Abstract
OBJECTIVE
Physical activity (PA) provides many benefits to adolescents with type 1 diabetes; however, these individuals tend to have lower fitness and PA levels than their disease-free counterparts. The purpose of this study was to examine the acute temporal associations between moderate-to-vigorous intensity PA (MVPA) and hypoglycemia (continuous glucose monitor [CGM] reading ≤70 mg/dL).
RESEARCH DESIGN AND METHODS
Nineteen participants (53% females) 14–20 years old with type 1 diabetes were recruited. Participant fitness was evaluated via indirect calorimetry using a maximal exercise test; body composition was measured using air displacement plethysmography. An accelerometer was worn continuously (3–5 days) and acceleration data used to estimate MVPA (minutes per day). Blood glucose values were simultaneously tracked using CGM. Controlling for sex, percent body fat (%BF), fitness, and concurrent MVPA, the likelihood of nighttime and next-day hypoglycemia due to MVPA was examined using logistic regression.
RESULTS
Participants were of average fitness (females: 43.9 mL/kg/min; males: 49.8 mL/kg/min) and adiposity (females: 26.2%; males: 19.2%); 63.2% met the U.S. federal guideline of accumulating 60 min/day of MVPA. Hypoglycemia was 31% more likely in those who accumulated 30 min/day more MVPA in the previous afternoon than those with less (95% CI 1.05–1.63; P = 0.017).
CONCLUSIONS
The results suggest that participating in afternoon MVPA increases the risk of overnight and next-day hypoglycemia, independent of sex, %BF, fitness, and concurrent MVPA. While promoting PA as a healthy behavior, it is important to educate adolescents with type 1 diabetes on prevention of hypoglycemia following PA.
There are many benefits of physical activity (PA) in adolescents, including improved blood lipid profiles, cardiovascular fitness, bone health, and psychological well-being. PA is also inversely associated with levels of adiposity and stress (1). Not only are these health outcomes immediately beneficial, but also health outcomes and physically active behavior track into adulthood, further increasing the importance of regular PA (1). The U.S. Department of Health and Human Services' 2008 Physical Activity Guidelines for Americans recommends a minimum of 60 min of moderate-to-vigorous intensity PA (MVPA) daily for children and adolescents (2). Despite the benefits of PA, the latest data from the 2011 Centers for Disease Control and Prevention Youth Risk Behavior Surveillance System indicate that only 28.7% of high school students are meeting the federal guideline of accumulating 60 min of MVPA daily (3). Research shows that these proportions are even lower for individuals with chronic diseases, including type 1 diabetes (4,5). Specifically, adolescents with type 1 diabetes spend 17 fewer min in MVPA per day than healthy adolescents and have 3–10 mL/kg/min lower VO2max values (5,6).
While regular PA is an important aspect of disease management for adolescents with type 1 diabetes, fear of hypoglycemia may lead to low levels of PA (7,8). This fear is valid because of the counterregulatory systems that control insulin levels not functioning properly, increasing the risk of exercise-induced hypoglycemia. Episodes of hypoglycemia have been associated in children and adolescents participating in prolonged moderate PA >60 min (9). Other factors that affect the glycemic response to exercise of an individual with type 1 diabetes include exercise duration, intensity, familiarity with type of activity, metabolic control, blood glucose levels, type and timing of insulin injections and food intake, insulin absorption, muscle mass required for activity, conditioning, degree of stress, and timing of activity (10). Robertson et al. (10) note that given consistent timing of exercise, amount of insulin, and pre-exercise meal, the response to 60 min of PA can be reproducible, indicating that it is safe for adolescents with type 1 diabetes to be physically active.
The relationship between PA and glycemic control is complex. Some studies have shown no benefit of PA on glycemic control, as measured by HbA1c (11). Others have shown that PA can reduce insulin requirements by 6–15% (11). This relationship, however, may be moderated by certain factors. Lukács et al. (5) have shown fitness to be inversely associated with HbA1c, while other studies have failed to show an association (12,13). Research on the relationship between adiposity and HbA1c has shown mixed results. In one study, BMI and adiposity were positively associated with HbA1c (14); however, other studies have failed to show a significant association (5,9). Sex may or may not be an important modifier in the relationship between PA and glycemic control. Some research shows a lack of association between sex and HbA1c (9), while others cite males as having more favorable HbA1c values, independent of PA, than females (14).
