Abstract
To provide an understanding of both the preclinical and clinical aspects of closed-loop artificial pancreas systems, we provide a discussion of this topic as part of this two-part Bench to Clinic narrative. Here, the Bench narrative provides an in-depth understanding of insulin-glucose-glucagon physiology in conditions that mimic the free-living situation to the extent possible in type 1 diabetes that will help refine and improve future closed-loop system algorithms. In the Clinic narrative, Doyle and colleagues compare and evaluate technology used in current closed-loop studies to gain further momentum toward outpatient trials and eventual approval for widespread use.
Introduction
The concept of a system that responds automatically to changing blood glucose concentrations by modulating insulin delivery in patients with type 1 diabetes (T1D) was born decades ago as the pioneering but cumbersome Biostator (1) and involved intravenous routes for both glucose sensing and insulin delivery. Ideally, such a system performs without human interventions operating as a “closed” process; however, attempts at “closing the loop” with Biostator were suboptimal in normalizing postprandial glucose excursions despite modulation of prandial rates of insulin delivery. For the system to be truly closed, technologic, algorithmic, and physiologic limitations must be overcome. While the Biostator had the ideal intravascular route for closing the loop, the lack of portability of the entire system precluded widespread use in patients with T1D in the free-living situation.
Over the past 15 years, the concept of closed-loop control (CLC) has been rekindled, thanks to significant advances in technological and algorithmic capabilities, renewed interest by funding agencies (both governmental and nongovernmental), and regulatory bodies, including the U.S. Food and Drug Administration, replacing lengthy preclinical animal studies with in silico (computer-based) simulations and “simulators” (2,3) to expedite the approval processes with integrated efforts of all parties involved. Consequently, several investigative teams worldwide have successfully conducted and published outpatient and inpatient clinical trials (4–6) that have demonstrated safety and efficacy in relatively short-term CLC using the subcutaneous route for both glucose sensing and insulin delivery in adults and children with T1D. While the subcutaneous compartment for glucose sensing and insulin delivery is nonphysiological and suboptimal involving definitive time delays at both ends, the practicality, usability, and simplicity of this route makes current efforts and realization of CLC promising to a potentially large segment of the T1D patient population.
Refinements in algorithms (proportional integral derivative, model predictive control, fuzzy logic), platforms (artificial pancreas system, Diabetes Assistant), continuous glucose monitors (CGMs), and insulin pumps, along with continuing miniaturization and portability of these devices, have enhanced the practicality and usability of contemporary CLC systems by adults and adolescents with T1D (7). Furthermore, recent studies (8) incorporating both insulin and glucagon infusions have extended the entire concept from an artificial β-cell closer to an artificial endocrine pancreas system.
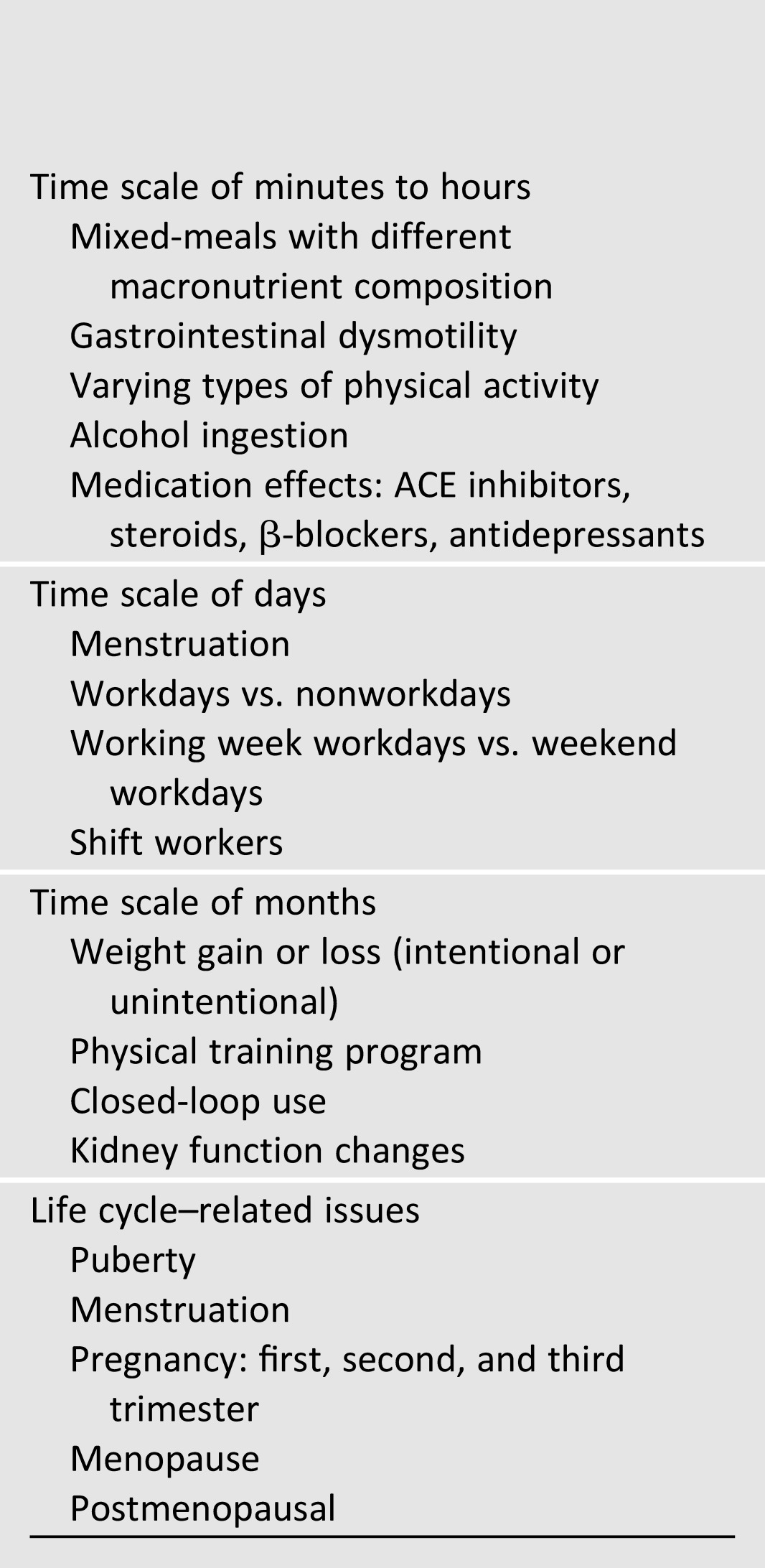
Formidable challenges remain before such a system can be safely used in free-living situations in T1D. These include adaptation (with possible individualization) of algorithms based on changes in physiological parameters (e.g., insulin sensitivity [SI], glucagon sensitivity) related to multiple natural perturbations induced by, among others, physical activity (varying types and intensities), meals (varying sizes and composition), intercurrent illness, dawn phenomenon, circadian variability, and biological factors (puberty, menstrual cycles, pregnancy, menopause) (Table 1). The logical way forward therefore would be to systematically determine the effect sizes of these relevant scenarios to pertinent physiological inputs that could “inform” existing control algorithms, followed by formal testing of these modified algorithms in clinical trials to determine safety and efficacy before broader clinical application.
