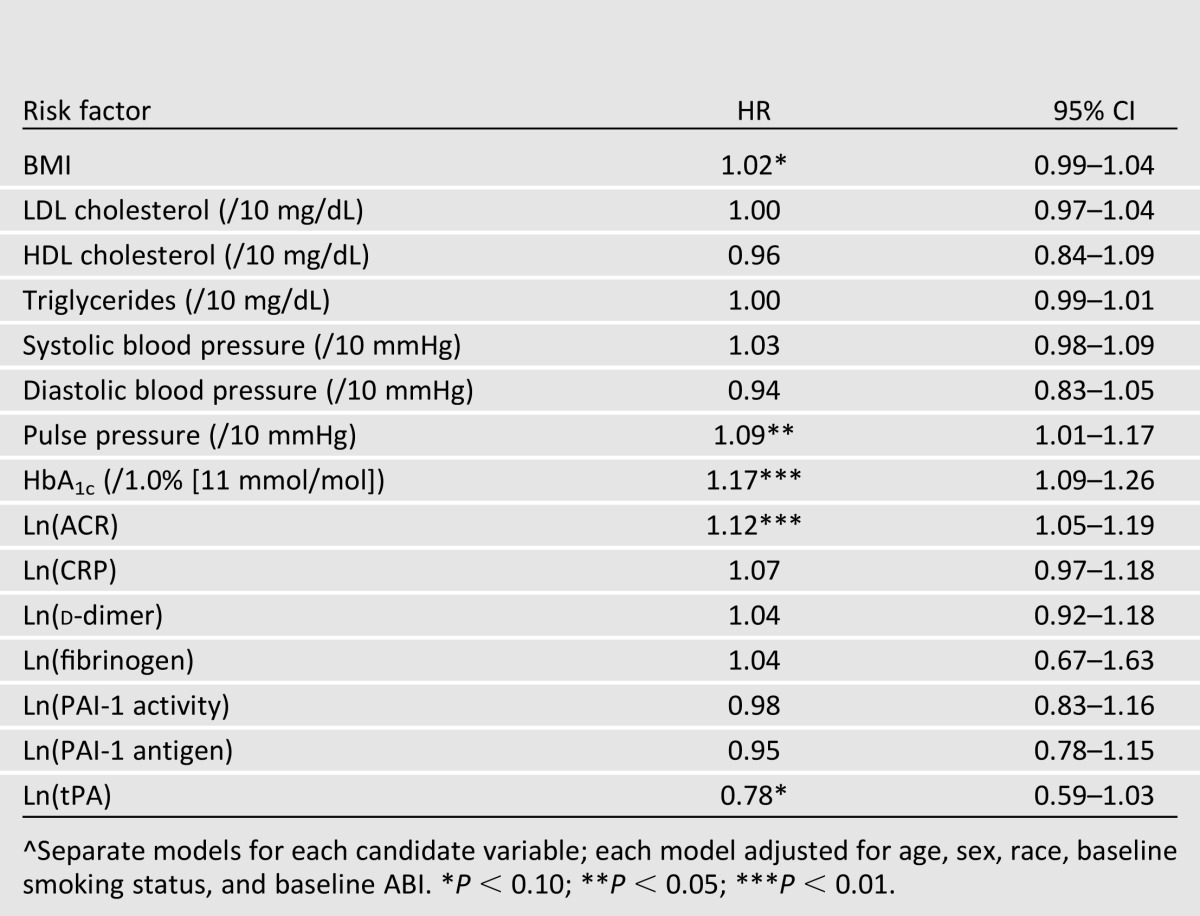
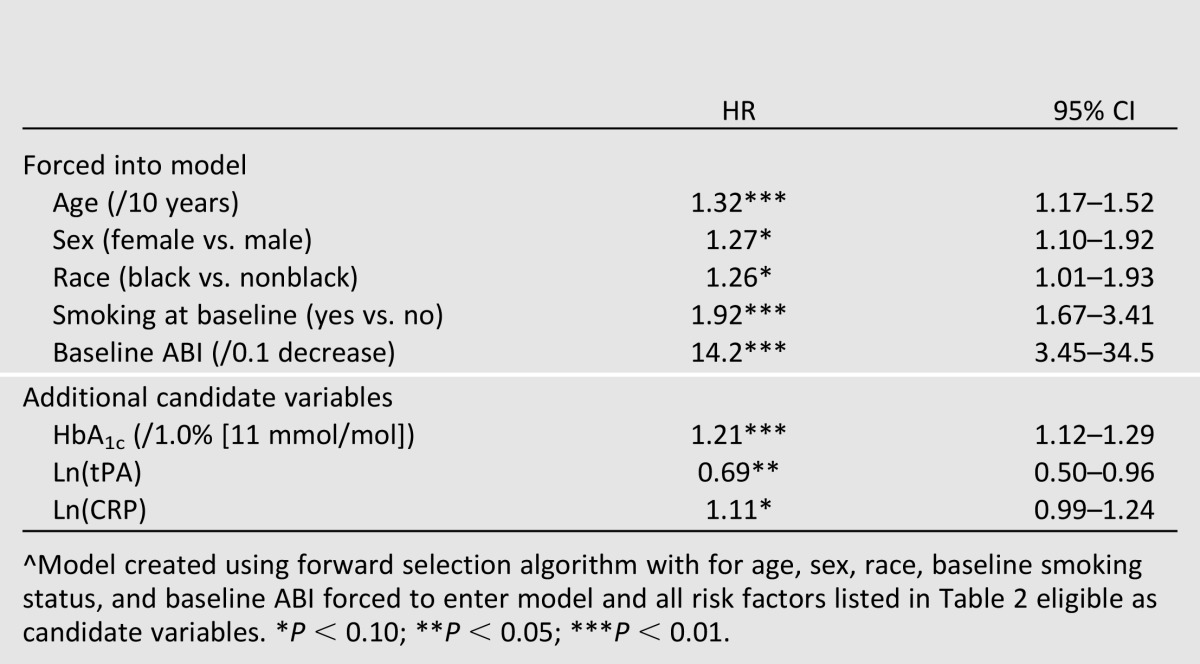
Table 3 displays the results of a forward selection algorithm with age, sex, race, baseline smoking, and baseline ABI forced to enter the model and all other variables shown in Table 2 eligible as candidate variables. Baseline HbA1c again shows the strongest association (HR 1.21; P < 0.01), followed by log-transformed tissue-type plasminogen activator (HR 0.69; P < 0.05) and then log-transformed CRP (HR 1.11; P < 0.10); the selection algorithm terminates after this step since no other variable is associated with outcome at P < 0.10 significance level with the aforementioned variables included in the model. Notably, tPA did not show a significant relationship at the 0.05 significance level in the first set of models, but was significantly associated with outcome in a model that also adjusted for baseline HbA1c.
Table 3.
Multivariate associations^ between baseline risk factors and incidence of PAD-related lower extremity outcomes
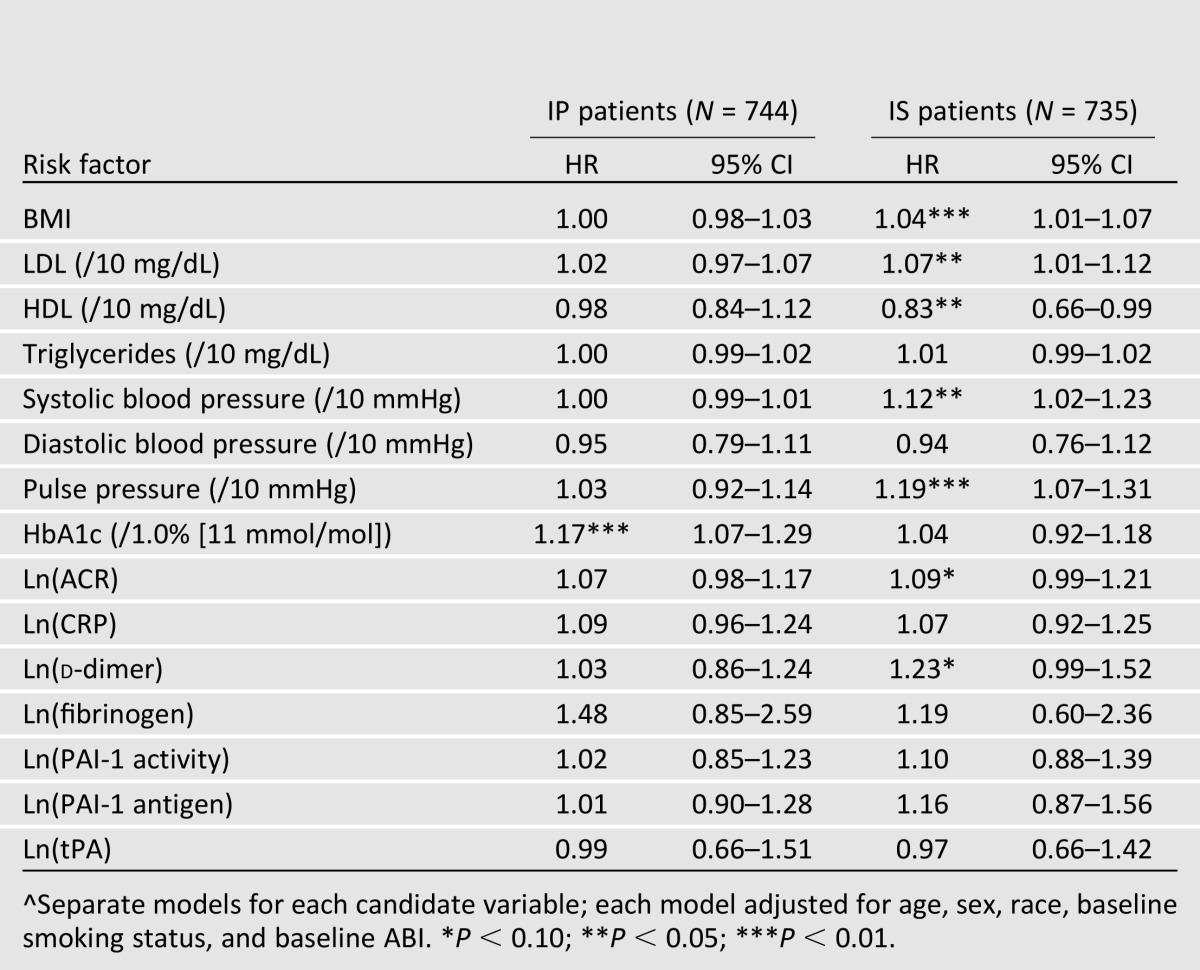