Studies have looked at the complex relationship between PA and glycemic control in young patients with type 1 diabetes, but most have used self-report methods, such as questionnaires, to assess daily PA participation (9,14–16). While questionnaires are a cost- and time-effective tool for assessing habitual PA, accelerometers are a more accurate measure of acute PA, as well as total PA level and energy expenditure. Additionally, accelerometers allow viewing of daily activity patterns (17).
The aim of this study was to analyze the relationship between MVPA and hypoglycemia through the assessment of their acute, temporal associations. We asked: 1) what are the acute, temporal associations between MVPA and hypoglycemia in adolescents with type 1 diabetes and 2) are these associations moderated by sex, fitness, or adiposity?
Research Design and Methods
Consent Procedures
The Institutional Review Board at the University of Iowa approved the study protocol, assent, and consent form. A parent or guardian provided written consent for all participants <18 years old, and participants provided assent. Participants >18 years old provided their own consent. Participants were also queried about their PA over the past 7 days.
Eligibility Criteria and Assessment
To be eligible for the study, the participant had to: 1) be between 14 and 20 years of age and at least Tanner stage III breast development for girls and at least Tanner stage III genitalia development for boys; 2) have a clinical diagnosis of type 1 diabetes of ≥1 year duration; 3) have a stable insulin regimen using an insulin pump or multiple daily injections for at least 12 months prior; 4) have an HbA1c ≤10.0% in the past 3 months measured with the DCA 2000 (Bayer Diagnostics, Tarrytown, NY); 5) have a BMI between the 5th and 95th percentile for age and sex; 6) have normal thyroid-stimulating hormone in the past 12 months; and 7) be willing and able to complete all study requirements. Participants were not eligible if they: 1) were hospitalized in the past month; 2) used systemic glucocorticoids in the past month; 3) had a musculoskeletal problem or physical or mental illness that may affect exercise performance; or 4) had a medical condition or were using a medication that, in the judgment of the investigator, could affect completion of the exercise protocol.
Study Procedures
The study consisted of two visits to the Clinical Research Unit of the Institute for Clinical and Translational Science (ICTS) at the University of Iowa separated by 3–5 days. During the first visit, the continuous glucose monitor (CGM) (SEVEN PLUS; Dexcom, San Diego, CA) was calibrated and inserted subcutaneously, anthropometric measures and VO2max testing were performed, and an accelerometer (device model 1.1, GENEActiv; Activinsights Ltd., Kimbolton, U.K.) was placed on the left wrist. Participants wore the CGM and accelerometer until they returned for the second study visit.
Hypoglycemic Events
Interstitial glucose values were recorded in milligrams per deciliter using CGM. Capillary glucose was checked during the initial visit using the patient’s home glucose meter at 2:00, 3:00, and 4:00 p.m., and these values were used to calibrate the CGM. The CGM sensor was placed subcutaneously during the initial visit to the ICTS and removed upon completion of the second visit to the ICTS 3–5 days later. The CGM collected glucose readings every 5 min, 24 h/day, and was calibrated with the patient’s home glucose meter two times daily. Data from the CGM were downloaded during the second visit, and glucose values were used to calculate the proportion of readings qualified as a hypoglycemic event, defined as any CGM reading ≤70 mg/dL. Alternative analyses were completed for hypoglycemic episodes, defined as successive CGM readings ≤70 mg/dL, at 5-min intervals, with an episode ending with at least two CGM readings >70 mg/dL.
Adiposity
Body fat and fat-free mass were measured using air displacement plethysmography via the BOD POD Gold Standard Body Composition Tracking System (model 2007A; COSMED USA, Inc., Concord, CA). The BOD POD uses body mass and body volume to calculate body density in grams per milliliter. Body mass is measured using a platform scale connected to the BOD POD, and body volume is measured using air displacement. Body density is then used to calculate percent body fat (%BF) using the Lohman density equation. The BOD POD has excellent validity when compared with dual energy X-ray absorptiometry in young females, with an intraclass correlation coefficient of 0.92 (18). In adolescent males, the BOD POD has a correlation coefficient of 0.90 when compared with hydrostatic weighing (19).