Table 1.
Examples of clinical contexts requiring physiologic exploration for refining the closed-loop algorithm
Why do we need physiological parameters to inform such algorithms? Because the causes of rising or falling glucose concentrations beyond the normal ranges in T1D vary with different circumstances, hence the solutions will also vary. As an example, hyperglycemia can occur postprandially (Fig. 1) and with stress (e.g., intercurrent illnesses) (Fig. 2). However, postprandial hyperglycemia results from the fact that the pharmacodynamics of subcutaneously injected insulin coupled with impaired suppression of postprandial glucagon concentrations cannot keep pace with the rapidity of systemic appearance of meal glucose. In contrast, illness induces hyperglycemia because of cytokine-induced insulin resistance (9). Similarly, hypoglycemia can occur either with exercise (Fig. 3) or following alcohol ingestion (Fig. 4) (10). While exercise induces hypoglycemia due to increased glucose uptake through insulin-dependent and -independent mechanisms together with inadequate glucagon secretion and/or hepatic glucagon sensitivity, alcohol inhibits hepatic gluconeogenesis, thus, in effect, enhancing hepatic SI and lowering hepatic glucose production. Hence, the solutions in each case will differ although the net results on glucose levels are comparable.
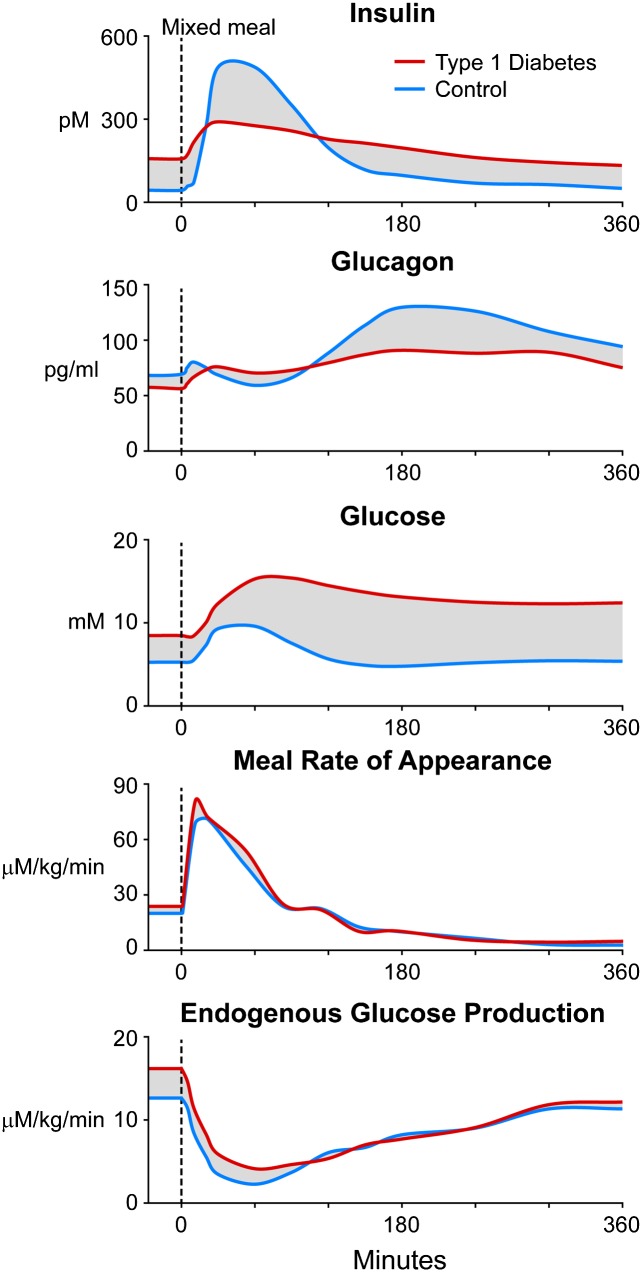
Figure 1.
Plasma insulin, glucagon, and glucose concentrations; meal rate of appearance of ingested carbohydrates; and rates of endogenous glucose production in control (blue) and T1D (red) subjects after a mixed-meal containing 50 g of carbohydrates (glucose). Data obtained from refs. 15 and 16 for the breakfast meal. The shaded parts represent differences between control and T1D subjects that could be targeted with informed algorithms.
