Abstract
OBJECTIVE
The conventional diet approach to gestational diabetes mellitus (GDM) advocates carbohydrate restriction, resulting in higher fat (HF), also a substrate for fetal fat accretion and associated with maternal insulin resistance. Consequently, there is no consensus about the ideal GDM diet. We hypothesized that, compared with a conventional, lower-carbohydrate/HF diet (40% carbohydrate/45% fat/15% protein), consumption of a higher-complex carbohydrate (HCC)/lower-fat (LF) Choosing Healthy Options in Carbohydrate Energy (CHOICE) diet (60/25/15%) would result in 24-h glucose area under the curve (AUC) profiles within therapeutic targets and lower postprandial lipids.
RESEARCH DESIGN AND METHODS
Using a randomized, crossover design, we provided 16 GDM women (BMI 34 ± 1 kg/m2) with two 3-day isocaloric diets at 31 ± 0.5 weeks (washout between diets) and performed continuous glucose monitoring. On day 4 of each diet, we determined postprandial (5 h) glucose, insulin, triglycerides (TGs), and free fatty acids (FFAs) following a controlled breakfast meal.
RESULTS
There were no between-diet differences for fasting or mean nocturnal glucose, but 24-h AUC was slightly higher (∼6%) on the HCC/LF CHOICE diet (P = 0.02). The continuous glucose monitoring system (CGMS) revealed modestly higher 1- and 2-h postprandial glucose on CHOICE (1 h, 115 ± 2 vs. 107 ± 3 mg/dL, P ≤ 0.01; 2 h, 106 ± 3 vs. 97 ± 3 mg/dL, P = 0.001) but well below current targets. After breakfast, 5-h glucose and insulin AUCs were slightly higher (P < 0.05), TG AUC was no different, but the FFA AUC was significantly lower (∼19%; P ≤ 0.01) on the CHOICE diet.
CONCLUSIONS
This highly controlled study randomizing isocaloric diets and using a CGMS is the first to show that liberalizing complex carbohydrates and reducing fat still achieved glycemia below current treatment targets and lower postprandial FFAs. This diet strategy may have important implications for preventing macrosomia.
Introduction
There is currently no consensus on the optimal diet for women diagnosed with gestational diabetes mellitus (GDM) (1). Yet the rapidly rising prevalence makes it critically important that carefully controlled studies clarify the optimal macronutrient composition for diet as a first-line treatment. A leading concern persuading the National Institutes of Health (NIH) not to adopt the International Association of the Diabetes and Pregnancy Study Groups/American Diabetes Association (ADA) diagnostic criteria for GDM (2), which predicts an 18% prevalence of GDM (3), was the lack of effective treatment strategies that could be easily implemented without incurring tremendous health care costs (4). Clarification of an optimal diet has the potential to effectively control glycemia and favorably affect lipid profiles, benefit both mother and infant health, and resolve the current dilemma of inconsistent diet recommendations. Due to limited resources to care for this expanding population, an effective, lower-cost treatment strategy that circumvents expensive medications and intensified fetal surveillance is critical.
The conventional approach to diet therapy in GDM has been carbohydrate restriction (30–40% of total calories), with the goal of blunting postprandial glucose (5,6), to mitigate glucose-mediated fetal macrosomia. However, this practice typically results in higher fat (HF) intake, given that protein intake is remarkably constant at 15–20% (7). Outside of pregnancy, an HF diet typically increases serum free fatty acids (FFAs), promoting insulin resistance (8). In nonhuman primates and in some human studies, a maternal HF diet increases fetal fat accretion and infant adiposity, promotes hepatic steatosis (9), increases inflammation and oxidative stress, and impairs skeletal muscle glucose uptake (10). Further, HF diets may cause placental dysfunction (11) and cultivate an obesogenic maternal microbiome that can be transferred to the infant (12). Despite the critical importance of dietary macronutrients on maternal–fetal metabolism, there is an absence of highly controlled randomized clinical trials (RCTs), resulting in consensus panels withdrawing specific diet recommendations until more definitive high-quality data are available (1).
To address this need, we undertook a randomized crossover trial in women with diet-controlled GDM to determine whether a diet that liberalized total carbohydrate (higher-complex, lower-glycemic index [GI] foods) and minimized fat could effectively control maternal glycemia and postprandial lipids. All food provision during the trial was precisely controlled through our metabolic kitchen. We hypothesized that, compared with the conventional lower-carbohydrate (LC) and HF diet (CONV), consumption of a higher-complex carbohydrate (HCC) and lower-fat (LF) Choosing Healthy Options In Carbohydrate Energy (CHOICE) diet would result in postprandial and 24-h glucose area under the curve (AUC) profiles within the current glycemic therapeutic targets. Further, we hypothesized that the postprandial lipid profile would be improved following the HCC/LF CHOICE diet compared with LC/CONV, an important consideration because maternal triglycerides (TGs) and FFAs are fundamental substrates for fetal growth (13,14).
Research Design and Methods
Subjects
This study was approved by the Colorado Multiple Institutional Review Board for University Hospital and the Kaiser Permanente Colorado Institutional Review Board, and women were recruited at both sites. Inclusion criteria were diagnosis of GDM at 24–28 weeks' gestation according to the Carpenter and Coustan criteria adopted by the American College of Obstetricians and Gynecologists (ACOG) (6), 20–36 years old, overweight/obese (BMI 26–39 kg/m2 at diagnosis), on diet alone, and English speaking. Exclusions were multiple gestation, hypertriglyceridemia (fasting TG >400 mg/dL), suspected overt diabetes (hemoglobin A1c ≥ 6.5), smoker, or likely to fail diet (fasting glucose >110 mg/dL (6)). Women with risk factors for placental insufficiency and growth restriction (hypertension, renal disease, thrombophilia, rheumatologic disease, use of β-blockers, or history of preeclampsia), or preterm delivery or with major medical disorders were excluded.
