Abstract
OBJECTIVE
Suboptimal adherence to diabetes medications is prevalent and associated with unfavorable health outcomes, but it remains unclear what intervention content is necessary to effectively promote medication adherence in diabetes. In other disease contexts, the Information–Motivation–Behavioral skills (IMB) model has effectively explained and promoted medication adherence and thus may have utility in explaining and promoting adherence to diabetes medications. We tested the IMB model’s hypotheses in a sample of adults with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Participants (N = 314) completed an interviewer-administered survey and A1C test. Structural equation models tested the effects of diabetes medication adherence-related information, motivation, and behavioral skills on medication adherence and the effect of medication adherence on A1C.
RESULTS
The IMB elements explained 41% of the variance in adherence, and adherence explained 9% of the variance in A1C. As predicted, behavioral skills had a direct effect on adherence (β = 0.59; P < 0.001) and mediated the effects of information (indirect effect 0.08 [0.01–0.15]) and motivation (indirect effect 0.12 [0.05–0.20]) on adherence. Medication adherence significantly predicted glycemic control (β = −0.30; P < 0.001). Neither insulin status nor regimen complexity was associated with adherence, and neither moderated associations between the IMB constructs and adherence.
CONCLUSIONS
The results support the IMB model’s predictions and identify modifiable and intervenable determinants of diabetes medication adherence. Medication adherence promotion interventions may benefit from content targeting patients’ medication adherence-related information, motivation, and behavioral skills and assessing the degree to which change in these determinants leads to changes in medication adherence behavior.
Introduction
Among adults with type 2 diabetes (T2DM), suboptimal medication adherence is common (1,2) and is associated with higher healthcare costs and worse health outcomes (2,3). Few interventions have been designed to promote adherence to diabetes medications, resulting in limited knowledge about what content should be included in interventions to improve this outcome (4). To date, interventions that have effectively improved medication adherence in diabetes (5–10) have coupled content with reminder systems (6,7) and/or disseminated content using labor-intensive contacts with healthcare providers (5,6,8–10). Although much has been gleaned from these interventions, it is unclear what content has been more or less effective.
The incorporation of theory-based behavior change content in interventions to improve medication adherence may offset the need for labor-intensive dissemination strategies, thereby increasing the likelihood of interventions being successfully implemented, adopted, and sustained. Furthermore, theories specifying modifiable and measurable determinants of behavior provide an opportunity to improve the mechanisms underlying behavioral performance, which allows for identifying why an intervention was more or less effective in changing behavior for future intervention efforts (11). However, to our knowledge, no theoretical framework detailing the content necessary for medication adherence promotion in diabetes has been validated, and no results from diabetes medication adherence promotion interventions with theory-based content have been published to date.
In HIV, interventions based on the Information–Motivation–Behavioral skills (IMB) model of medication adherence (12) have been successful (13–16). The IMB model of adherence has been validated with cross-sectional data collected from diverse samples of HIV-infected persons (17–20) and then used to design interventions that have successfully improved both medication adherence (13–16) and clinical outcomes (14,16). Diabetes medication adherence interventions may benefit from a similar trajectory.
Although medication adherence is just one of many recommended diabetes self-care behaviors, each recommended behavior (e.g., exercising and blood glucose testing) is complex, with specific determinants that should be identified and accounted for in interventions. The IMB model is a behavior-specific theory that posits the performance of a behavior requires behavior-specific information, motivation, and behavioral skills. While the IMB model has predicted and promoted adherence to exercise and dietary recommendations among adults with T2DM (21,22), it has not yet been validated as a useful framework to promote diabetes medication adherence.
According to the IMB model of adherence, medication adherence is determined by the extent to which an individual is informed about his/her regimen, is motivated to adhere, and possesses the necessary behavioral skills to adhere in a variety of situations (12,18–20) (Fig. 1). Adherence information includes accurate knowledge about a regimen (i.e., how and when to take medications and what dose to take), potential side effects and drug interactions (12,18–20), and having accurate heuristics and theories that support consistent adherence (as opposed to inaccurate heuristics such as “I only have to take medications when my blood sugar is high”) (12). Adherence motivation is a function of personal and social motivation to adhere (12). Personal motivation to adhere reflects an individual’s attitudes about adherence and is based on one’s beliefs that medications are helpful, and not taking medications as prescribed would have undesirable consequences. Social motivation to adhere rests on one’s perceptions of social norms endorsing adherence and/or social and instrumental support for adherence. Adherence behavioral skills includes objective and perceived abilities or self-efficacy to obtain, store, have accessible, and self-cue the use of medications as directed across situations and despite challenges (12).
Figure 1.
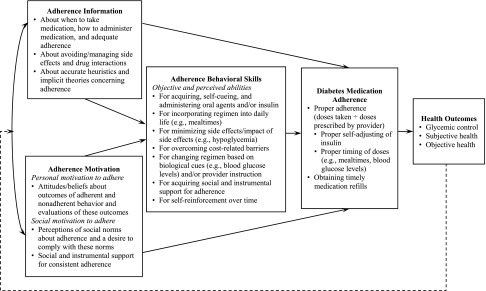
An IMB model of diabetes medication adherence, adapted from Fisher et al. (12) and Amico et al. (18). Solid lines indicate an effect between constructs, and the dashed line indicates a feedback loop in which health outcomes affect future levels of adherence information and motivation, which in turn affect adherence behavioral skills and subsequent diabetes medication adherence and health outcomes.