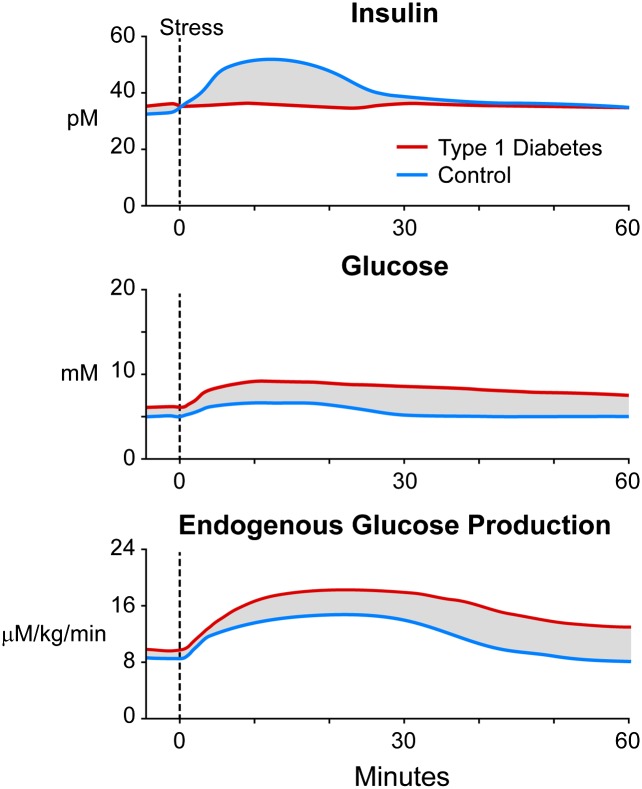
Figure 2.
Anticipated plasma insulin and glucose concentrations and changes in rates of endogenous glucose production during stress in control (blue) and T1D (red) subjects. The shaded parts represent estimated differences between control and T1D subjects that could be targeted with informed algorithms.
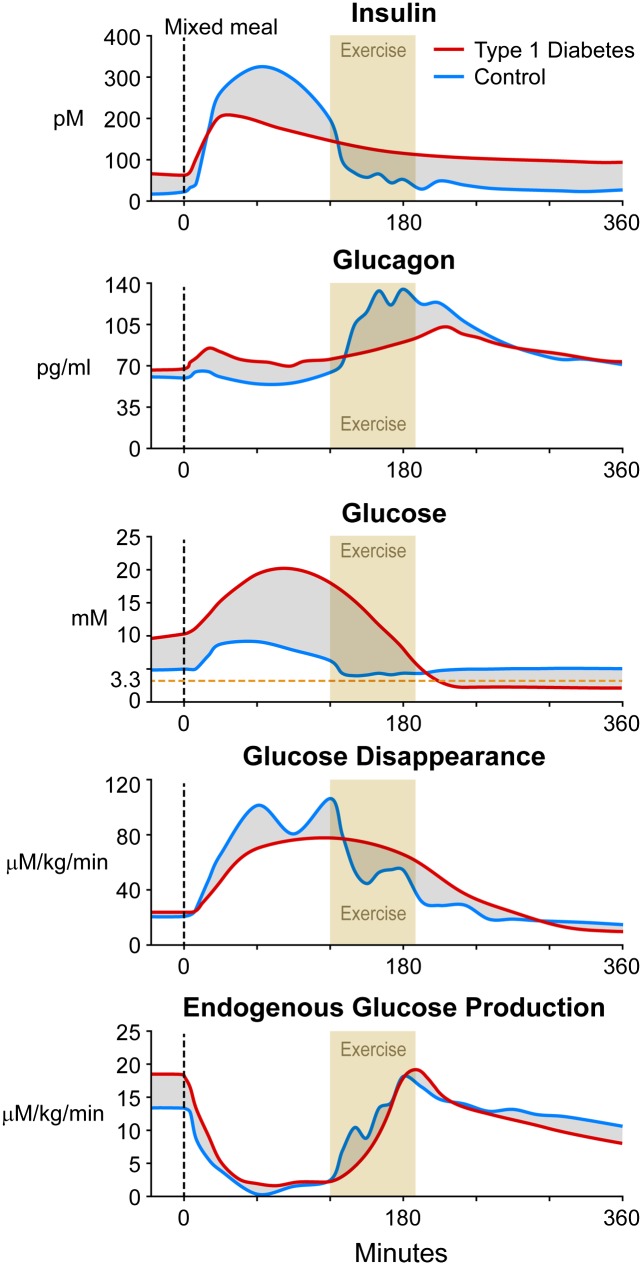
Figure 3.
Observed plasma insulin, glucagon, and glucose concentrations; rates of glucose disappearance; and endogenous glucose production in control (blue) subjects (data obtained from ref. 38) and estimated changes in these parameters in T1D (red) subjects after a mixed-meal containing 75 g of carbohydrates (glucose) with 50% Vo2max exercise for 75 min, starting 2 h after the meal. The shaded parts represent anticipated differences between control and T1D subjects that could be targeted with informed algorithms.
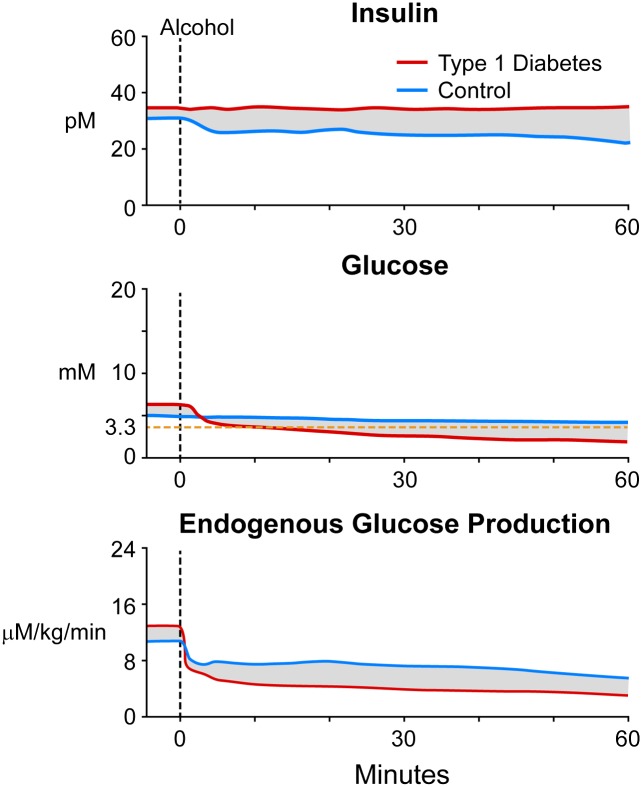
Figure 4.