Randomization
Participants were randomized through the study database using a predefined order created by a biostatistician with the Clinical Translational Research Center (CTRC). The process was fully concealed from the investigators until treatment order was revealed before starting the study.
Study Protocol
The randomized crossover diet protocol began between 29 and 32 weeks' gestation and lasted 12 days, during which 100% of food was provided by the CTRC Bionutrition Department. After informed consent, women reported to the CTRC clinic, where they had a fasting (≥10 h) venous blood sample collected (day 1) and were then placed on a washout/control diet for 2 days (described below). On day 3, subjects reported to the CTRC, where a continuous glucose monitoring system (CGMS) was placed before breakfast, and they began the first random diet assignment (LC/CONV or HCC/LF CHOICE) to be followed for 72 h as an outpatient. After 3 days of the first diet, on day 6, the women reported to the CTRC fasted; a baseline blood sample was collected, and the CGMS monitor was removed. A prepared breakfast meal, as outlined below, was administered consistent with the first diet assignment, and hourly blood samples were collected for 5 h to study the postprandial breakfast response. After completion, subjects followed a washout/control diet for the remainder of day 6 through day 8. The same 3-day protocol that was followed on days 3–6 was repeated on days 9–12, but providing the alternate diet.
Techniques
Diet Protocol
The caloric intake of both diets was matched at 24 kcal/kg for overweight or 18 kcal/kg for obese women (≥1,800 kcal/day) according to Institute of Medicine recommendations (15). Each participant completed a food preference questionnaire to optimize compliance, and meals were developed to meet individual daily caloric requirements. The initial 2-day and between-random assignment washout/control diet was composed of 50% carbohydrate/35% fat/15% protein. The macronutrient content of the LC/CONV diet was 40% carbohydrate/45% fat/15% protein based on the suggestion of Jovanovic-Peterson and Peterson (5,16) to limit carbohydrates to 33–40%; this approach has been standard of care in women with GDM and was recently supported in the 2013 ACOG Practice Bulletin (6). Macronutrient distribution for the HCC/LF (CHOICE) diet was 60% carbohydrate/25% fat/15% protein, enriched in complex carbohydrate, similar to that suggested by the ADA in 2004 (17) outside of pregnancy and most recently endorsed by the American Heart Association/American College of Cardiology for adults (55–59% carbohydrate, 26–27% fat) (18). We define “complex carbohydrate” as polysaccharides and starches primarily derived from grains, vegetables, and fruits that tend to attenuate a sharp postprandial rise in plasma glucose. Both diets provided foods enriched in complex carbohydrate and provided 15% protein (∼67.5 g/day), thereby meeting the U.S.-recommended daily allowance for protein. Distribution of dietary fatty acids was the same in both diets: 35% saturated fatty acid (SFA), 45% monounsaturated fatty acid, 20% polyunsaturated fatty acid. Simple sugars contributed <30% of total carbohydrate intake for both diets and never exceeded 18% of total calories. Daily kilocalories were provided as 25% at breakfast, 25% at lunch, 30% at dinner, and the remaining 20% divided into two snacks (afternoon and bedtime) distributed as 10% of calories each (16). Each meal/snack reflected the overall macronutrient distribution of each diet. During the CGMS monitoring period, women were asked to consume their meals within 30 min during similar windows of time. Physical activity was limited to walking. Because diets of low–moderate GI have shown potential in decreasing the need for insulin in GDM women (19), both diets consisted of mainly low–moderate GI foods.
Food items between the two study diets were very similar, but total complex carbohydrate or fat accounted for a different percentage of total calories. Typical breakfast meals included a whole egg, whole-wheat toast with jam or butter, and a side of fruit; yogurt with nuts; and oatmeal prepared with milk, brown sugar, nuts, and fruit. Typical lunches included a turkey/ham sandwich on whole-wheat bread, wraps, or salads using romaine lettuce and various vegetables. Typical dinners included whole-wheat pasta with a lean protein source, marinara or Alfredo sauce, vegetables, and a side salad; stir-fry dishes with vegetables and brown rice; and chicken with baked potato, broccoli, and a whole-wheat roll. The LC/CONV diet incorporated an average of 23.5 g/day of fiber, and the HCC/LF diet incorporated 29.3 g/day.
Blood Measures
Plasma glucose was measured using hexokinase (Beckman Coulter, Brea, CA). Plasma insulin was measured by radioimmunoassay (Millipore, Billerica, MA). Plasma TGs were measured using an enzymatic method (Beckman Coulter). FFAs were measured in serum using an enzymatic method (WaKo Chemicals, Richmond, VA). Lipids were measured by a spectrophotometric method (vanillin/sulfuric acid), and LDL cholesterol was measured directly by an enzymatic method (Beckman Coulter). Hemoglobin A1c was measured using potassium ferricyanide (Siemans DCA Vantage, Malvern, PA). Homeostasis model assessment of insulin resistance was calculated as (fasting insulin [µU/ml]) × (fasting glucose [mg/dL] × 0.05551) / 22.5. C-peptide was measured by radioimmunoassay (Siemans, Los Angeles, CA).
CGMS
The CGMS (Medtronic MiniMed) was used to monitor interstitial glucose every 5 min during the randomized diet assignments. Women were blinded to CGMS glucose concentrations. Identical glucometers were provided by the investigators (OneTouch, LifeScan Inc., Milpitas, CA); postprandial glucose was measured either 1 or 2 h after meals as per obstetrician preference, with the accepted targets of <140 mg/dL at 1 h and <120 mg/dL at 2 h (1). Preprandial glucose via glucometer four times daily was also measured for calibration of the CGMS sensor data (correlation between interstitial and meter glucose, r = 0.732; P < 0.0001; n = 840 measures). Women recorded meter glucose and meal start times. CGMS sensors were placed near the lateral iliac crest by an investigator. During the investigation, wireless CGMS equipment became available, but the sensor technology was unchanged. Given it was no longer possible with the wireless device to discern any sensor malfunction, women with the wireless CGMS monitors (n = 4) wore two sensors side-by-side, 1 inch apart in the same adipose tissue bed. The mean of interstitial glucose data from the two sensors was used for analysis in these cases. CGMS glucose variables were extracted and represent the mean over 48 h as we have described in detail previously (20,21).