The IMB model of adherence (12) suggests that adherence information and motivation often covary (17,19) (i.e., more knowledge may lead to increased motivation and motivated individuals may possess more knowledge), but adherence motivation may be present in the context of inaccurate or insufficient adherence information and vice versa (18,20). Adherence information and motivation affect medication adherence primarily through the enactment of adherence behavioral skills used to initiate and maintain medication adherence (18–20), but may also directly affect behavior (17) when complex behavioral skills are not required for the performance of the behavior (12). The model’s primary outcome is adherence, but it also stipulates that consistent adherence should be associated with favorable health outcomes (e.g., glycemic control), which, in turn, contribute to high levels of adherence information and motivation overtime (12) (Fig. 1).
Many factors consistently associated with adults’ diabetes medication adherence (23) are included in the IMB constructs. Information includes comprehension of the treatment regimen; personal motivation includes perceptions of benefits and adverse effects of treatment; and behavioral skills include patients’ ability to overcome cost-related barriers to adherence. Additional evidence suggests deficits in medication adherence–related information (24), motivation [i.e., personal attitudes (24,25) and social support (26)], and behavioral skills [i.e., objective skills and self-efficacy (24,25)] are associated with suboptimal adherence to diabetes medications and are more common among populations with lower socioeconomic status (SES) (24,27). Additional factors associated with diabetes medication adherence include insulin use (28), regimen complexity (23), and psychological distress (23). However, each of these factors alone cannot fully explain the presence, persistence, and complexity of nonadherence to diabetes medications. Thus, the IMB model of adherence may provide a multivariate explanation of nonadherence and guide to promoting medication adherence among people with diabetes.
Because populations with low SES are at increased risk of nonadherence to diabetes medications (24,27), we tested the IMB model’s predictions in a diverse, low SES sample from a Federally Qualified Health Center (FQHC). Consistent with the IMB model, we hypothesized that adherence-related information and motivation would determine adherence-related behavioral skills, which, in turn, would determine adherence behavior, and behavior would determine glycemic control. We also tested whether insulin status and/or regimen complexity explained any additional variance in medication adherence or moderated associations between adherence-related information, motivation, or behavioral skills and adherence behavior.
Research Design and Methods
We recruited adults (≥18 years old) who were diagnosed with T2DM and received outpatient care at an FQHC in Nashville, TN, between June 2010 and November 2012. Trained research assistants (RAs) worked with clinic personnel to consecutively recruit eligible patients who arrived for a clinic appointment, approaching patients in the clinic waiting room to describe the study and advertising the study on flyers in the clinic to prompt self-referrals. Eligible patients were English- or Spanish-speaking adults who were self-administering prescribed medications for T2DM. All study materials were translated from English to Spanish using the forward-backward technique by licensed translators (29). RAs consulted with clinic personnel to identify and exclude patients who did not have a social security number required for compensation or had an intellectual disability, unintelligible speech, a lack of orientation to person/place/time, or a severe hearing impairment.
Participants provided informed consent and completed an interviewer-administered survey in a private room before and/or after their clinic appointment. To accommodate high rates of limited literacy among patients treated at FQHCs in the southeastern U.S. (30), RAs read all items and response options aloud to ensure participants’ ability to read did not compromise their data. RAs also supplied a copy of each set of response options in large font, so participants could respond aloud and/or point to their response. Clinic nurses administered a point-of-care A1C test, and RAs reviewed medical records. The interview took ∼1 h to complete, and participants were compensated $20. The Vanderbilt University Institutional Review Board approved all study procedures.
Measures
Demographic characteristics included self-reported age, sex, race, ethnicity, income, education, and insurance status.
Clinical characteristics included self-reported duration of diagnosed diabetes in months and years and the number and type of prescribed diabetes medications obtained from the medical record. We dichotomized insulin status (prescribed insulin vs. oral agents only), and regimen complexity was indicated by the number of prescribed diabetes medications.
Adherence information was assessed with the five-item Diabetes Medication Knowledge Questionnaire (DMKQ), which assesses respondents’ knowledge of the: 1) name; 2) purpose; 3) dosing schedule; 4) side effects; and 5) appropriate management of a missed dose for a single diabetes medication in their regimen (31). To generate a more accurate estimate of adherence information, we administered the DMKQ for each diabetes medication in a participant’s regimen. In our sample, internal consistency reliability for the DMKQ was unacceptable (α = 0.36), so we examined Pearson correlation coefficients between individual DMKQ items and the measures of medication adherence (described below) to identify relevant adherence-related information. Significant DMKQ items asked respondents about their dosing schedule (i.e., how and when to take a medication) and handling of missed doses. The internal consistency reliability of these items across diabetes medications was good (α = 0.80), so we summed item scores for each medication and then averaged scores across medications to create a two-item DMKQ composite. Possible scores ranged from 0–3 with higher scores indicating greater adherence information.