Cardiovascular Fitness
Cardiovascular fitness (VO2max) was measured via maximal exercise testing to volitional fatigue by open-circuit spirometry using a metabolic cart (Parvo Medics, Sandy, UT). Participants were fitted with a heart rate chest strap (Polar USA, Lake Success, NY) before completing a modified Balke treadmill protocol. Immediately before testing, participants were seated for 5 min, after which resting heart rate and blood pressure were measured. Individuals then practiced walking on the treadmill while being given instructions on proper treadmill walking technique. Participants self-selected a brisk but comfortable walking pace, and speeds ranged from 3.0–4.0 mph at 0% grade, for an initial 4-min warm-up. While keeping the speed constant, the grade was then increased to 5% for 4 min. Speed was increased by 0.5 mph for 2 min, after which the grade was increased by 2% each minute until exhaustion (20). During the test, measures of gas exchange and heart rate were recorded. Rating of perceived exertion was assessed using Borg’s 15-point scale during each stage. Participants were considered to have reached their maximal effort when two of the following criteria were met: 1) heart rate ≥200; 2) respiratory exchange ratio >1.0; or 3) ≤2 mL/kg/min change in VO2 in final 60 s of test. VO2max in mL/kg/min was used in analysis. Insulin levels were not adjusted for exercise, as the exercise session was designed to mimic a typical exercise session, and our participants did not typically adjust for exercise. If a participant experienced a bout of hypoglycemia, he or she was treated with 15 g of quick-acting carbohydrate and then retested and retreated, if necessary, after 15 min.
MVPA
Prior to fitness testing, a GENEActiv accelerometer (Activinsights Ltd.) was placed on the left wrist, to be worn between the two clinic visits for 3–5 days. The GENEActiv has excellent criterion validity in both adults (r = 0.86) and children (r = 0.91) when worn at the left wrist (21,22). Accelerometers were programmed to collect data, starting on the initial visit day, at a frequency of 75 Hz. Participants were instructed to wear the monitor 24 h/day, including while sleeping and during water activities. Accelerometers were removed at the completion of the participants’ second visit to the ICTS, and data were downloaded. The raw acceleration output was converted to 60-s epochs using the GENEActiv Post-Processing PC Software (version 2.2, GENEActiv; Activinsights Ltd.). Next, the 60-s epoch data files were entered into an open source Excel macro (v2; Activinsights Ltd.) in order to classify activity as sedentary, light, moderate, or vigorous intensity. MVPA (min/day) was calculated for each participant-day. Validated acceleration magnitude cut points from Esliger et al. (21) were used to classify activity intensity. KineSoft software (version 3.3.75; KineSoft, Loughborough, U.K.) was used to produce a series of standardized accelerometry outcome variables following procedures similar to those described by Esliger and Tremblay (23) and Esliger et al. (24). KineSoft was also used to produce heat maps, which show the amount of time spent in each activity intensity each day. In addition to all-day MVPA (MVPATOT), defined as 6:00 a.m. through bedtime in minutes per day, hours of the day were split in two for analysis: daytime PA (MVPA06–15), defined as 6:00 a.m. through 3:00 p.m.; and afternoon/evening PA (MVPA15–BT), defined as the post–school day activity hours of 3:00 p.m. through bedtime. Data were collected between November 2011 and April 2012.