Expected plasma insulin and glucose concentrations and rates of endogenous glucose production in control (blue) and T1D (red) subjects after alcohol intake. The shaded parts represent anticipated differences between control and T1D subjects that could be targeted with informed algorithms.
Common Perturbations
Meals
As shown in Fig. 1, meals are a major daily contributor to glucose variability, especially in T1D where the natural prompt postprandial changes to portal insulin/glucagon ratio rapidly modulating hepatic glucose uptake and production to minimize glucose excursions are absent due to β-cell failure and accompanying α-cell dysfunction. Inherent delays in hepatic and peripheral insulin action necessarily resulting from subcutaneous insulin delivery in T1D aggravates the problem. Additional meal-related factors (composition, portion size, alcohol), accompanying abnormalities in rates of gastric emptying, and diurnal patterns of postprandial SI further complicate the situation. While studies have reported effects of changes in meal composition (11), including the effect of alcohol (12) as well as alterations in gastric emptying (13) to postprandial glucose excursions in people with and without T1D, quantitative estimation of effect sizes of these perturbations on postprandial SI has been challenging primarily due to methodological limitations. A better assessment of the effect size of these perturbations on SI could then be incorporated into existing control algorithms and tested for safety and efficacy in next-generation artificial pancreas systems. A challenge with integrating SI into control algorithms is variability in SI during the day, as well as within and between individuals. Using a triple tracer technique (14), the existence of a diurnal pattern of SI in T1D that differs from healthy nondiabetic subjects (15) has recently been reported (16). Although these studies underscored a threefold greater intraindividual variability of SI in T1D compared with anthropometrically matched control subjects despite identical experimental conditions, careful analyses of SI diurnal patterns suggest that approximately half of T1D subjects demonstrated rising SI as the day progressed. Unchanging SI or falling SI comprised the remaining half in roughly equal proportions. Such variability of diurnal SI is currently being tested in the University of Virginia-Padova simulator (2), which has been accepted by the U.S. Food and Drug Administration, before incorporation into algorithms for clinical testing. Investigations exploring the effects of varying meal composition on SI are needed to adequately inform control algorithms. While there has been an attempt to measure postprandial glucose turnover after complex carbohydrate ingestion (11) in T1D, the interpretation was confounded by limitations in the applied methods where the biochemical backbones of the meal tracer and tracee were dissimilar. However, such efforts will be further enhanced if prandial insulin dosing is determined not only by the meal carbohydrate content, as is the current practice, but also guided by the proportions of other noncarbohydrate nutrients, including fat and proteins.
Prior reports (13) have examined the effects of delayed gastric emptying on postprandial glucose turnover in healthy and T1D subjects. Research studies are under way to investigate the impact of pramlintide to delay gastric emptying on glucose fluxes and SI. Ongoing studies in T1D subjects will help delineate the effects of delayed gastric emptying on SI to better inform future generation control algorithms (17).
Exercise
Activity and exercise influences glucose concentrations in T1D not only during exercise but also several hours after exercise leading to late evening and nocturnal hypoglycemia (18,19). Even low-grade exercise mimicking activities of daily living impacts postprandial glucose concentrations (20). Different types of exercise (resistance vs. aerobic) have contrasting effects on the duration and severity of acute (21) and delayed (22) postexercise hypoglycemia. A recent review (23) has identified the importance of exercise as a major hurdle in current standard CLC efforts. While there is sufficient evidence to recommend exercise in the management of T1D (24), the duration, intensity, and form of exercise that should be recommended and whether such interventions would translate to better outcomes are presently unclear. A recent meta-analysis (25) suggests benefits of regular aerobic training interspersed with brief bouts of sprinting on glycated hemoglobin concentrations and incidence of delayed hypoglycemia in T1D. The American Diabetes Association does not provide specific suggestions regarding exercise in T1D, but recommends carbohydrate ingestion if pre-exercise glucose is <100 mg/dL in insulin-treated individuals (26).
Apart from applying commonsense tactics to prevent hypoglycemia during and after exercise in T1D, there have been few reports that have systematically examined therapeutic approaches to mitigate hypoglycemia during and after exercise in T1D until recently. These studies have compared multiple daily injections with open-loop continuous subcutaneous insulin infusion (CSII) (27), open-loop CSII with single hormone (insulin) CLC (28), and finally open-loop CSII with single hormone modular CLC (4). A recent observational study testing dual hormone (insulin and glucagon) CLC systems (8) has shown reduced rates of hypoglycemia with moderate exercise. However, none of the CLC systems were informed by a priori estimation of physiological parameters (i.e., SI) with subsequent incorporation into and refinement of the CLC. Such an approach needs urgent development and testing to improve the safety and efficacy of current CLC systems.
Prior publications have reported on the effects of exercise of varying intensity on glucose physiology. These have included pioneering work by exercise physiologists using isotope dilution techniques and glucose clamps predominantly in healthy adults with few recent reports in patients with T1D (29,30). Experiments (31,32) have demonstrated that carbohydrate feeding and water ingestion during prolonged exercise delay fatigue and increase neuromuscular power, partly by preventing hypoglycemia and muscle glycogen depletion. However, these studies, though elegantly designed, were all performed in healthy individuals and did not involve attempts to measure the effect size on glucose concentrations during or after exercise. Recently, Fahey et al. (29) demonstrated that a short sprint increased plasma glucose levels due to a decline in glucose uptake in both healthy and T1D individuals.