Adherence motivation was assessed with the Medicines for Diabetes Questionnaire (MDQ), which measures respondents’ beliefs about taking diabetes medications (32). The behavioral beliefs and normative beliefs subscales of the MDQ were used to assess personal and social motivation to adhere, respectively. The seven-item behavioral beliefs subscale asks respondents to report the degree to which they believe taking diabetes medications would “help me stay well” or “cause me to gain weight.” The three-item normative beliefs subscale asks respondents the degree to which their doctor/nurse, family/relatives, or partner/spouse would approve of them taking diabetes medications regularly. For both subscales, response options range from 1, strongly disagree, to 5, strongly agree, and are averaged to create a composite score ranging from 1–5, with higher scores indicating greater adherence motivation.
Adherence behavioral skills were assessed with the revised Medication Adherence Self-Efficacy Scale (MASES-R), which is a reliable and valid measure of medication adherence self-efficacy (33). The 13-item MASES-R asks respondents to report their degree of confidence in taking their medications in various situations/circumstances such as “when you are traveling” or “whatever they cost.” We added the word “diabetes” to each item to specify diabetes medications (e.g., “How confident are you that you can take your diabetes medications…”). Response options range from 1, not at all sure, to 4, extremely sure, and are averaged to create a composite score ranging from 1–4, with higher scores indicating greater adherence behavioral skills.
Diabetes medication adherence was assessed with two reliable and valid self-report measures: the Summary of Diabetes Self-Care Activities medications subscale (SDSCA-MS) (34) and the Adherence to Refills and Medications Scale for Diabetes (ARMS-D) (35). Although the SDSCA-MS is the most widely used self-report measure of medication adherence in diabetes, the ARMS-D is more sensitive to nonadherence and more predictive of glycemic control (35). Including both measures reduces the influence of measurement error (36).
The two-item SDSCA-MS asks respondents, “On how many of the last 7 days did you…take this medication?” and “…take the correct number of pills/injections for this medication?” requiring a 0–7 response for each item (34). We administered the SDSCA-MS for each diabetes medication in a participant’s regimen and then averaged these scores across medications to maintain the 0–7 scale, with higher scores indicating greater adherence. The SDSCA-MS composite had good interitem reliability across medications (average r = 0.86).
The 11-item ARMS-D asks respondents about their “daily experiences, on average” with taking their diabetes medications [e.g., “How often do you forget to take your diabetes medicine(s) when you feel sick?”] or refilling [e.g., “How often do put off refilling your diabetes medicine(s) because they cost too much money?”] (35). Response options range from 1, none of the time, to 4, all of the time, and are summed to create a score ranging from 11–44. We reverse-scored the ARMS-D so higher scores indicate greater adherence (in the same direction as the SDSCA-MS).
Glycemic control was assessed with a nurse-administered valid and reliable point-of-care A1C (%) test (37).
Analyses
We used Stata 12 to assess internal consistency reliability (i.e., Cronbach α and interitem correlation), calculate summary statistics, and explore correlations. We used AMOS 21, a structural equation modeling (SEM) program, to test statistical assumptions and estimate the models. There were no missing data.
First, we assessed whether our data met the assumptions of maximum likelihood estimation SEM. Multivariate nonnormality may result in an underestimate of data fit (i.e., inflated x2 values) and biased SEs (36). Three data characteristics contribute to multivariate nonnormality (36): 1) the distribution of any single variable is not normal (i.e., variables are skewed and kurtotic); 2) the joint distribution of any pair of the variables is not bivariate normal [i.e., Madia’s coefficient >1.96 (36)]; and 3) the presence of multivariate outliers [i.e., cases with an atypical pattern of scores; Mahalanobis distance P < 0.001 (36)]. All measures except the MDQ subscales were univariate nonnormal. Data were also multivariate nonnormal (Mardia’s coefficient, 29.3), and removal of five multivariate outliers improved, but did not correct, multivariate nonnormality (Mardia’s coefficient, 18.1). Therefore, we used bootstrapping to estimate robust SEs and bias-corrected P values for parameter estimates and 95% CIs for indirect effects (38). Stable parameter estimates are indicated by low bias (i.e., bootstrapped and maximum likelihood estimates are similar) and an unbiased good fitting model is indicated by a Bollen-Stine χ2 with P > 0.05 calculated using the bootstrapped distribution (38).
We used 5,000 bootstrapped samples (38) to estimate an IMB model of diabetes medication adherence with five multivariate outliers removed. Hypotheses regarding structural relations between the IMB model constructs were evaluated with an inspection of the direction and magnitude of the path coefficients (direct effects) and indirect effects, which indicate mediation. Significant indirect effects occur when the relationship between a predictor and an outcome is due to the predictor being associated with a third variable (i.e., all or part of the direct effect of A on C is due to a relationship between A and B). Model fit was tested with the comparative fit index (≥0.95 indicates good fit), the root mean square error of approximation (≤0.06 with CI 0.00–0.08 indicates good fit), and the standardized root mean square residual (<0.08 indicates acceptable fit, and 0 indicates perfect fit) (36). Agreement between multiple indices provides the best support a model has good data fit (36).