Statistical Analysis
Descriptive statistics, including mean, SD, and range, were calculated for HbA1c, MVPA (min/day), VO2 max (mL/kg/min), adiposity (%BF), and number of hypoglycemic events. Sex-specific differences were evaluated using independent sample t tests. Hypoglycemia was represented by the proportion of hypoglycemic events occurring overnight and the next day following activity. Statistically significant associations between MVPA, using 30-min increments, and bouts of hypoglycemia were assessed using logistic regression. Because we were interested in acute effects, the logistic regression used individual days of data collection rather than participants. Generalized linear mixed-model analysis was used to account for multiple outcome measures per participant. Models were constructed using previous-day MVPA: 1) MVPATOT; 2) daytime MVPA (MVPA06–15); and 3) afternoon/evening MVPA (MVPA15–BT) as exposure variables. Bouts of hypoglycemia outcome variables were: 1) overnight and next day; 2) overnight (only); and 3) next day (only). From the logistic regression, odds ratios (ORs) and 95% CIs were calculated. All models were adjusted for sex, VO2max, %BF, and concurrent MVPA (MVPA accumulated on the same day as hypoglycemia risk was assessed). Analysis was completed on all days and weekdays only; however, the results were not affected by removing weekend days from analysis. Final analysis includes all days of the week. P values of <0.05 were considered to be statistically significant. All statistical analyses were performed using SAS (SAS version 9.3; SAS Institute, Cary, NC).
Results
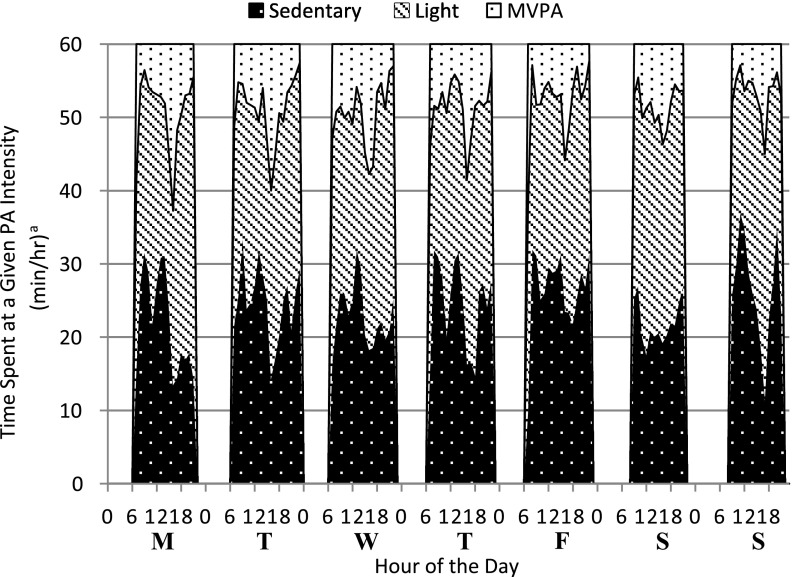
Twenty adolescents participated in the study; however, analyses are based on 19 participants (82 days of data), as 1 participant was lost to noncompliance with study procedures. Participant characteristics and covariates (age, height, weight, %BF, VO2max, and MVPA), HbA1c, and events of hypoglycemia are shown in Table 1. Average age of participants was 16.6 ± 1.6 years; 53% were female. Males and females had mean HbA1c values of 8.6 and 8.2%, respectively. Participants were of average fitness with a mean VO2max of 43.8 mL/kg/min for females (SD 6.8) and 49.8 mL/kg/min for males (SD 6.8) (25). Participants also had average levels of adiposity with a mean %BF in females of 26.1% (SD 4.8), and 19.1% in males (SD 9.3) (26). Sixty-three percent of participants met the U.S. federal guideline for adolescents of accumulating 60 min/day of MVPA (2). Of the participants who met the guideline, 75% (n = 9) reported participating in organized sports during the 7 days prior to the study. The mean MVPA per day was 107.2 ± 57.5 min in males and 117.8 ± 49.4 min in females. There were no statistically significant differences between male and female participants for MVPA and events of hypoglycemia. A heat map of average time spent in sedentary, light, moderate, and vigorous physical activities throughout the week by all participants is shown in Fig. 1. The heat map represents all 19 participants for each day that they wore the accelerometer and shows the time spent in each intensity of PA (minutes per hour) throughout each day. The majority of each hour is spent in sedentary and light-intensity activity, with the highest amounts of MVPA occurring in the late afternoon and early evening hours. Due to small sample size, our a priori analysis was between MVPA and hypoglycemia, as informed by previous research performed by the Direcnet Study Group (9). Therefore, we did not analyze sedentary and light-intensity PA.
