Abstract
In this two-part Bench to Clinic narrative, recent advances in both the preclinical and clinical aspects of artificial pancreas (AP) development are described. In the preceding Bench narrative, Kudva and colleagues provide an in-depth understanding of the modified glucoregulatory physiology of type 1 diabetes that will help refine future AP algorithms. In the Clinic narrative presented here, we compare and evaluate AP technology to gain further momentum toward outpatient trials and eventual approval for widespread use. We enumerate the design objectives, variables, and challenges involved in AP development, concluding with a discussion of recent clinical advancements. Thanks to the effective integration of engineering and medicine, the dream of automated glucose regulation is nearing reality. Consistent and methodical presentation of results will accelerate this success, allowing head-to-head comparisons that will facilitate adoption of the AP as a standard therapy for type 1 diabetes.
Introduction
From the time of closed-loop hospital setting studies in the 1970s (1), the automation of blood glucose (BG) control has been a grand challenge for type 1 diabetes treatment, i.e., the artificial pancreas (AP). The inconvenience of early intravenous BG sensing and insulin delivery motivated the development of subcutaneous (SC) continuous glucose monitors (CGM) and continuous SC insulin infusion (CSII) pumps, which remain the most widely used platforms for AP development. Initially, software algorithms utilizing CGM and CSII pumps operated with the goal of insulin suspension to prevent nocturnal hypoglycemia. Recent clinical trials have extended this approach with process control algorithms, often augmented with compensatory manual insulin boluses, to handle more complex challenges, such as meals and exercise. The application of advanced control algorithms has led to substantial improvements in BG control. The robustness of these systems has been verified by over 40 clinical studies in the last decade. Clinical trial protocols that will bring the AP from bench to clinic must, by necessity, move away from sedentary, clinic-based trials toward those that mimic everyday life, highlighting the need for standardization in reporting and verification protocols to allow for objective comparison of results.
Engineering Translation of Type 1 Diabetes Treatment
This article provides a discussion of the engineering design required for the AP to move from bench to clinic. Defining the design problem involves identifying key features, including medical objectives, physiologic variables, subject challenges, and system limitations. As is the case in most engineering problems, there is not one absolute solution, but several different options, each with advantages and disadvantages. Throughout the following sections, the engineering elements of clinically tested AP designs are reviewed.
Defining Design Objectives
The design objectives must be defined to meet the needs of the intended user, which will result in a controller design that is tailored to the target population. For example, children can benefit greatly from an AP because they do not have the capability to manage their own therapy and must rely on parental supervision; however, they are considered a high-risk group, so the AP will face more stringent safety requirements. Clinical studies that have been completed in the population of young children have tended to focus on nocturnal hypoglycemia prevention (2–6); thus, if these trials included meals, they used a manual bolus to avoid postprandial hyperglycemia. On the other hand, adults who suffer from chronic hyperglycemia due to the inability to consistently and accurately bolus for meals would benefit from a fully automated AP that required no user intervention. Consequently, the most appropriate objective should be chosen, such as maximization of time spent within a desired range, minimization of hypoglycemic events, prevention of postprandial hyperglycemia, or minimization of required patient intervention.
Controller Design Variables
Given a set of design objectives, a suitable controller can be designed. Nearly all of the possible design configurations can be represented by the generalized feedback control architecture shown in Fig. 1. For each of the components in this architecture, there are multiple options that allow optimized performance according to the design objectives. The options listed in Fig. 1 are representative of AP designs that have reached the clinical testing phase.
Figure 1.
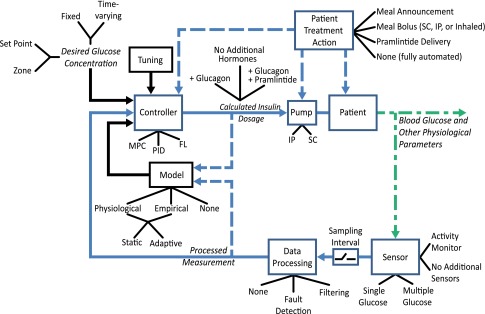
Taxonomy of the AP design. A specific AP configuration is created by selecting options for each of the major elements shown in the figure. Solid lines demonstrate connections that are always present and dashed lines represent connections that may only be present in some configurations. The tuning, model, and desired glucose concentration are all part of the controller, as signified by the black arrows. Green color distinguishes physiological states or properties from measured or digital signals. Black lines are used to indicate predetermined features of a block, and blue lines indicate signals or actions conducted during closed-loop operation.