The assigned glycemic control strategy may have had differential effects on certain risk factors during follow-up (e.g., HbA1c; Supplementary Fig. 2), so the analyses involving time-varying covariates were stratified by assigned glycemic control strategy. When the candidate risk factors are modeled as time-varying covariates, the observed associations are notably different between the two glycemic control arms (Table 4). Among those assigned to IP strategy, HbA1c is the most significant predictor (P < 0.01), and no other variable shows a significant relationship with the composite PAD outcome at the 0.05 level. Among those assigned to the IS strategy, several time-varying predictors show significant relationships with the composite PAD outcome including BMI, LDL, HDL, systolic blood pressure, and pulse pressure (P < 0.05 for each). Notably, time-varying change in HbA1c is not a significant predictor for those assigned to IS therapy, although it was highly significant for those assigned to IP therapy (Supplementary Table 2).
Table 4.
Associations^ between time-varying risk factors and incidence of PAD-related lower extremity outcomes, stratified by assigned glycemic treatment
Conclusions
The BARI 2D dataset was used to identify risk factors for incident PAD in patients with T2DM and stable coronary disease in order to gain a mechanistic understanding of how IS and IP medications affect the progression of atherosclerosis. We found that ∼20% of participants with normal ABI at study entry experienced at least one PAD-related incident within 5 years of follow-up. Age, sex, race, and baseline smoking were significantly associated with incidence of PAD outcomes. When adjusting for the aforementioned risk factors, baseline variables predictive of PAD outcomes were high pulse pressure, renal dysfunction (higher ACR), and poor glycemic control (higher HbA1c).
Increased pulse pressure is generally indicative of arterial stiffness, so it is interesting to note that pulse pressure demonstrates a strong relationship with PAD outcomes. Systolic blood pressure has emerged as a risk factor for PAD in prior research and would be expected to have some association with PAD risk (indeed, it is also marginally associated with PAD outcomes in several of the models in this article). Pulse pressure is strongly related to systolic blood pressure, and therefore, it is not surprising to see a strong association between pulse pressure and risk of PAD outcomes. This study confirms a previous study in which pulse pressure was associated with PAD progression (9).