Table 1.
Descriptive statistics for participant characteristics, MVPA, and hypoglycemic events
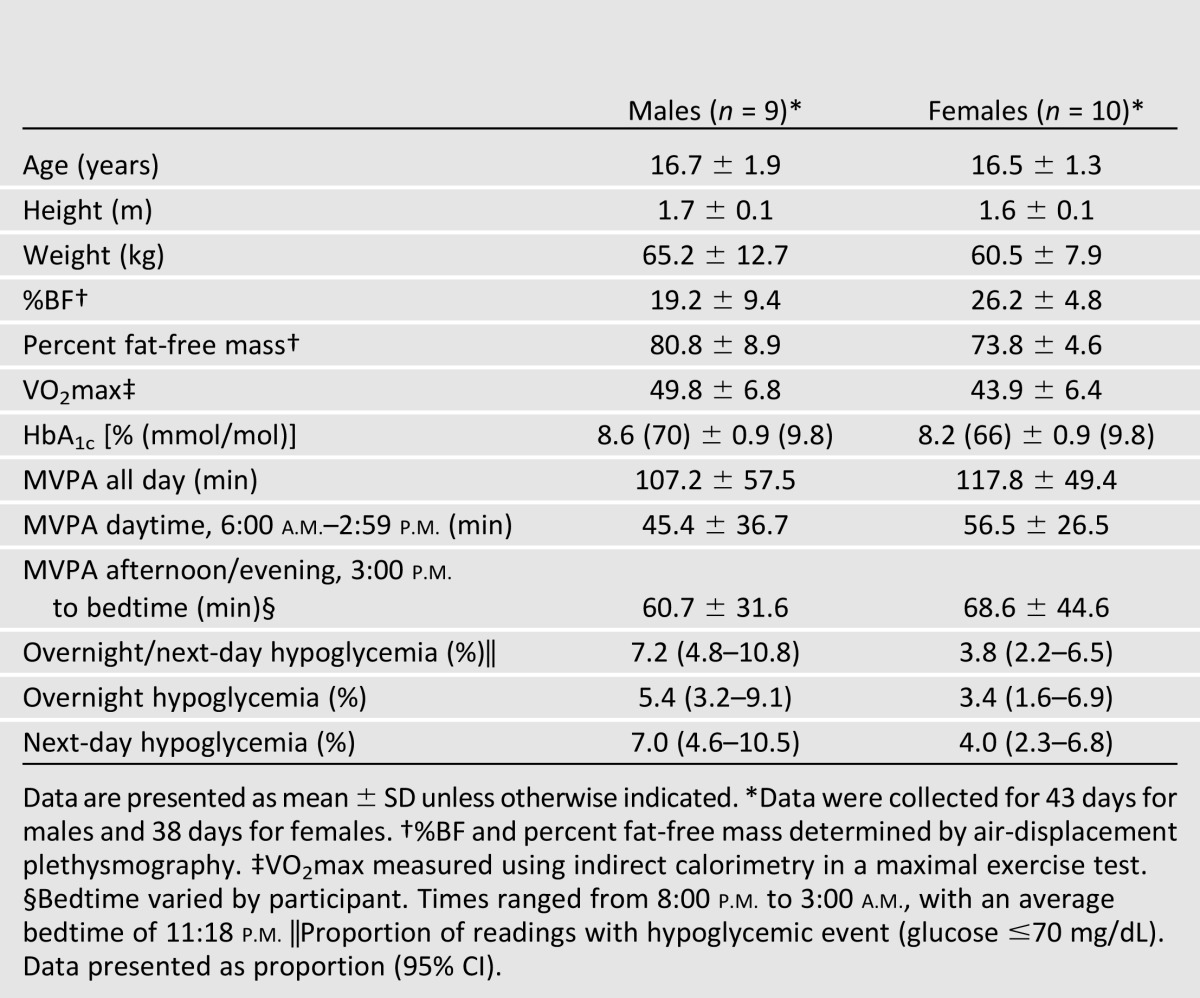
Figure 1.