The first option that must be specified is the desired BG. This can be either a specific value or a zone, and it can be fixed or time-varying. The controller receives the measured BG and compares it to the desired concentration. It may also incorporate a model of the BG as part of its algorithm. As shown in Table 1, several control algorithms have been tested, including model predictive control (MPC), proportional-integral-derivative control (PID), and fuzzy logic control (FL). The tuning of the control algorithm determines how aggressively it will react to BG deviations from the desired value. The control algorithm generates an output for insulin delivery, and may also calculate glucagon, pramlintide, and/or additional glucoregulatory hormone delivery. The controller output is calculated at discrete times determined by the controller action interval. The signal is then communicated to the pump, which may be SC or intraperitoneal (IP). The controller output and resulting glucose measurements may be used to update the controller or model parameters through adaptation, as demonstrated in run-to-run approaches (7) and adaptive learning schemes (8). The pump then delivers the calculated hormone doses to the patient.
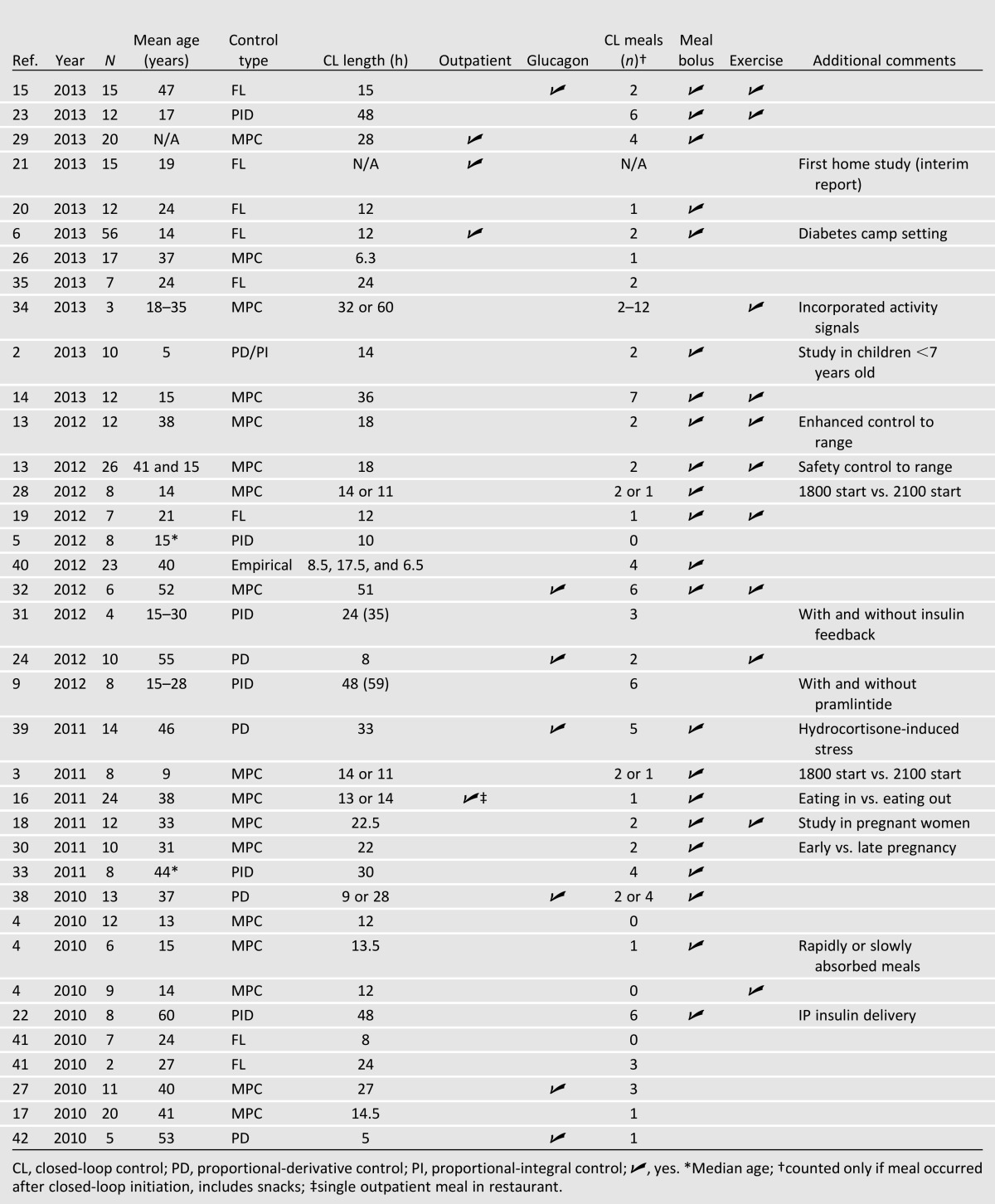
Table 1.