Breakfast Meal Study
After 72 h of diet, women reported to the CTRC after an overnight fast (≥10 h). An antecubital intravenous line was inserted, and baseline samples were collected. They then consumed a standardized breakfast meal (30% of total daily energy intake and slightly higher than the 25% of daily energy consumed while wearing the CGMS) and were matched to the macronutrient content of the current diet (followed for the previous 72 h). A typical breakfast meal for the LC/CONV diet consisted of an egg sandwich on whole-wheat toast with cheese and a side of yogurt. A typical HCC/LF CHOICE breakfast consisted of oatmeal prepared with low-fat milk with a side of nuts, fruit, or low-fat yogurt. Average GI for the mixed foods administered (not relative to food by weight) was 34.8 for CHOICE and 35.7 for LC/CONV. Blood was sampled hourly for 5 h to measure postprandial TG, FFA, glucose, C-peptide, and insulin.
Physical Activity
Physical activity was assessed during the protocol using the validated 36-item Pregnancy Physical Activity Questionnaire, for which 1-week test–retest reliability has been adequate (intraclass correlations 0.78–0.93) (22).
Diet Adherence
To ensure compliance that only provided foods were consumed, women completed a detailed food preference questionnaire prior to the study so that any unpleasant foods, liquids, or condiments were avoided. Women were strongly encouraged to consume all food provided; in rare cases, if consumption of a particular food was not 100%, a similar food of similar macronutrient content was substituted. The investigators, including highly skilled registered dietitians, had in-person contact five times throughout the 12-day study and remained in close contact with subjects throughout all of the study days.
Sample Size Determination and Statistical Methods
The a priori primary outcome was the difference in maternal 24-h glucose AUC between the two diet treatments (LC/CONV versus HCC/LF) within GDM subjects. Power was calculated at the time of study design based on data from Gannon et al. (23). Using a one-sample, two-sided t test with α at 0.05, a sample size of 16 was predicted to achieve 81% power to detect a statistically significant difference in AUC glucose between the diets. Glucose AUC was calculated using the trapezoid method to capture all potential glucose/substrate exposure to the fetus in the designated time periods (24 h, daytime, nocturnal, and postprandial). Normality of the outcome variables was tested using the Shapiro–Wilk test. Data are expressed as mean ± SEM; rounded values appear in text. Paired t tests were used to assess treatment effects. Two-sample t tests on halved crossover differences between sequences were used to assess the period effects. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Subjects and Randomization
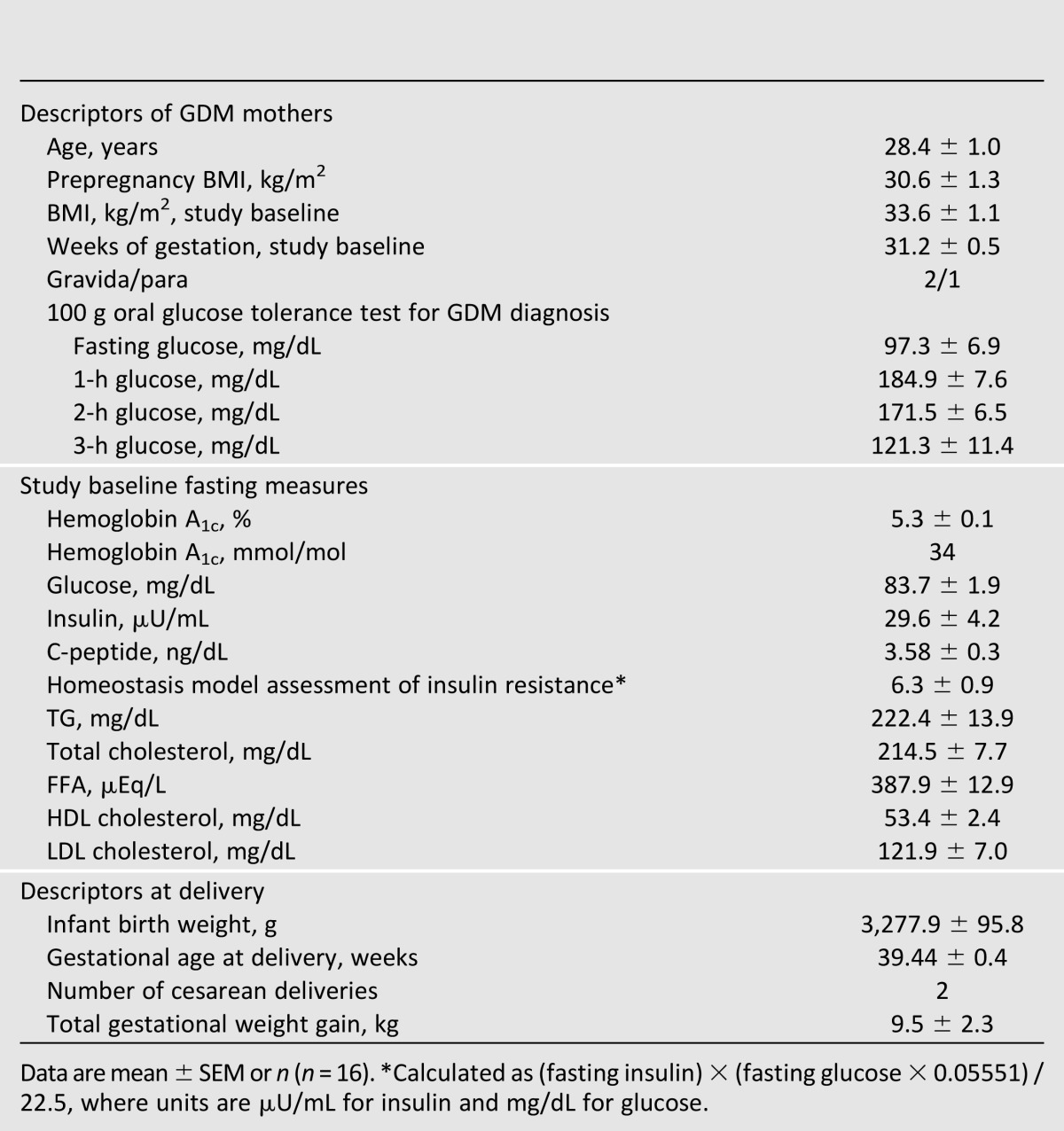
Nineteen women met inclusion criteria and were randomized. Two women were found to have elevated TG during the 12-day protocol and were removed from the study. An additional woman completed the 12-day protocol but was hyperglycemic on all three diets (washout/control, LC/CONV, CHOICE) and required immediate treatment with glyburide (data excluded). Sixteen women successfully completed the 12-day protocol, thereby receiving both study diets so each could serve as her own control. Eight of the 16 women were nulliparous.