Heat map of average physical activity patterns of all participants. aTimes based on average activity of all participants (n = 19).
All 19 participants experienced at least one bout of hypoglycemia over the 82 participant-days analyzed. Fourteen participants had one or more hypoglycemic events during the maximal exercise test. The median number of hypoglycemic readings per day was 8.5 (range 0–87). Specifically, the average number of hypoglycemic episodes overnight was 0.7 per subject per night (range 0–4); the median duration per episode was 20 min (range 5–145). The average number of hypoglycemic episodes the next day was 1.3 per subject per day (range 0–7); the median duration per episode was 20 min (range 5–135). The mean proportion of CGM readings with a hypoglycemic event was 4.45% of readings taken during sleep and 5.45% of readings taken during the day following activity.
Overnight and Next-Day Hypoglycemia
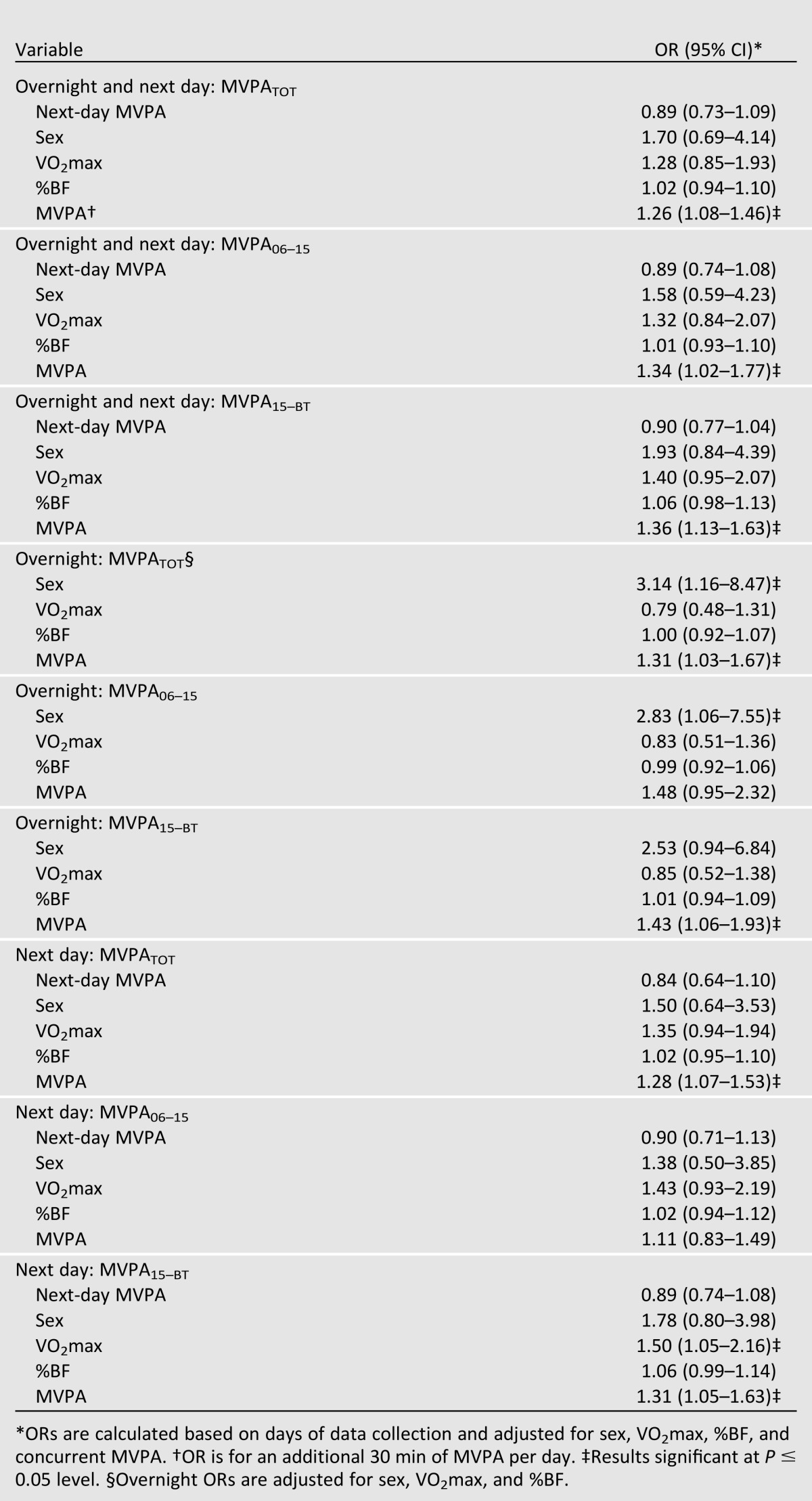
Risk of hypoglycemia logistic regression models described below were adjusted for sex, VO2max, %BF, and concurrent MVPA. Adjusted ORs for the full model are presented in Table 2. Previous-day MVPATOT (n = 50) was significantly associated with overnight and next-day hypoglycemia (OR 1.26 [95% CI 1.08–1.46]; P = 0.003). For every 30-min increase in previous-day MVPATOT, overnight and next-day hypoglycemia risk increased by 26%. When we segmented the previous day to more precisely examine temporal responses, MVPA06–15 (n = 50) was associated with a higher risk of overnight and next-day hypoglycemia (OR 1.34 [95% CI 1.02–1.77]; P = 0.036). Additionally, MVPA15–BT (n = 67) was significant, with an OR of 1.36 (95% CI 1.13–1.63; P = 0.001). (MVPA15–BT analyses have a higher sample size due to the inclusion of initial visit day MVPA, which was only available for afternoon hours.)
Table 2.
ORs for temporal associations between daily MVPA and hypoglycemia
Overnight Hypoglycemia