After evaluating the IMB model of adherence, we conducted a series of additional SEMs to examine the effects of insulin status and regimen complexity on the IMB model relationships. First, we added insulin status and, separately, regimen complexity to the model as predictors of adherence. Next, we evaluated each potential interaction with different models to assess whether insulin status or, separately, regimen complexity moderated associations between each of the IMB constructs and adherence. Regimen complexity was mean-centered to create interaction terms.
Results
Of the 588 patients with T2DM who attended a clinic appointment during recruitment, RAs approached 86.2% (507). Of these, 135 were not eligible, and 58 declined participation, resulting in 314 participants. As shown in Table 1, the sample of participants included in analyses (with outliers removed; n = 309) was diverse (53% were African American/black, and 8% reported Hispanic ethnicity), and 11 interviews were conducted in Spanish. Nearly half (45%) reported incomes <$10,000, and 46% were uninsured. On average, participants were prescribed 1.6 ± 0.7 diabetes medications (range 1–4), and 47% were prescribed insulin. Table 2 presents descriptive statistics for each variable of interest and bivariate correlations between these variables.
Table 1.
Participant characteristics (n = 309)
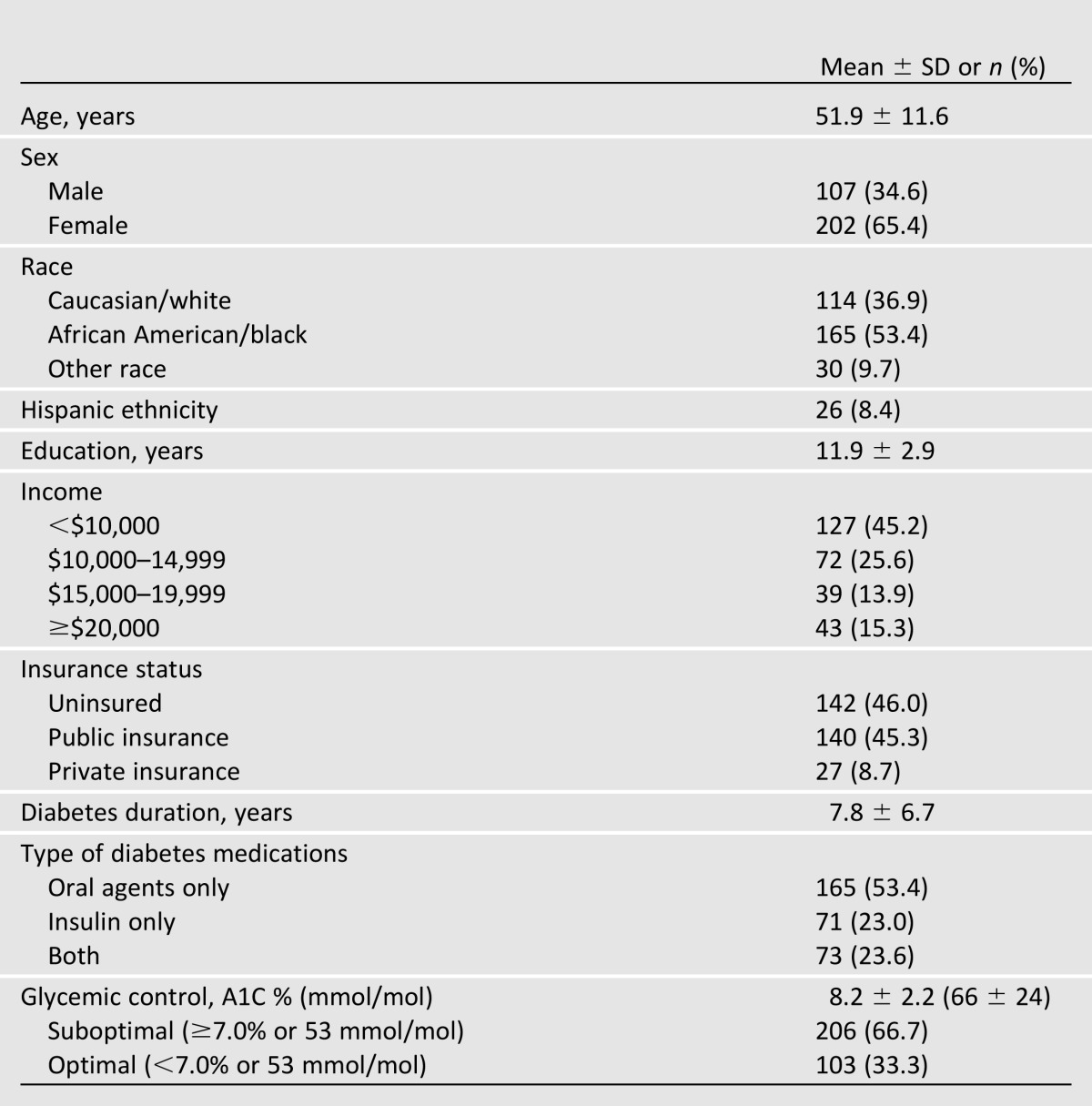
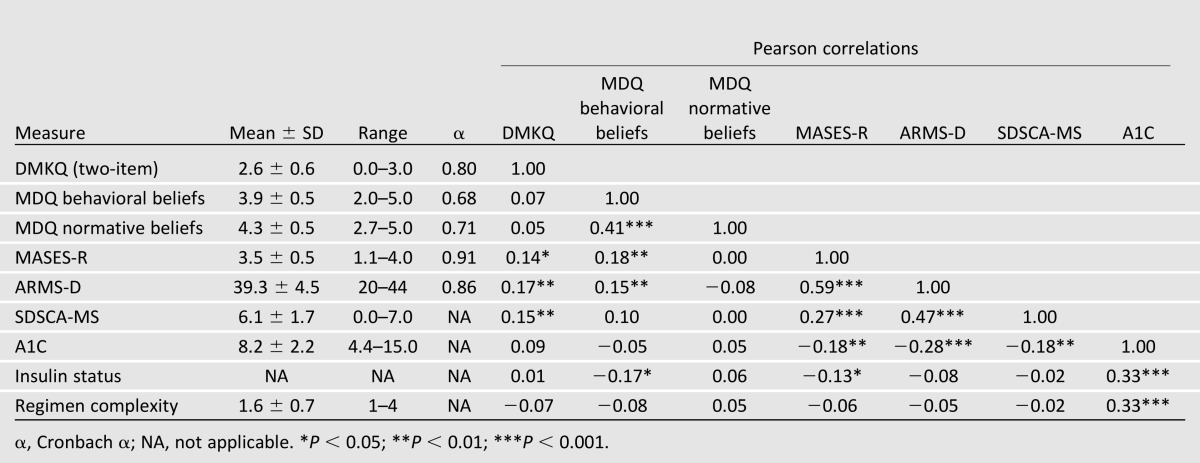
Table 2.
Descriptive statistics and correlations between model variables (n = 309)
First, we estimated an IMB model with the measures of personal and social motivation to adhere as indicators of a latent variable: adherence motivation. However, estimated variances on these factor loadings were negative, which is theoretically impossible and results from having only two indicator variables with small factor loadings (i.e., a Heywood case) (36). Therefore, we estimated a model in which personal and social motivation to adhere were permitted to covary, but were independent representations of diverse aspects of adherence motivation. This estimated IMB model of diabetes