Higher ACR, a marker of renal function, was also predictive of PAD outcomes in BARI 2D. Renal function is known to be associated with atherosclerotic events, both cardiac and peripheral. Cross-sectional data from the National Health and Nutrition Examination Survey (27) and the Cardiovascular Health Study (28) have demonstrated a relationship between different measures of kidney disease (measured by creatinine clearance in the National Health and Nutrition Examination Survey and estimated glomerular filtration rate in the Cardiovascular Health Study) and abnormal ABI; however, as cross-sectional studies, these data do not address temporality. The BARI 2D data suggest that higher baseline ABI was predictive of future PAD outcomes, suggesting that renal insufficiency may influence the progression of atherosclerosis. Potential physiological mechanisms by which renal dysfunction might affect the atherosclerotic process include altered calcium-phosphorus metabolism, homocysteine metabolism, lipoprotein(a) metabolism, and alterations in inflammatory and coagulation pathways (29,30).
Our results showed a 21% increased hazard for each 1% (11 mmol/mol) increase in baseline HbA1c in multivariate models, similar to that which might be expected based on results from previous studies. The UK Prospective Diabetes Study showed that each 1% (11 mmol/mol) increase in HbA1c was associated with a 28% increased risk of PAD (31), later confirmed by a meta-analysis showing the same magnitude of risk (32). A novel finding from our study is the different magnitude of time-varying HbA1c’s relationship with PAD according to glycemic treatment in the stratified analyses. Adjusting for age, sex, race, and smoking status, our results revealed a statistically significant 17% increased HR in those assigned to IP therapy for each 1% (11 mmol/mol) increase in HbA1c, but a corresponding nonsignificant 4% increased hazard in those assigned to IS therapy. One possibility is that the better overall glycemic control in the IS arm dampened the effects of HbA1c on PAD outcomes by pushing the majority of participants into an HbA1c range in which there was relatively little effect of glycemic control on the development of new atherosclerosis, while a greater proportion of participants in the IP arm remained in a higher range of HbA1c.
A second possibility is that the glycemic control medications have different physiological effects on the development of new atherosclerosis. In our time-varying analyses, there are notable differences in which risk factors are associated with PAD outcomes among the respective glycemic control strategies. Among patients assigned to IP strategy, when adjusting for known baseline risk factors (age, sex, race, smoking status, and ABI), time-varying HbA1c is the only significant predictor of with PAD outcomes. In contrast, among patients assigned to IS strategy, several time-varying risk factors (BMI, LDL, HDL, systolic blood pressure, and pulse pressure) are significantly associated with PAD outcomes. Each of these relationships point in the direction that would be expected; higher BMI, higher LDL, lower HDL, higher systolic blood pressure, and higher pulse pressure are all established cardiovascular risk factors.
The physiological reasons for the discrepancies between the glycemic control strategies are unclear, but this research combined with our previous finding that patients assigned to IS strategy had lower incidence of PAD (23) suggests that the different classes of glycemic control medications used in BARI 2D have differing effects on the progression of atherosclerosis in this population. For example, the anti-inflammatory effects of thiazolinediones (used by 62% of the patients assigned to IS therapy in BARI 2D) may retard the development of atherosclerosis, contributing to the lower incidence of PAD in the IS group. The BARI 2D trial was designed to examine mechanistically different treatment strategies rather than individual drugs, so we cannot say for certain whether thiazolinediones alone were responsible for the reduction in PAD risk.
It should be noted that therapeutic regimens other than glycemic control strategies may have differential effects on the progression of PAD. Blood pressure medications, lipid-lowering medications, antiplatelet therapy, exercise conditioning, and smoking cessation have been proposed as potentially viable therapies to reduce the progression of PAD (9,33). It should be noted that all BARI 2D patients received intensive medical therapy and that 93–95% of BARI 2D patients were receiving blood pressure medication, statins, and aspirin, respectively, as well as counseling regarding exercise and smoking cessation provided to all patients. Therefore, the results presented in this study must be considered in appropriate context; the relationships between each risk factor and PAD-related outcomes hold true against the backdrop of intensive therapy in a population with pre-existing T2DM and stable CAD.