Summary of clinical trial protocols from 2010 to 2013
The glucose concentration is measured by one or more glucose sensors at a specified time interval. Sensors to measure other physiological information (e.g., heart rate, accelerometry, skin temperature) may also be included (34). The sensor signals are processed by filters or fault detection algorithms before being communicated to the controller, and then the entire process is repeated at the next sample time.
The patient may or may not be directly involved in the control loop. In some designs, the patient is required to make a meal announcement including the size of the meal consumed. The controller may then use that information to deliver a full or partial bolus. In other designs, the patient does not announce the meal but calculates and delivers a meal bolus manually. This bolus is delivered using an external pump to the SC or IP space. One study included manual preprandial pramlintide delivery (9). Better control can often be achieved with the addition of a meal bolus or announcement due to delays in insulin action after SC insulin delivery and glucose sensing. Since these additions require that a human is involved in a control loop, there is a higher potential for safety concerns, given the unpredictable nature of human behavior. The trade-off between the degree of automation and the control performance is a critical issue in current AP design and clinical testing.
Challenges and Limitations on Controller Design
Most studies use commercially available glucose sensors and insulin pumps, and thus are constrained by their features and performance. For example, it is inconvenient to place more than one sensor on a person for use in daily life, even though extra sensors might improve system robustness. New sensors may be developed that are smaller or consist of an array of multiple sensors, allowing for redundant glucose measurements to overcome sensor malfunctions. The AP also relies on currently available insulin, glucagon, and pramlintide formulations. Glucagon is often difficult to work with due to its inability to remain stable in solution, although there has been research into creating improved glucagon formulations (10). The controller must be implemented using available laptops, tablets, smartphones, or other small computer devices. Battery life is an important factor to consider for all of these options, especially when Bluetooth or other wireless communication is needed. As current AP designs rely on existing commercial devices, it is important for such designs to be robust against difficulties that arise due to the communication between different hardware components, as well as to include failure modes in case of problems such as signal interruptions due to transmission or intrinsic sensor loss. Moreover, it is critical for current systems to demonstrate the ability to manage these problems during their validation steps prior to clinical implementation. However, all of these issues are symptoms of technology development, and future APs will be based on integrated devices with an embedded controller that will eliminate the communication overhead and excessive power requirements.
Some challenges are immutable because they are inherent to human physiology. For example, insulin cannot be removed from the body once it has been delivered; thus, controllers must be designed to account for insulin on board (11). Additionally, insulin sensitivity varies due to factors such as time of day, stress, and exercise, so controllers must be sufficiently robust to this variation. The companion review article by Kudva et al. (12) provides further explanation of the need for physiological inputs in control algorithms to achieve superior glucose regulation.