medication adherence (Fig. 2) had excellent data fit: comparative fit index, 1.00; root mean square error of approximation, 0.00 (90% CI 0.00–0.04); and standardized root mean square residual, 0.02. The parameter estimates were stable and unbiased by multivariate nonnormality (bias ranged from −0.001 to 0.003), and the Bollen-Stine χ2 (8, 309), P = 1.00 indicated an unbiased model. Fig. 2 shows the parameter coefficients and the proportion of variance explained in each endogenous variable (R2).
Figure 2.
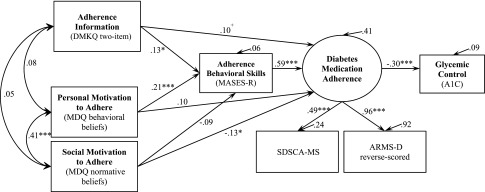
An empirical test of the IMB model of diabetes medication adherence using SEM with standardized path coefficients and bootstrapped bias-corrected P values. +P < 0.08; *P ≤ 0.05; ***P < 0.001. n = 309.
Paths from information to behavioral skills and from personal motivation to behavioral skills were significant and in the predicted direction. Behavioral skills were significantly related to medication adherence, and also mediated the effects of information (indirect effect 0.08 [CI: 0.01–0.15]) and personal motivation (indirect effect 0.12 [CI: 0.05–0.20]) on adherence. There was also a trend (P < 0.08) toward a direct effect of information on adherence. The IMB constructs explained 41% of the variance in adherence, and adherence explained 9% of the variance in glycemic control.
Social motivation was significantly related to adherence (−0.13; P < 0.05), indicating participants with greater perceived social norms for adherence reported worse adherence. Given this unexpected finding, we examined post hoc models with each item separately (i.e., doctor/nurse, family/relatives, and partner/spouse) and found only the item about family/relatives was related to less adherence (−0.15; P < 0.01).
Neither insulin status nor regimen complexity was associated with adherence (not shown in Fig. 2), and no additional variance in adherence was explained by their inclusion in the model. Moreover, none of the relationships between information, personal or social motivation, or behavioral skills and adherence were moderated by insulin status or regimen complexity.