All enrolled women were effectively managed with diet alone from GDM diagnosis until delivery (Table 1). As a group, their baseline BMI was 33.6 ± 1.1 kg/m2. Of the 16 women, 5 were Hispanic, 1 was Asian, 1 was African American, and 9 were Caucasian. During the 12-day protocol, all women were weight stable. The Pregnancy Physical Activity Questionnaire showed that they did not engage in rigorous physical activity. Each woman delivered a healthy full-term infant (38–41 weeks). Period effects were tested for each outcome variable, and none were statistically significant (each P > 0.05), suggesting that there were no systematic differences between the two periods of the trial other than the dietary differences.
Table 1.
Characteristics of women with diet-controlled GDM
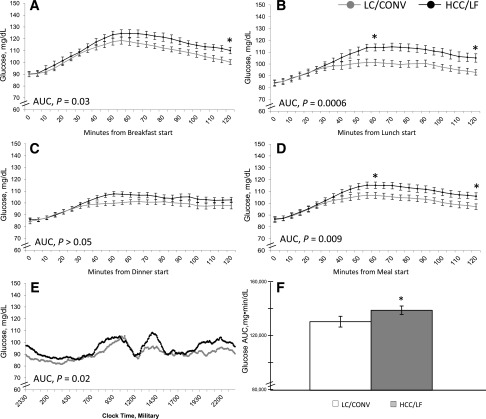
Patterns of Glycemia by CGMS
The CGMS revealed that there were no between-diet differences within the women for fasting or preprandial glucose (Table 2). When meals were considered individually, the 1-h postprandial glucose was higher on the CHOICE diet for lunch only (HCC/LF 115 ± 3 vs. LC/CONV 101 ± 3 mg/dL; P ≤ 0.001) (Fig. 1B), but not for breakfast or dinner. The 2-h postprandial glucose was higher on CHOICE for breakfast (HCC/LF 111 ± 4 vs. LC/CONV 99 ± 3 mg/dL; P ≤ 0.01) (Fig. 1A) and lunch (HCC/LF 104 ± 3 vs. LC/CONV 93 ± 3 mg/dL; P ≤ 0.001) (Fig. 1B), but not dinner. All values on both diets were lower than the current recommended glycemic targets (<140 mg/dL at 1 h, <120 mg/dL at 2 h) (1). When meals were considered together as a mean across three meals, both 1- and 2-h postprandial glucose were modestly higher on the CHOICE diet (1 h, HCC/LF 115 ± 2 vs. LC/CONV 107 ± 3 mg/dL, P ≤ 0.01; 2 h, HCC/LF 106 ± 3 vs. LC/CONV 97 ± 3 mg/dL, P = 0.001) (Fig. 1D). The 2-h postprandial glucose AUC was moderately higher for breakfast and lunch on CHOICE (HCC/LF 13,597 ± 346 vs. LC/CONV 12,894 ± 306 breakfast, P = 0.03 [Fig. 1A]; HCC/LF 12,613 ± 320 vs. LC/CONV 11,586 ± 335 lunch, P = 0.0006 [Fig. 1B]), but not for dinner (Fig. 1C). The 2-h postprandial glucose AUC across meals was modestly higher on CHOICE versus the LC/CONV diet (HCC/LF 12,780 ± 337 vs. 12,086 ± 325 mg·min/dL; P = 0.009) (Fig. 1D). There was no significant difference in the time to postprandial glucose peak between the diets.
Table 2.