Recent Advancements in Clinical Trials
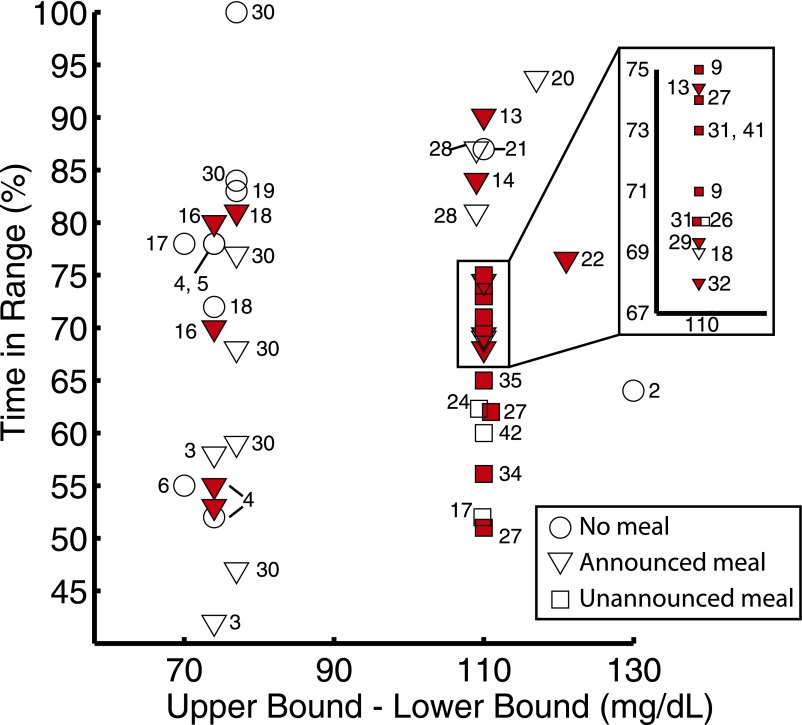
Clinical testing of an AP system has been reported in over 40 publications since 2004, although this article emphasizes those published since 2010. The recent growth in clinical studies by many groups within a short period of time has meant that there were no clear precedents set for trial designs. With the exception of overnight-only control, no two studies have followed the same protocol for closed-loop initiation, meal size and timing, and exercise. In addition, results are often reported as percent of time in hyperglycemic, hypoglycemic, and euglycemic ranges, but there is no consistent definition of these intervals. Figure 2 provides a summary of the results of trials conducted from 2010 to 2013. Each published trial has been a success in that it has met its own objective; however, due to protocol variations and the lack of a standardized metric to measure AP performance, it is difficult to directly compare results across different studies without resorting to qualitative descriptions. Still, the limited analyses and comparisons that can be done indicate encouraging progress over time. The number of trials published per year has been steadily increasing, and the average percentage of closed-loop time maintained within the euglycemic range (using 70–180 mg/dL) has remained near 70%, despite later studies incorporating more difficult challenges into the protocol. Most importantly, studies that have been designed to compare control by the AP to conventional treatment have summarily concluded that AP control is the same or better than conventional therapy (2,4–6,13–24). Table 1 shows a selection of details from published clinical studies spanning 2010–2013. Please refer to the online database at www.thedoylegroup.org/APdatabase for a searchable database of published clinical trials from 2004 to the present.
Figure 2.
Summary of the percentage of time in desired range reported by studies published in the years 2010–2013. The results are plotted against the size of the desired range (upper bound − lower bound), with all studies but one using between 63 and 71 mg/dL as the lower bound (59 mg/dL in ref. 22). A circle, triangle, or square indicates that there were no meals, announced meals, or meals with no announcement/prebolus, respectively, during the time period used to calculate the percent time in range. A red shaded icon indicates that the time period used to calculate percent time in range was longer than 12 h. Multiple icons are used for a single study if there were different protocol branches or if results were reported only for discrete segments of the study (e.g., overnight and postprandial results reported separately).
Control Algorithms
MPC and PID algorithms have undergone the most extensive evaluation through clinical trials, with 14 and 6 studies, respectively. Applications of both controller types have shown strong performance across all metrics, with MPC controllers having slightly fewer instances of hypoglycemia per subject and PID controllers having slightly greater time in range for a wide variety of experimental conditions. Both controller types maintained, on average, 71% of the experimental period within the euglycemic range from 2010 forward (3–5,9,13,16–18,22,25–34). FL has experienced an increase in usage in recent years (6,15,19,20,35). In fact, FL was one of the first algorithms to be demonstrated in an outpatient camp study (6), and a recent interim analysis has shown successful application of this controller in a home setting (21).
Insulin Delivery Methods
The two routes for insulin delivery that have undergone clinical evaluation in closed-loop trials are SC and IP delivery. SC insulin was most widely used, with all but a few published studies (22,36,37) using this route. As technology improves, the IP route is likely to see an increase in usage because it can eliminate problematic SC delays.