Exercise increases glucose uptake through both insulin-dependent and -independent mechanisms and that endogenous glucose production (EGP) must increase to meet the increased metabolic demands of the exercising muscle to prevent hypoglycemia (33–36). These changes in glucose fluxes are facilitated by falling insulin and rising glucagon and catecholamine levels during exercise in healthy individuals (37). In the only human study comparing individuals with and without T1D during moderate and high intensity exercise, Petersen et al. (30), applying magnetic resonance technology, determined that compared with healthy individuals T1D had higher rates of EGP, which was entirely due to increased gluconeogenesis. However, as in prior investigations, lack of development of physiological models of insulin action and glucose uptake during exercise of varying intensities precludes quantification of the effect of exercise on SI, especially in T1D. This represents a significant knowledge gap with potentially large effect size to enhance currently available CLC algorithms. A recent study in healthy individuals undergoing moderate grade exercise (50% Vo2max) 2 hours after a mixed-meal reported 75% increase in SI (38). This was due to an exercise-induced increase in peripheral SI and was accompanied by a doubling of glucagon concentrations and an eightfold increase in rates of hepatic glucose production (Fig. 3). The increase in SI with exercise has been incorporated into the CLC simulator and experiments run in silico on virtual patients with T1D simulating identical experimental conditions as above. Various bolus and basal insulin adjustments are being simulated with responses analyzed using control-variability grid analyses (39) for refinement of current generation CLC. Sophisticated models are needed to determine the extent of insulin-independent effects of exercise on glucose excursions.
However, there are currently limited data on exercise physiology in T1D and in particular, there are no studies that have measured whole-body SI in T1D during sustained or intermittent exercise before and after a training period. Such studies are therefore sorely needed to better understand exercise effects on carbohydrate physiology in T1D that could help further inform and refine future CLC algorithms.
Stress/Intercurrent Illness
Mental stress and intercurrent illnesses affect glucose control through peripheral and hepatic insulin resistance leading to hyperglycemia induced by cytokine and stress hormones (e.g., catecholamine, glucocorticoids). Figure 2 shows anticipated changes in metabolic parameters induced by stress. Systematic investigations are necessary to assess the effect size, reproducibility, and interindividual variabilities on glucose concentrations and the remedial factors (e.g., increasing insulin delivery rates based on changes to SI) to inform and refine next-generation CLC algorithms.
Biological Factors
Dawn Phenomenon
Although perturbations induced by meals and exercise are a major factor for daytime glucose variability, the putative dawn phenomenon could contribute to nocturnal glucose variability in T1D. A better understanding of the frequency (every night), prevalence (every patient with T1D), and causes (cortisol, growth hormone) of this phenomenon (40), and additional investigations of modulators that could determine the effect size and predictability of dawn (sleep pattern, antecedent exercise, bedtime snacks, antecedent hypoglycemia, etc.) would inform next-generation algorithms if effect size is substantial. We are currently conducting studies in T1D subjects that are designed to examine the effect sizes of some of these factors. Subsequently, based on effect sizes and simulation, decisions will be made regarding further human studies before individualizing the control algorithm or proceeding directly to control system use.
Sex Steroids
A large gap currently exists in our understanding of the effects and effect sizes of physiological and pharmacological changes in sex steroids on carbohydrate metabolism in T1D. These include physiological hormonal changes that occur during puberty, menstrual cycle, pregnancy, and menopause, and pharmacological changes due to hormone-replacement therapies. As clinicians, we routinely encounter patients with T1D with changes in insulin requirements that occur often with puberty, menstruation, and menopause, and always occur during pregnancy. It is also widely known that due to fetal and maternal risks, tight maternal glucose control is required from conception to delivery in all pregnant women with T1D. To do so, considerable resources and expertise are used to care for a mother with T1D throughout pregnancy and in the postpartum period. While some studies in healthy women have shown no effects of menstruation on insulin action (41), others (42) have noted alterations in insulin action and secretion and glucose effectiveness. Studies in T1D have been sparse (43), showing subtle decrease in insulin action during the follicular phase. A recent study using CLC and dual isotope technique to measure postprandial glucose turnover (44) demonstrated hepatic and peripheral insulin resistance during late versus early pregnancy in a cohort of T1D. However, systematic examination, using robust state-of-the-art techniques, to estimate the effect sizes of puberty, menstruation, pregnancy, menopause, and hormone-replacement therapy on parameters of insulin action are needed before individualized next-generation control algorithms in T1D could reliably and safely be used, especially during pregnancy. While there has been emerging interest on use of CGM in pregnancy and labor (45), widespread use of modern diabetes technology has been limited in the pregnant adult.
Hypoglycemia
Hypoglycemia is a major limiting factor for optimal management of T1D with conventional insulin therapies. While short-term CLC clinical trials with single hormone (insulin) and dual hormone (insulin and glucagon) therapies have shown reduction in time spent in hypoglycemia (4–6,8) compared with conventional therapy, we need to better understand the factors that determine hepatic glucagon sensitivity in T1D. These factors include hepatic glycogen content, antecedent hypoglycemia, and prevailing glucose and insulin concentrations, etc. Investigations exploring the effects of such modulators of hepatic glucagon action (by stimulating glycogenolysis) are needed so that algorithms could be adequately informed for not only treatment of (impending) hypoglycemia but also prevention of hypoglycemia in the first place. A recent approach (46) has highlighted the efficacy of discontinuing insulin infusion based on CGM trends to lower hypoglycemia frequency.