CGMS-derived glucose variables within 16 women during 3 days each of diet treatment (LC/CONV vs. HCC/LF CHOICE, in random order)
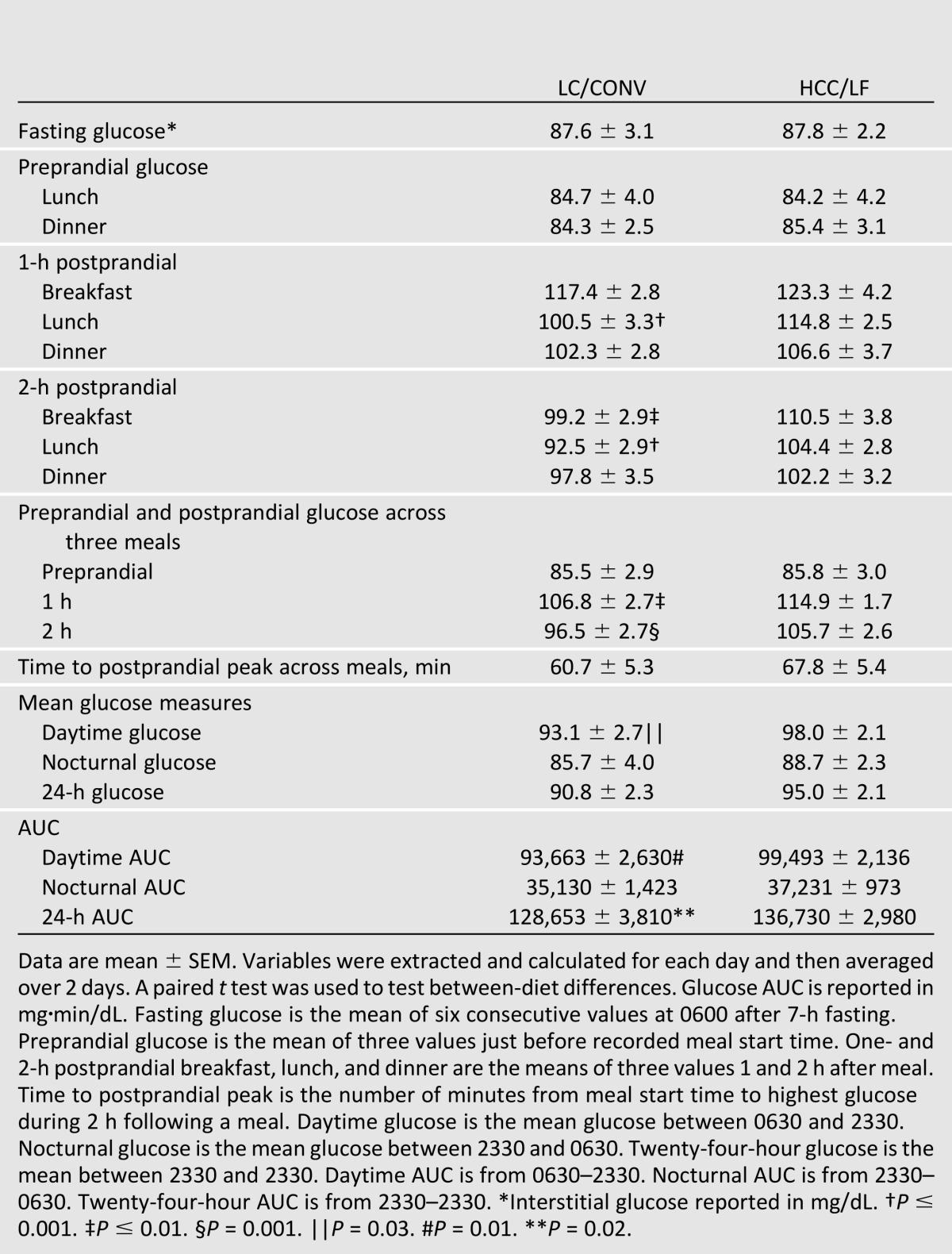
Figure 1.
CGMS data portraying patterns of glycemia within 16 pregnant women with GDM during 3 days each of diet treatment (LC/CONV [gray] vs. HCC/LF CHOICE [black]). Two-hour postprandial glucose response for (A) breakfast, (B) lunch, and (C) dinner and (D) average of three meals (mean over 2 days). Data are aligned for meal start times. (E and F) Twenty-four-hour pattern of glycemia and glucose AUC with difference between diets. Data are aligned for time beginning at 2330; not aligned for meals although women consumed meals during similar time frames. Mean ± SEM. *, P < 0.05.
We additionally used the CGMS to derive mean glucose measures and total AUC. While there were no between-diet differences within the women on mean nocturnal and 24-h glucose, the daytime mean glucose was slightly higher on CHOICE compared with LC/CONV (98 ± 2 vs. 93 ± 3 mg/dL, respectively; P = 0.03) (Table 2). The nocturnal glucose AUC was not different between diets, while the daytime glucose AUC was higher on CHOICE compared with LC/CONV (99,493 ± 2,136 vs. 93,663 ± 2,630 mg·min/dL, respectively; P = 0.01), as was the 24-h total glucose AUC by ∼6% (136,730 ± 2,980 vs. 128,653 ± 3,810 mg·min/dL, respectively; P = 0.02) (Fig. 1E and F, Table 2). Despite these modest differences, the patterns of glycemia were remarkably similar (Fig. 1E) and were well within current treatment targets for daytime, nocturnal, postprandial, and mean glycemia.
Postprandial Plasma/Serum
In response to breakfast, glucose (Fig. 2A), C-peptide (data not shown), and insulin (Fig. 2B) AUC were significantly higher on CHOICE compared with LC/CONV (glucose, 30,000 ± 574 vs. 27,323 ± 602 mg·min/dL, P ≤ 0.001; C-peptide, 2,121 ± 132 vs. 1,709 ± 93 ng·min/dL, P ≤ 0.001; insulin, 29,374 ± 2,973 vs. 20,713 ± 2,344 µU·min/mL, P = 0.0001; respectively). There were no between-diet differences in the TG AUC (Fig. 2C). However, the FFA AUC was significantly lower on CHOICE by 19% compared with LC/CONV (93,684 ± 6,153 vs. 115,449 ± 6,856 µEq·min/L, respectively; P ≤ 0.01) (Fig. 2D). As shown, postprandial plasma glucose was within current treatment targets at both 1 and 2 h after breakfast.
Figure 2.
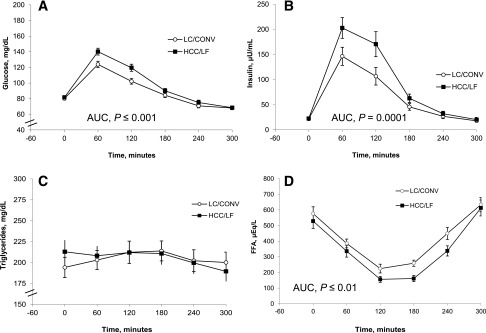
(A–D) Five-hour postprandial response to breakfast (30% of total EI) within 16 diet-controlled women with GDM. The meal study took place on the research unit after 3 days each of diet treatment (LC/CONV versus HCC/LF CHOICE). Data are plasma/serum, mean ± SEM. EI, energy intake.