Conclusions
The IMB model of adherence (12), which has been largely used to conceptualize HIV medication adherence (17–20), was applied to conceptualize the determinants of diabetes medication adherence among a low-income, diverse sample of adults with T2DM. Consistent with the IMB model’s predictions, adherence information and motivation were associated with adherence behavioral skills, which were, in turn, associated with diabetes medication adherence, and adherence was associated with glycemic control. In short, the IMB model was well positioned to explain the sample’s medication adherence behavior, accounting for 41% of the variability in this outcome. Neither insulin status nor regimen complexity was associated with adherence. Furthermore, none of the associations between the IMB constructs and adherence were moderated by insulin status or regimen complexity. Specific findings and conclusions about each of the IMB components are discussed in turn.
We used an objective assessment of adherence information and found that knowing how and when to take a medication and how to handle a missed dose were the types of information relevant to adherence. Consistent with other empirical tests of the IMB model of adherence (18–20), the effect of information on adherence was mediated by behavioral skills. We also found a trend toward a direct effect of information on adherence, which may be because we did not assess more complex knowledge (e.g., knowing how and when to get prescriptions refilled/reauthorized) that may affect adherence through the enactment of behavioral skills, nor did we assess heuristics or implicit theories that may also influence adherence (12,18). Due to internal consistency concerns with our measure of information, only limited knowledge about medications was accounted for. Thus, identification of other types of information important for adherence is needed.
We examined the personal and social aspects of adherence motivation as separate constructs. As others have found in the context of adherence to HIV medications (18,20), information was not associated with personal or social motivation to adhere, suggesting the assessed adherence information was insufficient to motivate participants to adhere, and being motivated to adhere did not ensure participants had accurate and sufficient adherence information. Personal motivation to adhere, which we operationalized with a measure of personal beliefs toward taking diabetes medications, was associated with more adherence behavioral skills as predicted. We operationalized social motivation to adhere with a measure of social norms for adherence and found that having greater social norms for adherence was not associated with behavioral skills and was negatively associated with adherence. This unexpected finding might be due to an incomplete operationalization of social motivation. The MDQ normative beliefs subscale has not been validated and, while it assesses participants’ perceptions of others’ approval of adherence, it does not assess the degree to which participants desire such approval (i.e., valence). Furthermore, we did not assess social support for medication adherence. Post hoc analyses indicated the item inquiring about family/relatives’ approval for adherence was driving the significant negative association with adherence behavior. This may be because positive family support (e.g., “my family members help me remember to take my medications”) often co-occurs with negative family support (e.g., “my family members nag/argue with me about my medications”) (26,39), whereas only negative family support has been associated with less medication adherence (26). Thus, the validity of the item inquiring about family members’ approval for adherence may be affected by the complex relationships between positive and negative family support and adherence. Future studies should measure: 1) respondents’ perceptions of social norms to adhere from meaningful others; 2) respondents’ desire to please these referents; and 3) referents’ positive and negative support for medication adherence.
Adherence behavioral skills had a strong relationship with medication adherence and mediated the effects of information and motivation on adherence. Like others (17–20), we used a measure of self-efficacy to operationalize behavioral skills because of the impracticality of observing one’s skills across situations and the high correlations between actual and perceived skills, but objective skills or factors that might serve as proxies (e.g., problem-solving) for behavioral skills may operate differently.
The remaining 59% of unexplained variance in diabetes medication adherence may be attributed to unmeasured aspects of information, motivation, and behavioral skills and/or to unmeasured constructs previously associated with adherence. However, several other constructs previously associated with adherence [e.g., access to healthcare and depressive symptoms (23)] would be considered moderating factors in the IMB model (12). Others (23,28) have found associations between diabetes medication adherence and insulin status and regimen complexity, but we did not find these associations in our sample, nor did we find these to be moderating factors. Hertz et al. (28) reported that patients prescribed insulin upon diagnosis were more likely to quit taking diabetes medications, but all of our participants were taking prescribed diabetes medications as a condition of enrollment and therefore may represent a different patient population. Relationships between regimen complexity and nonadherence have been found using dosing schedules to assess regimen complexity and ecological momentary assessment or pharmacy records to assess adherence (23). Furthermore, adherence to diabetes medications explained only 9% of the variance in A1C. Other factors that account for the remaining variance in glycemic control include (but are not limited to) adherence to other recommended self-care behaviors, the class and dose of antidiabetes medications, age, obesity, and diabetes-related complications (40).
Limitations
In general, our use of self-report measures may have introduced recall and social desirability bias. We selected these measures because they were the best available for assessing the IMB constructs specific to diabetes medications (rather than general measures of diabetes knowledge, motivation, and self-efficacy) and captured previously identified barriers to diabetes medication adherence, but neither the DMKQ nor the MDQ have been formally validated. Furthermore, cross-sectional data prevent conclusions about true causal mediation and the ability to test the IMB model’s proposed feedback loop from health outcomes to information and motivation (12). Our estimated model was theoretically justified, but, statistically, there are competing models (including those with opposite directionality) that would have generated comparable path coefficients and a good data fit. Moreover, the relationships between the IMB constructs may be different in other patient populations and when using other measures of adherence. Different measures of the IMB constructs may be necessary for patients managing multiple medications on different refill/reauthorization schedules and for those who have to self-adjust their insulin dose. Finally, the IMB model (12) suggests potential moderators (e.g., unstable living situation or depression) we did not explore.