Glucose Sensor Issues
A necessary prerequisite of an effective CLC algorithm is accurate continuous glucose sensing accomplished by CGMs that measure interstitial fluid (ISF) glucose concentrations through subcutaneously placed glucose-sensing probes. While algorithms, insulin delivery devices, and faster insulin preparations are being constantly refined, such initiatives are limited in the glucose-sensing arena—a critical initial component of any effective artificial pancreas system. In fact, some current control algorithms for artificial pancreas include adjusting for inaccuracy and the putative delay inherent to CGM sensing as an independent module.
While there have been reports (47,48) that have attempted to examine the temporal relationship between changes in plasma glucose concentrations to ISF glucose concentrations in subjects with and without diabetes (suggesting a time delay of 4–50 min, but with significant variability across published reports), detailed analyses of the kinetics of glucose from the intravascular compartment across the vessel wall and into the ISF compartment has not been performed in humans. There is one report (49) that has used fluorescein kinetics between vascular and ISF compartments and found a 2–4 min delay between the two compartments after a bolus dose. Others (50) have used microdialysis to examine the effects of insulin on adipose tissue glucose disposal without examining glucose kinetics. Hence, the relevance of these observations to glucose kinetics across the vascular wall is speculative at best.
An assessment of these physiological variables is crucial before the accuracy of CGM devices could improve and help shorten the delay that is purported to occur across compartmental barriers (48,51). A better understanding of the kinetics of subcutaneous glucose transport (i.e., delay of glucose transport from the vascular system across the capillaries into the ISF) will lead to the development of next-generation CLC algorithms. In a study using glucose isotopes and microdialysis techniques, the estimated mean time lag of appearance of tracer glucose into the ISF after intravenous bolus was between 5.3 and 6.2 min in healthy adults in the overnight fasted state (52). Furthermore, the effects of meals, exercise, insulin administration, hypoglycemia, hyperglycemia, obesity, and other variables on the putative time lag between blood and CGM glucose changes are currently being systematically examined in ongoing studies, and will need to be tested in silico before conducting clinical trials when appropriate to enable development of next-generation algorithms incorporating these changes. Nevertheless, based on analyses of glucose patterns derived from CGM and insulin infusion rates derived from insulin pump, a recent report tested the validity of a novel index of postprandial SI in T1D subjects (53). This index requires further field testing before incorporation into algorithms.
Furthermore, medications like Tylenol are known to interfere with CGM measurements, thereby providing erroneous ISF glucose readings. Ongoing experiments are currently examining the effect size of such interferences in T1D. Further studies are necessary to better understand the cause and nature of this interference, not only with Tylenol but with other pharmaceuticals commonly used in the management of T1D, for next-generation CGM devices that use enzymatic and nonenzymatic approaches for CGM.
Different Remedies for Hyper- and Hypoglycemia
As mentioned above and as depicted in Figs. 1 and 2, the physiological consequence of both meals and stress is hyperglycemia. Meal-induced hyperglycemia results predominantly due to the digestive process leading to meal glucose appearance, stimulation of glucose uptake due to insulin secretion (in a nondiabetic person) or infusion (in a T1D subject), and suppression of endogenous glucose production brought about by the normal postprandial suppression of glucagon secretion resulting in net improved SI. In contrast, stress induces hyperglycemia through stimulation of stress hormones and cytokines secretions that increases hepatic glucose production and reduces peripheral glucose uptake leading to worsening SI. Hence, although the net effect of both meals and stress is hyperglycemia, in the former scenario, SI increases; while in the latter, SI decreases. Hence, the controller will need to be adequately informed and trained to distinguish between the different situations by appropriate rapid changes to SI, insulin on board, or correction factors based on multimodality inputs or cues (e.g., meal announcement and/or heart rate or cutaneous sweat sensors).
On the other hand, as shown in Figs. 3 and 4, hypoglycemia can be a consequence of either exercise or alcohol consumption, respectively. The former is due to enhanced exercise-induced insulin-dependent and -independent peripheral glucose uptake leading to increased peripheral SI as simultaneously hepatic glucose production increases due to glucagon counterregulatory effects. Alcohol effect is, however, predominantly due to suppression of hepatic glucose production by alcohol inhibition of gluconeogenesis, thereby enhancing hepatic SI. Hence, although the net effect of both exercise and alcohol is hypoglycemia, in the former scenario, peripheral SI increases but hepatic SI decreases, while in the latter, hepatic SI increases with little or no effects on peripheral insulin action. Hence, the controller once again will need to be adequately informed to distinguish between the different scenarios by appropriate changes to SI, insulin on board, or correction factors, with or without glucagon delivery, based on multimodality inputs or cues (e.g., exercise announcement, accelerometer/heart rate inputs, and alcohol announcement). Control strategies could also exploit the rate of change of glucose that may be unique. For example, the rate of decrease in glucose achieved by moderate or strenuous physical activity is unique and not matched by any other physiological situations.
Hormone Delivery Factors
Current limitations in single- and dual-hormone CLC systems include delay in insulin action after subcutaneous administration and the instability of glucagon in solution. Apart from the inherent delay in insulin kinetics due to time lag of insulin absorption from the subcutaneous depots, factors that potentially aggravate the delay in insulin action include local lipohypertrophy, edema, and fibrosis at insulin injection sites. Instability of glucagon in solution relates to fibril formation and consequent loss of biological efficacy. Such drawbacks will hopefully soon be overcome with faster-acting insulin from local heating of injection sites (54), intraperitoneal route of insulin delivery (55), and more stable glucagon formulations (56).