Conclusions
This prospective, randomized crossover trial demonstrated for the first time that a short-term diet with liberalized complex carbohydrate and limited fat (CHOICE) effectively controlled maternal glycemia to within current therapeutic targets and significantly reduced postprandial FFA. This is important because higher FFA may worsen maternal insulin resistance (8), and maternal lipids (TG/FFA) have further been associated with excess offspring growth (13). A highly controlled randomized crossover design in which all meals were provided was used in order to clarify the effects of the two diets within the same woman. The study was limited to 12 days so that women would be exposed to each diet during a finite period of time to minimize the effect of increasing insulin resistance with advanced gestation (24,25). We hypothesized that even if the CHOICE diet resulted in modestly higher postprandial glucoses, the 24-h glucose AUC, which reflects total potential fetal glucose exposure, would be minimally different and all glycemic profiles would meet current therapeutic targets. Further, we postulated that postprandial lipids would be lower on CHOICE compared with the LC/CONV diet. Our intent was that if the CHOICE diet was well-tolerated and met short-term glycemic targets, we would embark on a longer trial for the remainder of pregnancy requiring greater resources in order to examine maternal and infant outcomes with prolonged dietary exposure.
Using both a CGMS and plasma sampling, we provide evidence that the CHOICE diet effectively and safely controlled maternal glycemia. The plasma glucose response to breakfast was characterized in a highly controlled setting, while CGMS was used to characterize postprandial glucose on the controlled diet over 24 h, but in the free-living situation. By a CGMS across meals, CHOICE compared with the LC/CONV diet resulted in slightly higher 1- and 2-h postprandial glucose (115 vs. 107 mg/dL [rounded] at 1 h and 106 vs. 97 mg/dL at 2 h, respectively, [Table 2]), but values on both diets were well within the recommended postprandial targets of <140 mg/dL (1 h) and <120 mg/dL (2 h) (1) (Fig. 1D). During the breakfast test meal, the GIs were low and similar between diets, implying that the modest difference in glycemic response may be explained by exposure to carbohydrate-rich foods. In our recent review of data describing glycemia in normal pregnancy, we were impressed that patterns of glycemia are perhaps lower than previously appreciated (26). Although the 24-h glucose AUC was slightly higher on the CHOICE diet (Fig. 1E and F), there was no difference in nocturnal or fasting glucose (88 mg/dL, both diets), and the patterns were remarkably similar on both diets (Fig. 1E). Whether this statistically significant difference in 24-h glucose AUC is clinically meaningful and sustainable for the duration of pregnancy requires further study. In this study, the mean 24-h glucose on both the CHOICE and the LC/CONV diet (Table 2) fell within the range of 87–104 mg/dL (95.0 ± 2.1 vs. 90.8 ± 2.3 mg/dL, respectively; P > 0.05), which has been observed to minimize both small-for-gestational-age and large-for-gestational-age (LGA) risk (27). Thus our primary hypothesis that liberalizing complex carbohydrate intake and lowering fat would achieve glucose levels within the recommended therapeutic targets was confirmed.
A second goal of this study was to challenge the historic practice of limiting carbohydrate intake in GDM, which focuses only on control of maternal glucose (6). This is salient given increasing evidence from our group and others demonstrating that maternal TG, which is sensitive to dietary fat intake, can be hydrolyzed and FFA transported across the placenta as important substrates for fetal fat accretion (13,14,28). We have observed GDM women to be so fearful of macrosomia that they replace carbohydrate with primarily fat-rich foods in hopes of blunting their postprandial glucose excursion and avoiding medical treatment. Originally, data from nonrandomized trials supported carbohydrate restriction (16) by demonstrating blunted insulin secretion in response to a high-SFA test meal (29) and less need for insulin therapy with carbohydrate intake <42% (30). However, carbohydrate restriction to <39% has been linked with higher infant birth weight (31). In our recent systematic review of prospective RCTs of diet interventions in GDM over the past 30 years (32), we found that only six studies (250 women across 4 countries) met criteria for inclusion due to lack of compliance and confounding insulin use. A remarkable finding across these RCTs was that improvements in glucose tolerance were seen in as little as 4 days (33) and that women tolerated HCC/low-GI diets (55–70% carbohydrate) (19,33,34). In fact, higher unrefined/complex carbohydrate diets effectively blunted postprandial glycemia (33), reduced the need for insulin therapy (19), lowered fasting LDL cholesterol (33,35) and FFA (33), and improved insulin sensitivity (36), A1C (35) and systolic blood pressure (35). Moreover, a recent RCT demonstrated that carbohydrate restriction did not reduce the need for insulin therapy (37). Our data are not confounded by the use of medications and support the idea that women with diet-controlled GDM are able to tolerate a more liberal amount of complex, low-GI carbohydrate and still maintain glycemic control within the recommended targets.