Variations on Meal Challenge and Compensation
Clinical trials were further delineated based on the postprandial hyperglycemia compensation strategy, which remains one of the most difficult challenges in glucose control. The timing and size of meals varied from trial to trial, with some providing more challenging disturbances than others. Studies that included prandial announcement or manual bolus (full or partial) showed the greatest amount of time in range, as shown in Fig. 2 (2–6,13,15,16,18–20,22,25,28–30,32,33,38–40). Fully automated closed-loop control showed a comparatively lower time in range, although these systems require considerably less patient effort (9,17,24,26,27,31,34,35,41,42). The number of fully automated AP designs is increasing, with over 42% of clinical trials in 2013 including at least one unannounced meal challenge (2,6,14,15,20,21,23,26,29,34,35). To date, no studies have incorporated a strategy to explicitly compensate for the effects of noncarbohydrate nutrients on BG concentration. A further understanding of these effects may improve closed-loop control. This idea is explored further in the companion review article by Kudva et al. (12).
Hypoglycemia Prevention
The biggest risk in the AP system is hypoglycemia due to overdelivery of insulin, exercise, or consumption of alcohol. Many AP designs have incorporated safety systems, such as a low glucose prediction module or insulin-on-board calculation (2,5,9,11,13,22,31,33). All studies utilizing a PID controller from 2010 onward have incorporated insulin feedback models to prevent hypoglycemia induced by overdelivery of insulin, which is necessary because PID controllers do not have explicit predictive capabilities (5,9,22,23,31,33). Rescue carbohydrates were administered if hypoglycemia could not be prevented by reduced insulin delivery (or increased glucagon delivery in bihormonal systems); however, the threshold varied anywhere from 50 mg/dL (40) to 90 mg/dL (24). As discussed in Kudva et al. (12), hypoglycemia prevention may be improved by studying its physiological causes and effects, as reduced insulin delivery may be ineffective in preventing hypoglycemia due to a relative excess of insulin. Hypoglycemia prevention remains one area that can use improvement, as a majority of studies reported at least one episode during closed-loop operation. Consequently, it is critical for safety systems supporting the control algorithms to incorporate multiple procedures for hypoglycemia prevention, such as escalating alarms and alerts to the user (43,44), suspension of insulin delivery, recommendation of carbohydrate ingestion, and initiation of glucagon delivery.
Additional Clinical Challenges
Several events have been identified as additional challenges in type 1 diabetes treatment. First, improvement in nocturnal glucose control is a critical area of need due to the danger posed by hypoglycemia during sleep. Thus, it is not surprising that closed-loop AP systems were first tested during overnight periods with the sole purpose of eliminating hypoglycemic events, and indeed, almost every clinical trial of the AP has since included nocturnal BG control as one of its challenges. AP systems have been shown to be robust and effective in prevention of nocturnal hypoglycemic events, with dramatic reductions in hypoglycemia in comparison with basal bolus therapy (6,16,19–21,23,40).
Exercise is the next widely tested challenge, with 11 studies reporting an exercise component and all such trials reporting successful management of the challenge (4,13–15,18,19,23–25,29,32,34). Since exercise does not follow a universal regimen, it is difficult to design a test that will evaluate controller response while still reflecting everyday scenarios. In addition, most studies include the exercise challenge before a meal, which may confound the results by supporting the controller with the additional carbohydrates.
One clinical trial demonstrated successful management of alcohol consumption with an MPC controller (16), and another included adaptation by a bihormonal controller to a stress response induced by hydrocortisone (39). Two studies have shown successful application of an MPC AP system during pregnancy, and one recent study demonstrated improved control in children less than 7 years old (2). Recent studies have begun attempting to modularize the system, with three studies taking place in an outpatient environment (6,21,25,29). Outpatient studies represent an important step forward for AP technology, and their number will increase greatly in the coming years as the AP moves from clinic to the home.