Conclusions
There is a critical need for a better and more complete understanding of insulin-glucose-glucagon physiology in conditions that mimic the free-living situation to the extent possible in T1D that will help refine and improve future CLC algorithms. Further studies directed at understanding the role of the nervous system (brain, autonomic system) on glucose regulation in T1D should also enhance future generation CLC algorithms. Perturbations with large effect sizes, for example on SI, then need to be tested in the simulator to provide the algorithm with stipulations and guidelines that would help inform and modify the controller for adjustments in rates of insulin and/or glucagon delivery. It is important to underscore that the ideal CLC algorithm may ultimately need to account for all or some of these perturbations occurring simultaneously. Each perturbation could be introduced into “simulations,” and ultimately only key ones with large effect sizes would be included in the control system, a working model that engineers do for most or all machine systems.
Development, validation, and incorporation of such physiological models into control algorithms after adequate in silico testing followed by randomized clinical trials will pave the way for fully automated systems to treat patients with T1D as we approach a century after the iconic discovery of insulin!
Article Information
Acknowledgments. The authors are indebted to Mr. Brent McConahey and Dr. Ling Hinshaw of the Endocrine Research Unit, Mayo Clinic, Rochester, MN, for assistance with the figures.
Funding. This work was supported by National Institutes of Health grants DK-R01-085561 to A.B. and Y.C.K., DK-DP3-094331 to A.B., and DK-029953 to R.B.; Helmsley Charitable Trust 2012PG-TID005 to R.B.; and grant UL1-TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, to the Mayo Clinic. C.C. is partially funded by Italian Ministero dell’Istruzione, dell’Università e della Ricerca (Progetto FIRB 2009).
Duality of Interest. This work is partially supported by a grant from Dexcom, Inc. to R.B. No other potential conflicts of interest relevant to this article were reported.
Footnotes
References
- 1.Rizza RA, Gerich JE, Haymond MW, et al. Control of blood sugar in insulin-dependent diabetes: comparison of an artificial endocrine pancreas, continuous subcutaneous insulin infusion, and intensified conventional insulin therapy. N Engl J Med 1980;303:1313–1318 [DOI] [PubMed] [Google Scholar]
- 2.Kovatchev BP, Breton M, Man CD, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Tech 2009;3:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalla Man C, Micheletto F, Lv D, Breton M, Kovatchev B, Cobelli C. The UVA/Padova type 1 diabetes simulator: new features. J Diabetes Sci Tech 2014;8:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breton M, Farret A, Bruttomesso D, et al. International Artificial Pancreas Study Group Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes 2012;61:2230–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillip M, Battelino T. Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–833 [DOI] [PubMed] [Google Scholar]
- 6.Cobelli C, Renard E, Kovatchev BP, et al. Pilot studies of wearable outpatient artificial pancreas in type 1 diabetes. Diabetes Care 2012;35:e65–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle FJ, III, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care 2014;37:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care 2012;35:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanya KJ, Jacobi D, Liu S, et al. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest 2013;123:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kehlenbrink S, Tonelli J, Koppaka S, Chandramouli V, Hawkins M, Kishore P. Inhibiting gluconeogenesis prevents fatty acid-induced increases in endogenous glucose production. Am J Physiol Endocrinol Metab 2009;297:E165–E173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elleri D, Allen JM, Harris J, et al. Absorption patterns of meals containing complex carbohydrates in type 1 diabetes. Diabetologia 2013;56:1108–1117 [DOI] [PubMed] [Google Scholar]
- 12.Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011;342:d1855 [DOI] [PMC free article] [PubMed]
- 13.Woerle HJ, Albrecht M, Linke R, et al. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am J Physiol Endocrinol Metab 2008;294:E103–E109 [DOI] [PubMed] [Google Scholar]
- 14.Basu R, Di Camillo B, Toffolo G, et al. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab 2003;284:E55–E69 [DOI] [PubMed] [Google Scholar]
- 15.Saad A, Dalla Man C, Nandy DK, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012;61:2691–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinshaw L, Dalla Man C, Nandy DK, et al. Diurnal pattern of insulin action in type 1 diabetes: implications for a closed-loop system. Diabetes 2013;62:2223–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Micheletto F, Dalla Man C, Kolterman O, et al. In silico design of optimal ratio for co-administration of pramlintide and insulin in type 1 diabetes. Diabetes Technol Ther 2013;15:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maran A, Pavan P, Bonsembiante B, et al. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther 2010;12:763–768 [DOI] [PubMed] [Google Scholar]
- 19.Iscoe KE, Riddell MC. Continuous moderate-intensity exercise with or without intermittent high-intensity work: effects on acute and late glycaemia in athletes with type 1 diabetes mellitus. Diabet Med 2011;28:824–832 [DOI] [PubMed] [Google Scholar]
- 20.Manohar C, Levine JA, Nandy DK, et al. The effect of walking on postprandial glycemic excursion in patients with type 1 diabetes and healthy people. Diabetes Care 2012;35:2493–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yardley JE, Kenny GP, Perkins BA, et al. Resistance versus aerobic exercise: acute effects on glycemia in type 1 diabetes. Diabetes Care 2013;36:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yardley JE, Kenny GP, Perkins BA, et al. Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care 2012;35:669–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Bon AC, Verbitskiy E, von Basum G, Hoekstra JB, DeVries JH. Exercise in closed-loop control: a major hurdle. J Diabetes Sci Tech 2011;5:1337–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 2012;55:542–551 [DOI] [PubMed] [Google Scholar]