In recent years, provocative data have demonstrated a positive correlation between maternal lipids and LGA (13,14). Interestingly, studies in which GDM women were randomized to insulin based on ultrasound evidence of excess fetal growth despite normal blood glucose (“fetal-based strategy”) have shown a decrease in LGA (38). It is possible this may be due to greater insulin suppression of lipolysis, resulting in less fetal FFA availability and attenuation of excess growth, rather than simply decreasing blood glucoses. Although TG excursions were similar (Fig. 2C) between the two diets in this study, the 5-h postprandial FFA AUC was 19% lower on CHOICE versus LC/CONV (Fig. 2D). The higher FFA AUC on LC/CONV could provide excess fuel for fetal growth during the fed state in these women. The slightly higher postprandial insulin (Fig. 2B) following the CHOICE breakfast meal not only mitigated postprandial glucose (Fig. 2A), but also suppressed lipolysis, resulting in a significantly lower 5-h FFA AUC (Fig. 2D). Therefore, short-term treatment with a HCC/LF diet resulted in slightly higher postprandial glycemia (Fig. 2A) but appears to have an advantage over LC/CONV on FFA concentrations. One could argue that increasing maternal postprandial insulin levels are concerning (if persistent) because they may further stress the β-cells, increasing risk of future type 2 diabetes (39). However, it is important to point out that postprandial insulin on CHOICE returned to baseline and was no different than LC/CONV at 3 h, likely due to the high-quality carbohydrate content (rather than simple sugars).
Results from prolonged treatment with these diets on maternal insulin resistance and fetal growth (particularly infant adiposity) are clearly necessary. The classic glucocentric Pedersen hypothesis was expanded in 1980 by Freinkel (40) to include the concept that maternal substrates leading to increased fetal growth include TG, FFA, and amino acids, with maternal insulin resistance proposed to be the gatekeeper of maternal–fetal substrate flux across the placenta. It is thought that women with GDM begin pregnancy on a “background” of chronic insulin resistance (24,25), on which the effects of pregnancy-induced insulin resistance are additive. If continued exposure to a diet higher in fat-rich foods results in greater insulin resistance, it is possible that a diet higher in complex carbohydrate-rich/lower in fat-rich foods that improves insulin sensitivity might attenuate the increasing insulin resistance of pregnancy, result in less β-cell insulin secretion demand, and limit fetal substrate availability. Preliminary data from the second phase of this investigation (which continued the second diet assignment per randomization through delivery) suggested an overall reduction in measures of insulin resistance and neonatal adiposity following prolonged exposure to the CHOICE diet (41). These preliminary data require confirmation in a larger sample size.
A limitation of this investigation is its short duration, highly controlled diet exposure, and small sample size. However, the study was appropriately powered on the main outcome using a crossover design, allowing exposure to both diets. The LC/CONV diet (40% carbohydrate/45% fat) was recreated based on published reports (5,16), and the CHOICE diet (60% complex carbohydrate/25% fat) was designed to be different enough to challenge that prescription but remain within guidelines outside of pregnancy (17). The current lack of consensus in diet recommendations is striking: the 2013 ACOG Practice Bulletin references the low-carbohydrate diet (33–40%) for GDM women (6), the Fifth International ADA Workshop withdrew macronutrient recommendations for GDM women (1), the 2013 ADA diet recommendations for nonpregnant individuals with diabetes do not advocate for a specific macronutrient content (42), and the American Heart Association recently endorsed a macronutrient prescription similar to our CHOICE diet for nonpregnant individuals (18). All of the panels recommend minimizing simple sugars and SFAs and increasing fiber. Simple sugars and fat were tightly controlled, and the diets were isocaloric in this study, theoretically making them healthier compared with diet options in the free-living environment.
This is the first highly controlled diet study in women with GDM in which a randomized crossover design was implemented to provide isocaloric diets while using CGMS technology. The data support a diet that liberalizes total complex (lower GI) carbohydrate intake and lowers fat intake. The reassuring glycemic and lipemic profiles support the need for more definitive, adequately powered prospective RCTs of longer duration to determine the effects of diet on maternal insulin resistance, pregnancy outcomes, and fetal growth. As the incidence of GDM continues to rise globally, it is critical that we carefully design RCTs to test which diet most effectively controls glycemia, favorably affects lipids, attenuates excess fetal growth, and minimizes expensive medical treatment.
Article Information
Acknowledgments. The authors acknowledge the support of Robert H. Eckel, Bryan R. Haugen, and Catherine Chartier-Logan (Department of Medicine, University of Colorado); Jill Davies (Department of Obstetrics and Gynecology, University of Colorado); William R. Hay (Department of Pediatrics, University of Colorado); Coni Francis, Therese Ida, Archana Mande, and Susan P. Gross (Colorado Clinical Translational Research Institute); Elizabeth Long (University of Colorado Hospital); and the women participants on whom the success of this study rests. The authors finally acknowledge Janine Higgins and her outstanding team in the University of Colorado CTRC Bionutrition Department: Ann Wilson, Iris Williams, Gary Smith, Russell Rosborough, and Lois Nuechterlein.
Funding. The authors acknowledge funding support from the NIH (R21 DK 088324), the University of Colorado Diabetes and Endocrinology Research Center, the NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Research Institute (UL1 TR000154), the Colorado Nutrition and Obesity Research Center (NORC, NIH P30 DK 048520-15), and the Colorado Program for Nutrition and Healthy Development.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.L.H. and L.A.B. researched data and wrote, reviewed, and edited the manuscript. R.E.V.P., M.A.A., N.A.W., and J.E.F. researched data, reviewed and edited the manuscript, and contributed to discussion. L.J.D. and W.T.D. researched data and reviewed and edited the manuscript. T.L.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01719029, clinicaltrials.gov.