Future Directions
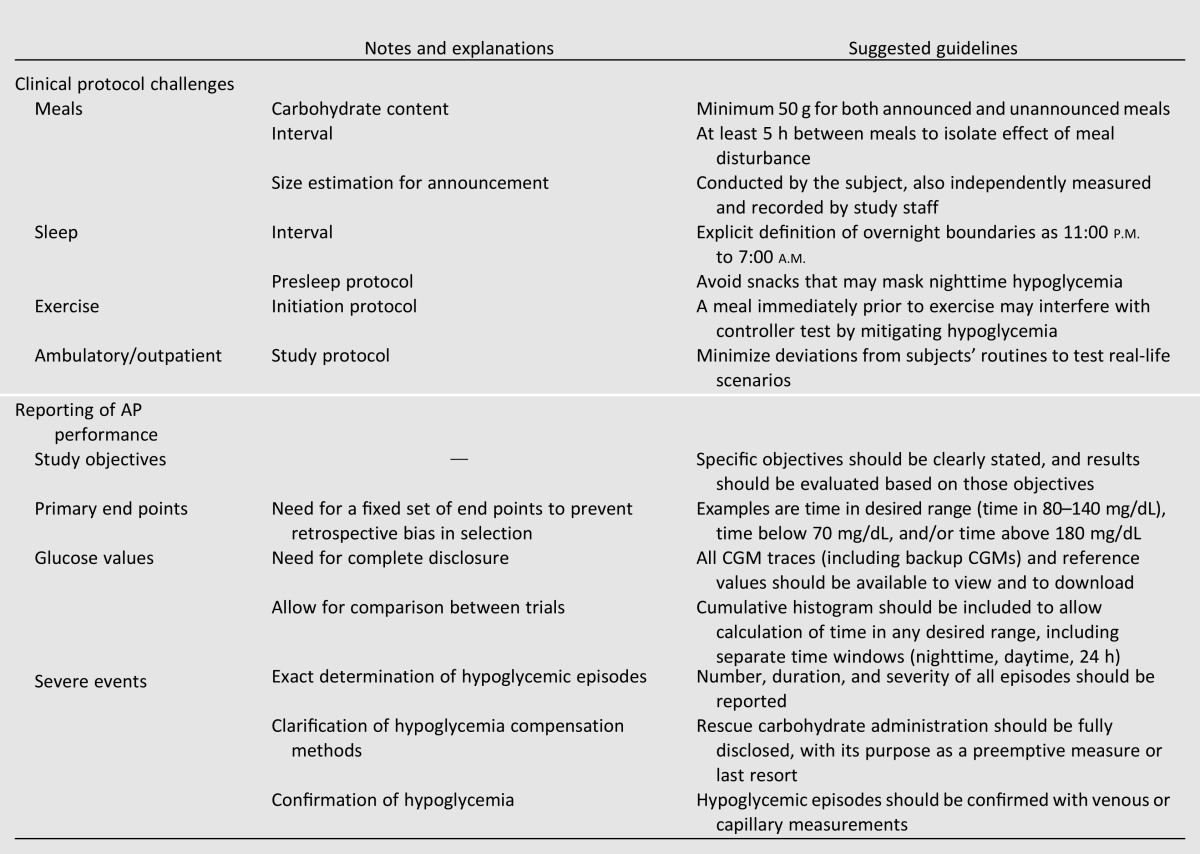
Multiple obstacles remain in the development of the AP. Some of these challenges, such as CGM performance, insulin delays, and robust communication between system components, are related to technological advancements, and must be resolved prior to commercialization of any AP system. These challenges are particularly relevant in the light of U.S. Food and Drug Administration’s recent interest in the AP, as its recently released guidance document addressing the AP also identifies these issues as some of the primary barriers to commercialization (45). Another challenge in the coming years will be to improve controller evaluation in clinic. The variety of protocols and reported metrics makes it difficult to compare results across different AP configurations. As shown in Fig. 2, the desired ranges reported in clinical publications have varied greatly, making it difficult to compare controller performance across trials. Agreement on a standardized definition of the desired euglycemic range is unlikely, as there is no clear choice for such a demarcation. Rather than reporting time in a few selected ranges, the focus should be on presenting data in a way that allows readers to draw their own conclusions. As a central common reporting requirement, the time in range data should be presented as a cumulative histogram, allowing the percent time in any range to be calculated. These cumulative histograms should be reported separately for overnight, postprandial, and 24-h periods. Protocol variations such as meal size, timing, and compensation strategy should be described as clearly as possible to allow for accurate comparison between trials. As trials become longer in length, standardized measurements, such as HbA1c, can be used to report the quality of BG control resulting from AP use. Table 2 represents a compiled list of suggested common requirements for clinical protocol challenges and for reporting of AP performance.