Summary
Our results suggest patients’ adherence-related information, motivation, and behavioral skills are important targets for interventions promoting adherence to diabetes medications, regardless of their medication regimen. IMB model-based intervention content might include strategies to ensure patients possess appropriate knowledge about their medication regimen (i.e., dosing schedules and handling missed doses) and skills to obtain adherence information when needed (e.g., to access trustworthy sources and ask providers about medications). Motivation may be enhanced by targeting negative or false beliefs about adherence, juxtaposing consequences of nonadherence with a patients’ goals and interests, and teaching patients how to elicit support for adherence (e.g., asking for dose reminders when their routine changes). Intervention content that helps patients identify strategies to adhere in a variety of situations (e.g., setting alarm reminders to take/refill medications while traveling) may increase adherence behavioral skills. Evaluations of IMB model–based interventions can and should identify the extent to which intervention content improved patients’ adherence-related information, motivation, and behavioral skills and, in turn, medication adherence to inform future intervention efforts.
Article Information
Acknowledgments. The authors thank the research staff (Cecilia C. Quintero, Sahbina Ebba, Karen Calderon, Leo Cortes, Anne Crook, and Carmen Mekhail), the Vine Hill Community Clinic personnel, and the participants for contributions to this research.
Funding. This research was funded with support from the Vanderbilt Clinical and Translational Science Award (UL1-TR-000445) from the National Center for Advancing Translational Sciences. L.S.M. was supported by a National Research and Service Award (F32-DK-097880) from the National Institute of Diabetes and Digestive and Kidney Diseases, and C.Y.O. was supported by a Career Development Award (K01-DK-087894) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.S.M. managed data, conducted analyses, and wrote the manuscript. C.Y.O. designed the parent study; supervised all aspects of data collection, cleaning, and management; guided the analyses; and reviewed and edited the manuscript. C.Y.O. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
Footnotes
The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent official views of the National Institutes of Health.
References
- 1.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care 2004;27:1218–1224 [DOI] [PubMed] [Google Scholar]
- 2.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166:1836–1841 [DOI] [PubMed] [Google Scholar]
- 3.Aikens JE, Piette JD. Longitudinal association between medication adherence and glycaemic control in type 2 diabetes. Diabet Med 2013;30:338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med 2012;157:785–795 [DOI] [PubMed] [Google Scholar]
- 5.Piette JD, Weinberger M, McPhee SJ, Mah CA, Kraemer FB, Crapo LM. Do automated calls with nurse follow-up improve self-care and glycemic control among vulnerable patients with diabetes? Am J Med 2000;108:20–27 [DOI] [PubMed] [Google Scholar]
- 6.Rosen MI, Rigsby MO, Salahi JT, Ryan CE, Cramer JA. Electronic monitoring and counseling to improve medication adherence. Behav Res Ther 2004;42:409–422 [DOI] [PubMed] [Google Scholar]
- 7.Skaer TL, Sclar DA, Markowski DJ, Won JK. Effect of value-added utilities on prescription refill compliance and Medicaid health care expenditures—a study of patients with non-insulin-dependent diabetes mellitus. J Clin Pharm Ther 1993;18:295–299 [DOI] [PubMed] [Google Scholar]
- 8.Cherry JC, Moffatt TP, Rodriguez C, Dryden K. Diabetes disease management program for an indigent population empowered by telemedicine technology. Diabetes Technol Ther 2002;4:783–791 [DOI] [PubMed] [Google Scholar]
- 9.Wolever RQ, Dreusicke M, Fikkan J, et al. Integrative health coaching for patients with type 2 diabetes: a randomized clinical trial. Diabetes Educ 2010;36:629–639 [DOI] [PubMed] [Google Scholar]
- 10.Bogner HR, de Vries HF. Integrating type 2 diabetes mellitus and depression treatment among African Americans: a randomized controlled pilot trial. Diabetes Educ 2010;36:284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell M, Fitzpatrick R, Haines A, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher JD, Fisher WA, Amico KR, Harman JJ. An Information-Motivation-Behavioral Skills model of adherence to antiretroviral therapy. Health Psychol 2006;25:462–473 [DOI] [PubMed] [Google Scholar]
- 13.Kalichman SC, Cherry J, Cain D. Nurse-delivered antiretroviral treatment adherence intervention for people with low literacy skills and living with HIV/AIDS. J Assoc Nurses AIDS Care 2005;16:3–15 [DOI] [PubMed] [Google Scholar]
- 14.Mannheimer SB, Morse E, Matts JP, et al. Terry Beirn Community Programs for Clinical Research on AIDS Sustained benefit from a long-term antiretroviral adherence intervention. Results of a large randomized clinical trial. J Acquir Immune Defic Syndr 2006;43(Suppl. 1):S41–S47 [DOI] [PubMed] [Google Scholar]