- 25.Tonoli C, Heyman E, Roelands B, et al. Effects of different types of acute and chronic (training) exercise on glycaemic control in type 1 diabetes mellitus: a meta-analysis. Sports Med 2012;42:1059–1080 [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yardley JE, Iscoe KE, Sigal RJ, Kenny GP, Perkins BA, Riddell MC. Insulin pump therapy is associated with less post-exercise hyperglycemia than multiple daily injections: an observational study of physically active type 1 diabetes patients. Diabetes Technol Ther 2013;15:84–88 [DOI] [PubMed] [Google Scholar]
- 28.Elleri D, Allen JM, Kumareswaran K, et al. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care 2013;36:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahey AJ, Paramalingam N, Davey RJ, Davis EA, Jones TW, Fournier PA. The effect of a short sprint on postexercise whole-body glucose production and utilization rates in individuals with type 1 diabetes mellitus. J Clin Endocrinol Metab 2012;97:4193–4200 [DOI] [PubMed] [Google Scholar]
- 30.Petersen KF, Price TB, Bergeron R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: impact of type 1 diabetes. J Clin Endocrinol Metab 2004;89:4656–4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coyle EF, Hagberg JM, Hurley BF, Martin WH, Ehsani AA, Holloszy JO. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J Appl Physiol 1983;55:230–235 [DOI] [PubMed] [Google Scholar]
- 32.Fritzsche RG, Switzer TW, Hodgkinson BJ, Lee SH, Martin JC, Coyle EF. Water and carbohydrate ingestion during prolonged exercise increase maximal neuromuscular power. J Appl Physiol 2000;88:730–737 [DOI] [PubMed] [Google Scholar]
- 33.Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest 1971;50:2715–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felig P, Wahren J. Fuel homeostasis in exercise. N Engl J Med 1975;293:1078–1084 [DOI] [PubMed] [Google Scholar]
- 35.Wasserman DH, Williams PE, Lacy DB, Green DR, Cherrington AD. Importance of intrahepatic mechanisms to gluconeogenesis from alanine during exercise and recovery. Am J Physiol 1988;254:E518–E525 [DOI] [PubMed] [Google Scholar]
- 36.Coggan AR, Swanson SC, Mendenhall LA, Habash DL, Kien CL. Effect of endurance training on hepatic glycogenolysis and gluconeogenesis during prolonged exercise in men. Am J Physiol 1995;268:E375–E383 [DOI] [PubMed] [Google Scholar]
- 37.Wolfe RR, Nadel ER, Shaw JH, Stephenson LA, Wolfe MH. Role of changes in insulin and glucagon in glucose homeostasis in exercise. J Clin Invest 1986;77:900–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiavon M, Hinshaw L, Mallad A, et al. Postprandial glucose fluxes and insulin sensitivity during exercise: a study in healthy individuals. Am J Physiol Endocrinol Metab 2013;305:E557–E566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magni L, Raimondo DM, Man CD, et al. Evaluating the efficacy of closed-loop glucose regulation via control-variability grid analysis. J Diabetes Sci Tech 2008;2:630–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll MF, Schade DS. The dawn phenomenon revisited: implications for diabetes therapy. Endocr Pract 2005;11:55–64 [DOI] [PubMed] [Google Scholar]
- 41.Toth EL, Suthijumroon A, Crockford PM, Ryan EA. Insulin action does not change during the menstrual cycle in normal women. J Clin Endocrinol Metab 1987;64:74–80 [DOI] [PubMed] [Google Scholar]
- 42.Yeung EH, Zhang C, Mumford SL, et al. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. J Clin Endocrinol Metab 2010;95:5435–5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott AR, Macdonald IA, Bowman CA, Jeffcoate WJ. Effect of phase of menstrual cycle on insulin sensitivity, peripheral blood flow and cardiovascular responses to hyperinsulinaemia in young women with type 1 diabetes. Diabet Med 1990;7:57–62 [DOI] [PubMed] [Google Scholar]
- 44.Murphy HR, Elleri D, Allen JM, et al. Pathophysiology of postprandial hyperglycaemia in women with type 1 diabetes during pregnancy. Diabetologia 2012;55:282–293 [DOI] [PubMed] [Google Scholar]
- 45.Kumareswaran K, Elleri D, Allen JM, et al. Accuracy of continuous glucose monitoring during exercise in type 1 diabetes pregnancy. Diabetes Technol Ther 2013;15:223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergenstal RM, Klonoff DC, Garg SK, et al. ASPIRE In-Home Study Group Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 47.Schoonen AJ, Wientjes KJ. A model for transport of glucose in adipose tissue to a microdialysis probe. Diabetes Technol Ther 2003;5:589–598 [DOI] [PubMed] [Google Scholar]
- 48.Wientjes KJ, Schoonen AJ. Determination of time delay between blood and interstitial adipose tissue glucose concentration change by microdialysis in healthy volunteers. Int J Artif Organs 2001;24:884–889 [PubMed] [Google Scholar]
- 49.Smith A, Yang D, Delcher H, Eppstein J, Williams D, Wilkes S. Fluorescein kinetics in interstitial fluid harvested from diabetic skin during fluorescein angiography: implications for glucose monitoring. Diabetes Technol Ther 1999;1:21–27 [DOI] [PubMed] [Google Scholar]
- 50.Rooyackers O, Myrenfors P, Nygren J, Thorell A, Ljungqvist O. Insulin stimulated glucose disposal in peripheral tissues studied with microdialysis and stable isotope tracers. Clin Nutr 2004;23:743–752 [DOI] [PubMed] [Google Scholar]
- 51.Hullegie LM, Lutgers HL, Dullaart RP, et al. Effects of glucose and insulin levels on adipose tissue glucose measurement by microdialysis probes retained for three weeks in Type 1 diabetic patients. Neth J Med 2000;57:13–19 [DOI] [PubMed] [Google Scholar]
- 52.Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes 2013;62:4083–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schiavon M, Dalla Man C, Kudva YC, Basu A, Cobelli C. Quantitative estimation of insulin sensitivity in type 1 diabetic subjects wearing a sensor-augmented insulin pump. Diabetes Care 2014;37:1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cengiz E, Weinzimer SA, Sherr JL, et al. Acceleration of insulin pharmacodynamic profile by a novel insulin infusion site warming device. Pediatr Diabetes 2013;14:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care 2010;33:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson MA, Caputo N, Castle JR, David LL, Roberts CT, Jr, Ward WK. Stable liquid glucagon formulations for rescue treatment and bi-hormonal closed-loop pancreas. Curr Diab Rep 2012;12:705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]