Table 2.
Proposed minimal common requirements for AP clinical trials
Conclusions
AP technology is advancing quickly. The number of publications detailing clinical evaluations of AP devices has increased steadily over the past 4 years and will increase even more rapidly as more designs reach the clinical testing stage. The number of potential controller configurations is high, and the number of potential protocols is even higher, especially as trials move to the outpatient setting. As the scenarios become more complex, it is important to keep the fundamental engineering design considerations in mind. Organizing protocol standards and reporting metrics will ensure that all clinical trials will result in knowledge that can help in the shared goal of developing an AP to improve patient health outcomes.
Article Information
Funding. This work was supported by grants from JDRF (22-2009-797, 22-2009-796, 17-2011-515, 17-2010-765, and 22-2011-637) and the National Institutes of Health (DP3-DK-094331 and R01-DK-085628).
Duality of Interest. H.C.Z. is a consultant for Animas Corp., Cellnovo, Insulet Corp., MannKind Corp., and Hoffmann-La Roche Ltd.; has received research grant and product support from Animas Corp., Abbot Laboratories, Dexcom, Inc., Eli Lilly and Co., GluMetrics, Inc., Insulet Corp., LifeScan, Inc., Medtronic, Inc., Novo Nordisk, Hoffmann-La Roche Ltd., and Sanofi; and is an employee of Insulet Corp. No other potential conflicts of interest relevant to this article were reported.
Footnotes
References
- 1.Marliss EB, Murray FT, Stokes EF, et al. Normalization of glycemia in diabetics during meals with insulin and glucagon delivery by the artificial pancreas. Diabetes 1977;26:663–672 [DOI] [PubMed] [Google Scholar]
- 2.Dauber A, Corcia L, Safer J, Agus MS, Einis S, Steil GM. Closed-loop insulin therapy improves glycemic control in children aged <7 years: a randomized controlled trial. Diabetes Care 2013;36:222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elleri D, Allen JM, Nodale M, et al. Automated overnight closed-loop glucose control in young children with type 1 diabetes. Diabetes Technol Ther 2011;13:419–424 [DOI] [PubMed] [Google Scholar]
- 4.Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 5.O’Grady MJ, Retterath AJ, Keenan DB, et al. The use of an automated, portable glucose control system for overnight glucose control in adolescents and young adults with type 1 diabetes. Diabetes Care 2012;35:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–833 [DOI] [PubMed] [Google Scholar]
- 7.Zisser H, Palerm CC, Bevier WC, Doyle FJ, 3rd, Jovanovič L. Clinical update on optimal prandial insulin dosing using a refined run-to-run control algorithm. J Diabetes Sci Tech 2009;3:487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller S, Nimri R, Atlas E, Grunberg EA, Phillip M. Automatic learning algorithm for the MD-logic artificial pancreas system. Diabetes Technol Ther 2011;13:983–990 [DOI] [PubMed] [Google Scholar]
- 9.Weinzimer SA, Sherr JL, Cengiz E, et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care 2012;35:1994–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner SS, Li M, Hauser R, Pohl R. Stabilized glucagon formulation for bihormonal pump use. J Diabetes Sci Tech 2010;4:1332–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellingsen C, Dassau E, Zisser H, et al. Safety constraints in an artificial pancreatic beta cell: an implementation of model predictive control with insulin on board. J Diabetes Sci Tech 2009;3:536–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudva YC, Carter RE, Cobelli C, Basu R, Basu A. Closed-loop artificial pancreas systems: physiological input to enhance next-generation devices. Diabetes Care 2014;37:1184–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breton M, Farret A, Bruttomesso D, et al. International Artificial Pancreas Study Group Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes 2012;61:2230–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elleri D, Allen JM, Kumareswaran K, et al. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care 2013;36:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haidar A, Legault L, Dallaire M, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ 2013;185:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011;342:d1855 [DOI] [PMC free article] [PubMed]
- 17.Kovatchev B, Cobelli C, Renard E, et al. Multinational study of subcutaneous model-predictive closed-loop control in type 1 diabetes mellitus: summary of the results. J Diabetes Sci Tech 2010;4:1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy HR, Kumareswaran K, Elleri D, et al. Safety and efficacy of 24-h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes: a randomized crossover case series. Diabetes Care 2011;34:2527–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimri R, Atlas E, Ajzensztejn M, Miller S, Oron T, Phillip M. Feasibility study of automated overnight closed-loop glucose control under MD-logic artificial pancreas in patients with type 1 diabetes: the DREAM Project. Diabetes Technol Ther 2012;14:728–735 [DOI] [PubMed] [Google Scholar]