- 15.Pearson CR, Micek MA, Simoni JM, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr 2007;46:238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher JD, Amico KR, Fisher WA, et al. LifeWindows Team Computer-based intervention in HIV clinical care setting improves antiretroviral adherence: the LifeWindows Project. AIDS Behav 2011;15:1635–1646 [DOI] [PubMed] [Google Scholar]
- 17.Kalichman SC, Rompa D, DiFonzo K, et al. HIV treatment adherence in women living with HIV/AIDS: research based on the Information-Motivation-Behavioral Skills model of health behavior. J Assoc Nurses AIDS Care 2001;12:58–67 [DOI] [PubMed] [Google Scholar]
- 18.Amico KR, Toro-Alfonso J, Fisher JD. An empirical test of the Information, Motivation and Behavioral Skills model of antiretroviral therapy adherence. AIDS Care 2005;17:661–673 [DOI] [PubMed] [Google Scholar]
- 19.Amico KR, Barta W, Konkle-Parker DJ, et al. The Information-Motivation-Behavioral Skills model of ART adherence in a Deep South HIV+ clinic sample. AIDS Behav 2009;13:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starace F, Massa A, Amico KR, Fisher JD. Adherence to antiretroviral therapy: an empirical test of the Information-Motivation-Behavioral Skills model. Health Psychol 2006;25:153–162 [DOI] [PubMed] [Google Scholar]
- 21.Osborn CY, Rivet Amico K, Fisher WA, Egede LE, Fisher JD. An Information-Motivation-Behavioral Skills analysis of diet and exercise behavior in Puerto Ricans with diabetes. J Health Psychol 2010;15:1201–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborn CY, Amico KR, Cruz N, et al. A brief culturally tailored intervention for Puerto Ricans with type 2 diabetes. Health Educ Behav 2010;37:849–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med 2005;118(Suppl. 5A):27S–34S [DOI] [PubMed] [Google Scholar]
- 24.Duru OK, Gerzoff RB, Selby JV, et al. Identifying risk factors for racial disparities in diabetes outcomes: the Translating Research into Action for Diabetes (TRIAD) study. Med Care 2009;47:700–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann DM, Ponieman D, Leventhal H, Halm EA. Predictors of adherence to diabetes medications: the role of disease and medication beliefs. J Behav Med 2009;32:278–284 [DOI] [PubMed] [Google Scholar]
- 26.Mayberry LS, Osborn CY. Family support, medication adherence, and glycemic control among adults with type 2 diabetes. Diabetes Care 2012;35:1239–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the health and retirement study. Arch Intern Med 2007;167:1853–1860 [DOI] [PubMed] [Google Scholar]
- 28.Hertz RP, Unger AN, Lustik MB. Adherence with pharmacotherapy for type 2 diabetes: a retrospective cohort study of adults with employer-sponsored health insurance. Clin Ther 2005;27:1064–1073 [DOI] [PubMed] [Google Scholar]
- 29.Behling O, Law KS. Translating questionnaires and other research instruments: Problems and solutions. In Quantitative Applications in the Social Sciences. Lewis-Beck M, Ed. Thousand Oaks, Sage, 2000, p. 1–70 [Google Scholar]
- 30.Arnold CL, Rademaker A, Bailey SC, et al. Literacy barriers to colorectal cancer screening in community clinics. J Health Commun 2012;17(Suppl. 3):252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPherson ML, Smith SW, Powers A, Zuckerman IH. Association between diabetes patients’ knowledge about medications and their blood glucose control. Res Social Adm Pharm 2008;4:37–45 [DOI] [PubMed] [Google Scholar]
- 32.Farmer A, Kinmonth AL, Sutton S. Measuring beliefs about taking hypoglycaemic medication among people with Type 2 diabetes. Diabet Med 2006;23:265–270 [DOI] [PubMed] [Google Scholar]
- 33.Fernandez S, Chaplin W, Schoenthaler AM, Ogedegbe G. Revision and validation of the medication adherence self-efficacy scale (MASES) in hypertensive African Americans. J Behav Med 2008;31:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 35.Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The ARMS-D out performs the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Res Clin Pract 2013;102:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kline RB. Principles and Practices of Structural Equation Modeling. New York, NY, The Guilford Press, 2005 [Google Scholar]
- 37.Kennedy L, Herman WH, GOAL A1C Study Team Glycated hemoglobin assessment in clinical practice: comparison of the A1cNow point-of-care device with central laboratory testing (GOAL A1C Study). Diabetes Technol Ther 2005;7:907–912 [DOI] [PubMed] [Google Scholar]
- 38.Chernick MR. Bootstrap Methods: A Practitioner's Guide . 2nd ed New York, Wiley, 2008 [Google Scholar]
- 39.Glasgow RE, Toobert DJ. Social environment and regimen adherence among type II diabetic patients. Diabetes Care 1988;11:377–386 [DOI] [PubMed] [Google Scholar]
- 40.Chan JC, Gagliardino JJ, Baik SH, et al. IDMPS Investigators Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care 2009;32:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]