References
- 1.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl. 2):S251–S260 [DOI] [PubMed] [Google Scholar]
- 2.Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy Study Groups Consensus Panel International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks DA, Hadden DR, Maresh M, et al. HAPO Study Cooperative Research Group Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012;35:526–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health. National Institutes of Health Consensus Development Conference: Diagnosing Gestational Diabetes Mellitus, 2013. Available from http://prevention.nih.gov/cdp/conferences/2013/gdm/resources.aspx
- 5.Jovanovic-Peterson L, Peterson CM. Dietary manipulation as a primary treatment strategy for pregnancies complicated by diabetes. J Am Coll Nutr 1990;9:320–325 [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Gestational Diabetes Mellitus Vol. 137. Washington, DC, American College of Obstetricians and Gynecologists, 2013, p. 1-11 [Google Scholar]
- 7.Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev 2005;6:133–142 [DOI] [PubMed] [Google Scholar]
- 8.Lichtenstein AH, Schwab US. Relationship of dietary fat to glucose metabolism. Atherosclerosis 2000;150:227–243 [DOI] [PubMed] [Google Scholar]
- 9.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009;119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol 2010;299:R711–R722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frias AE, Morgan TK, Evans AE, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 2011;152:2456–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012;150:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whyte K, Kelly H, O’Dwyer V, Gibbs M, O’Higgins A, Turner MJ. Offspring birth weight and maternal fasting lipids in women screened for gestational diabetes mellitus (GDM). Eur J Obstet Gynecol Reprod Biol 2013;170:67–70 [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen KM, Yaktine AL, eds.; Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines Washington, DC, National Academies Press, 2009, p. 1–250 [PubMed]
- 16.Peterson CM, Jovanovic-Peterson L. Percentage of carbohydrate and glycemic response to breakfast, lunch, and dinner in women with gestational diabetes. Diabetes 1991;40(Suppl. 2):172–174 [DOI] [PubMed] [Google Scholar]
- 17.Franz MJ, Bantle JP, Beebe CA, et al. American Diabetes Association Nutrition principles and recommendations in diabetes. Diabetes Care 2004;27(Suppl. 1):S36–S46 [DOI] [PubMed] [Google Scholar]
- 18.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 12 November 2013 [Epub ahead of print]24222015 [Google Scholar]
- 19.Moses RG, Barker M, Winter M, Petocz P, Brand-Miller JC. Can a low-glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care 2009;32:996–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez TL, Barbour LA. A standard approach to continuous glucose monitor data in pregnancy for the study of fetal growth and infant outcomes. Diabetes Technol Ther 2013;15:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmon KA, Gerard L, Jensen DR, et al. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care 2011;34:2198–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc 2004;36:1750–1760 [DOI] [PubMed] [Google Scholar]
- 23.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr 2003;78:734–741 [DOI] [PubMed] [Google Scholar]
- 24.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 1999;180:903–916 [DOI] [PubMed] [Google Scholar]
- 25.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007;30(Suppl. 2):S112–S119 [DOI] [PubMed] [Google Scholar]
- 26.Hernandez TL, Friedman JE, Van Pelt RE, Barbour LA. Patterns of glycemia in normal pregnancy: should the current therapeutic targets be challenged? Diabetes Care 2011;34:1660–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langer O, Mazze R. The relationship between large-for-gestational-age infants and glycemic control in women with gestational diabetes. Am J Obstet Gynecol 1988;159:1478–1483 [DOI] [PubMed] [Google Scholar]
- 28.Barbour LA, Hernandez TL, Reece MS, et al. Change in fasting triglycerides from early to late gestation are highly predictive of neonatal adiposity independent of maternal BMI (Abstract). Diabetes 2009;58:A84 [Google Scholar]
- 29.Ilic S, Jovanovic L, Pettitt DJ. Comparison of the effect of saturated and monounsaturated fat on postprandial plasma glucose and insulin concentration in women with gestational diabetes mellitus. Am J Perinatol 1999;16:489–495 [DOI] [PubMed] [Google Scholar]
- 30.Major CA, Henry MJ, De Veciana M, Morgan MA. The effects of carbohydrate restriction in patients with diet-controlled gestational diabetes. Obstet Gynecol 1998;91:600–604 [DOI] [PubMed] [Google Scholar]
- 31.Romon M, Nuttens MC, Vambergue A, et al. Higher carbohydrate intake is associated with decreased incidence of newborn macrosomia in women with gestational diabetes. J Am Diet Assoc 2001;101:897–902 [DOI] [PubMed] [Google Scholar]
- 32.Hernandez TL, Anderson MA, Chartier-Logan C, Friedman JE, Barbour LA. Strategies in the nutritional management of gestational diabetes. Clin Obstet Gynecol 2013;56:803–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nolan CJ. Improved glucose tolerance in gestational diabetic women on a low fat, high unrefined carbohydrate diet. Aust N Z J Obstet Gynaecol 1984;24:174–177 [DOI] [PubMed] [Google Scholar]
- 34.Louie JC, Markovic TP, Perera N, et al. A randomized controlled trial investigating the effects of a low-glycemic index diet on pregnancy outcomes in gestational diabetes mellitus. Diabetes Care 2011;34:2341–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. Br J Nutr 2013;109:2024–2030 [DOI] [PubMed] [Google Scholar]
- 36.Lauszus FF, Rasmussen OW, Henriksen JE, et al. Effect of a high monounsaturated fatty acid diet on blood pressure and glucose metabolism in women with gestational diabetes mellitus. Eur J Clin Nutr 2001;55:436–443 [DOI] [PubMed] [Google Scholar]
- 37.Moreno-Castilla C, Hernandez M, Bergua M, et al. Low-carbohydrate diet for the treatment of gestational diabetes mellitus: a randomized controlled trial. Diabetes Care 2013;36:2233–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaefer-Graf UM, Kjos SL, Fauzan OH, et al. A randomized trial evaluating a predominantly fetal growth-based strategy to guide management of gestational diabetes in Caucasian women. Diabetes Care 2004;27:297–302 [DOI] [PubMed] [Google Scholar]
- 39.Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 2006;55:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980;29:1023–1035 [DOI] [PubMed] [Google Scholar]
- 41.Hernandez TL, Anderson MA, van Pelt RE, et al. Women with gestational diabetes randomized to a low-carbohydrate/higher fat diet demonstrate greater insulin resistance and infant adiposity (Abstract). Diabetes 2013;62:A19 [Google Scholar]
- 42.Evert AB, Boucher JL, Cypress M, et al. American Diabetes Association Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013;36:3821–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]