- 20.Nimri R, Danne T, Kordonouri O, et al. The “Glucositter” overnight automated closed loop system for type 1 diabetes: a randomized crossover trial. Pediatr Diabetes 2013;14:159–167 [DOI] [PubMed] [Google Scholar]
- 21.Nimri R, Muller I, Atlas E, et al. Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis. Pediatr Diabetes. 15 August 2013 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care 2010;33:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherr JL, Cengiz E, Palerm CC, et al. Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care 2013;36:2909–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Bon AC, Jonker LD, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a bihormonal closed-loop system to control postexercise and postprandial glucose excursions. J Diabetes Sci Tech 2012;6:1114–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cobelli C, Renard E, Kovatchev BP, et al. Pilot studies of wearable outpatient artificial pancreas in type 1 diabetes. Diabetes Care 2012;35:e65–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dassau E, Zisser H, Harvey RA, et al. Clinical evaluation of a personalized artificial pancreas. Diabetes Care 2013;36:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2010;2:27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elleri D, Allen JM, Biagioni M, et al. Evaluation of a portable ambulatory prototype for automated overnight closed-loop insulin delivery in young people with type 1 diabetes. Pediatr Diabetes 2012;13:449–453 [DOI] [PubMed] [Google Scholar]
- 29.Kovatchev BP, Renard E, Cobelli C, et al. Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care 2013;36:1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy HR, Elleri D, Allen JM, et al. Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care 2011;34:406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz JL, Sherr JL, Cengiz E, et al. Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Tech 2012;6:1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care 2012;35:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab 2011;96:1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Çinar A. Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther 2013;15:386–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauseth R, Hirsch IB, Bollyky J, et al. Use of a “fuzzy logic” controller in a closed-loop artificial pancreas. Diabetes Technol Ther 2013;15:628–633 [DOI] [PubMed] [Google Scholar]
- 36.Renard E. Clinical experience with an implanted closed-loop insulin delivery system. Arq Bras Endocrinol Metabol 2008;52:349–354 [DOI] [PubMed] [Google Scholar]
- 37.Renard E, Costalat G, Chevassus H, Bringer J. Artificial beta-cell: clinical experience toward an implantable closed-loop insulin delivery system. Diabetes Metab 2006;32:497–502 [DOI] [PubMed] [Google Scholar]
- 38.Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Youssef J, Castle JR, Branigan DL, et al. A controlled study of the effectiveness of an adaptive closed-loop algorithm to minimize corticosteroid-induced stress hyperglycemia in type 1 diabetes. J Diabetes Sci Tech 2011;5:1312–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patte C, Pleus S, Galley P, Weinert S, Haug C, Freckmann G. Feasibility of overnight closed-loop control based on hourly blood glucose measurements. J Diabetes Sci Tech 2012;6:902–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care 2010;33:1072–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Bon AC, Hermanides J, Koops R, Hoekstra JB, DeVries JH. Postprandial glycemic excursions with the use of a closed-loop platform in subjects with type 1 diabetes: a pilot study. J Diabetes Sci Tech 2010;4:923–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dassau E, Jovanovič L, Doyle FJ, III, Zisser HC. Enhanced 911/global position system wizard: a telemedicine application for the prevention of severe hypoglycemia–monitor, alert, and locate. J Diabetes Sci Technol 2009;3:1501–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shivers JP, Mackowiak L, Anhalt H, Zisser H. “Turn it off!”: diabetes device alarm fatigue considerations for the present and the future. J Diabetes Sci Tech 2013;7:789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Food and Drug Administration The Content of Investigational Device Exemption (IDE) and Premarket Approval (PMA) Applications for Artificial Pancreas Device Systems Silver Spring, MD, 2012 (publ. no. 1759) [Google Scholar]