Our study findings must be considered carefully in context of the trial’s strengths and limitations as well. This is a post hoc secondary analysis of a randomized controlled trial in which all participants had CAD and T2DM at study entry; the effects of these risk factors may be different in the general population. Several of the biomarkers included in this analysis, including CRP and fibrinogen, were only collected through 24 months of follow-up, and thus, their relationships with PAD risk in our analyses using time-varying covariates do not account for possible late changes in these measures. We also acknowledge that some other inflammatory markers such as interleukin-6 and adhesion molecules were not measured in this study, nor were plasma homocysteine levels, which may have influenced the risk of PAD. We present no information on family history of PAD because those data were not collected in BARI 2D; however, adjustment for family history of CVD did not alter the results presented in this article. It also should be noted that our primary outcome was a composite of low ABI, lower extremity revascularization, and lower extremity amputation used in a prior BARI 2D publication on PAD. Intermittent claudication was not considered as an outcome because of the lack of a validated claudication questionnaire in BARI 2D, so some patients who developed clinical PAD may not have been included as outcomes.
We used a composite outcome of low ABI, lower extremity revascularization, or lower extremity amputation in an effort to capture all patients with PAD-related lower extremity events, in case a patient had an event without a measured low ABI. However, we acknowledge that amputations may occur for reasons other than PAD, such as ulcers or peripheral neuropathy, and therefore, we performed sensitivity analyses by repeating the models in Tables 2, 3, and 4 using only patients with low ABI as outcomes. The statistical significance of each risk factor’s relationship with outcome remained consistent with those presented here. This is not surprising given that >90% of our lower extremity events were patients experiencing a low ABI.
Summary
Many previous studies have identified risk factors for PAD, but fairly few have examined them specifically in the high-risk population of patients with type 2 diabetes. This article reports the associations between traditional and nontraditional risk factors for PAD and related lower extremity outcomes over time in patients with T2DM. After adjusting for known PAD risk factors, our data showed that higher baseline pulse pressure, ABI, and HbA1c were positively associated with risk of PAD outcomes. In addition, glycemic control strategy may have differential effects on the progression of atherosclerosis in patients with type 2 diabetes.
Article Information
Funding. BARI 2D is funded by the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases (U01-HL-061744, U01-HL-061746, U01-HL-061748, and U01-HL-063804).
Duality of Interest. BARI 2D receives significant supplemental funding from GlaxoSmithKline and additional funding from Lantheus Medical Imaging, Inc. (formerly Bristol-Myers Squibb Medical Imaging, Inc.), Astellas Pharma US, Inc., Merck, Abbott Laboratories, and Pfizer. Medications and supplies were donated by Abbott Laboratories, MediSense Products, Bayer Diagnostics, BD Biosciences, J.R. Carlson Laboratories, Centocor, Inc., Eli Lilly, LipoScience, Inc., Merck Santé, Novartis Pharmaceuticals, and Novo Nordisk. Unrestricted grant support for the Nuclear Core Laboratory was provided by Astellas Healthcare and Lantheus Imaging. A.D.F. reports receiving support from Amylin, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Genentech, GlaxoSmithKline, Janssen, Lexicon, Merck, Novartis Pharmaceuticals, Pfizer, Hoffmann-La Roche, Omthera, Sanofi, Shionogi, and Takeda. S.M. reports receiving support from Abbott Vascular. M.M.B. reports receiving support from Sanofi.
Author Contributions. A.D.A. performed statistical analyses and wrote the manuscript. J.D.A., A.D.F., M.B., E.B.-M., R.C.T., S.M., and V.A. contributed to discussion and reviewed and edited the manuscript. M.M.B. contributed to statistical analyses, discussion, and reviewed and edited the manuscript. A.D.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This research was presented by A.D.A. at the American Heart Association 2012 Scientific Sessions, Los Angeles, CA, 3–7 November 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-2303/-/DC1.
Clinical trial reg. no. NCT00006305, clinicaltrials.gov.
References
- 1.O’Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation 2006;113:388–393 [DOI] [PubMed] [Google Scholar]
- 2.Sutton-Tyrrell K, Venkitachalam L, Kanaya AM, et al. Relationship of ankle blood pressures to cardiovascular events in older adults. Stroke 2008;39:863–869 [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2010;56:1506–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med 2002;136:873–883 [DOI] [PubMed] [Google Scholar]
- 5.Brach JS, Solomon C, Naydeck BL, et al. Cardiovascular Health Study Research Group Incident physical disability in people with lower extremity peripheral arterial disease: the role of cardiovascular disease. J Am Geriatr Soc 2008;56:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nehler MR, McDermott MM, Treat-Jacobson D, Chetter I, Regensteiner JG. Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc Med 2003;8:115–126 [DOI] [PubMed] [Google Scholar]
- 7.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation 2004;110:738–743 [DOI] [PubMed] [Google Scholar]
- 8.Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care 2001;24:1433–1437 [DOI] [PubMed] [Google Scholar]
- 9.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation 2006;113:2623–2629 [DOI] [PubMed] [Google Scholar]
- 10.Newman AB, Sutton-Tyrrell K, Kuller LH. Lower-extremity arterial disease in older hypertensive adults. Arterioscler Thromb 1993;13:555–562 [DOI] [PubMed] [Google Scholar]
- 11.Fowkes FG, Housley E, Riemersma RA, et al. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. Am J Epidemiol 1992;135:331–340 [DOI] [PubMed] [Google Scholar]
- 12.Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J 1999;20:344–353 [DOI] [PubMed] [Google Scholar]
- 13.Olin JW. Hypertension and peripheral arterial disease. Vasc Med 2005;10:241–246 [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Lloyd-Jones DM. The role of biomarkers and genetics in peripheral arterial disease. J Am Coll Cardiol 2009;54:1228–1237 [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001;285:2481–2485 [DOI] [PubMed] [Google Scholar]
- 16.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation 2008;117:823–831 [DOI] [PubMed] [Google Scholar]
- 17.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 2005;112:976–983 [DOI] [PubMed] [Google Scholar]
- 18.McDermott MM, Ferrucci L, Guralnik JM, et al. Elevated levels of inflammation, d-dimer, and homocysteine are associated with adverse calf muscle characteristics and reduced calf strength in peripheral arterial disease. J Am Coll Cardiol 2007;50:897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott MM, Liu K, Ferrucci L, et al. Circulating blood markers and functional impairment in peripheral arterial disease. J Am Geriatr Soc 2008;56:1504–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDermott MM, Ferrucci L, Liu K, et al. D-dimer and inflammatory markers as predictors of functional decline in men and women with and without peripheral arterial disease. J Am Geriatr Soc 2005;53:1688–1696 [DOI] [PubMed] [Google Scholar]
- 21.McDermott MM, Liu K, Guralnik JM, et al. Functional decline in patients with and without peripheral arterial disease: predictive value of annual changes in levels of C-reactive protein and D-dimer. J Gerontol A Biol Sci Med Sci 2006;61:374–379 [DOI] [PubMed] [Google Scholar]
- 22.Sobel BE, Hardison RM, Genuth S, et al. BARI 2D Investigators Profibrinolytic, antithrombotic, and antiinflammatory effects of an insulin-sensitizing strategy in patients in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation 2011;124:695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Althouse AD, Abbott JD, Sutton-Tyrrell K, et al. BARI 2D Study Group Favorable effects of insulin sensitizers pertinent to peripheral arterial disease in type 2 diabetes: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Diabetes Care 2013;36:3269–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks MM, Frye RL, Genuth S, et al. Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial Investigators Hypotheses, design, and methods for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006;97(Suppl. 12A):9G–19G [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association Peripheral arterial disease in people with diabetes. Diabetes Care 2003;26:3333–3341 [DOI] [PubMed] [Google Scholar]
- 26.Lin D, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika 1993;80:557–572 [Google Scholar]
- 27.O’Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999-2000. Circulation 2004;109:320–323 [DOI] [PubMed] [Google Scholar]
- 28.Ix JH, Katz R, De Boer IH, et al. Association of chronic kidney disease with the spectrum of ankle brachial index the CHS (Cardiovascular Health Study). J Am Coll Cardiol 2009;54:1176–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoccali C, Mallamaci F, Tripepi G. Traditional and emerging cardiovascular risk factors in end-stage renal disease. Kidney Int Suppl 2003:S105–S110 [DOI] [PubMed] [Google Scholar]
- 30.O’Hare AM, Johansen K. Lower-extremity peripheral arterial disease among patients with end-stage renal disease. J Am Soc Nephrol 2001;12:2838–2847 [DOI] [PubMed] [Google Scholar]
- 31.Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, Holman RR. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care 2002;25:894–899 [DOI] [PubMed] [Google Scholar]
- 32.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421–431 [DOI] [PubMed] [Google Scholar]
- 33.Gandhi S, Weinberg I, Margey R, Jaff MR. Comprehensive medical management of peripheral arterial disease. Prog Cardiovasc Dis 2011;54:2–13 [DOI] [PubMed] [Google Scholar]