We also examined the risk of hypoglycemia during overnight (as opposed to overnight and next day). Previous-day MVPATOT (n = 58) was significantly associated with bouts of overnight hypoglycemia (OR 1.31 [95% CI 1.03–1.67]; P = 0.026). A nonsignificant association was seen for the previous day for MVPA06–15 (n = 58) (OR 1.48 [95% CI 0.95–2.32]; P = 0.082); however, MVPA15–BT (n = 70) was significant (OR 1.43 [95% CI 1.06–1.93]; P = 0.021). Additionally, males had a higher risk than females of experiencing a bout of hypoglycemia overnight (OR 3.14 [95% CI 1.16–8.47]; P = 0.026).
Next-Day Hypoglycemia
Finally, we examined the risk of hypoglycemia during the next day following activity (as opposed to overnight and next day). Previous-day MVPATOT (n = 51) was significantly associated with next-day hypoglycemia (OR 1.28 [95% CI 1.07–1.53]; P = 0.008). Increasing MVPA by 30 min/day significantly increased risk of next-day hypoglycemia by 28%. When the previous day’s MVPA was segmented, MVPA06–15 (n = 51) was not significant (OR 1.11 [95% CI 0.83–1.49]; P = 0.454); however, MVPA15–BT (n = 68) was (OR 1.31 [95% CI 1.05–1.63]; P = 0.017). Increasing MVPA15-BT by 30 min/day significantly increased risk of next-day hypoglycemia by 31%. In addition, participants with a higher VO2max had a higher risk of experiencing next-day hypoglycemia than those with a lower VO2max. This relationship exists for MVPA15–BT (OR 1.50 [95% CI 1.05–2.16]; P = 0.030).
Conclusions
While PA provides many benefits to adolescents with type 1 diabetes, including improvements in cardiovascular risk profile, cardiovascular fitness, bone health, and adiposity, there is a risk of hypoglycemia after activity. In our study, MVPA was assessed as total minutes per day, daytime hours and afternoon/evening hours. This strategy was used to assess the effect of MVPA accumulated during afterschool hours, which is likely the most common time to participate in MVPA, and is also the time of most organized school athletics practices. We found the greatest risk for hypoglycemic events to be associated with afternoon/evening MVPA. This was the time that our participants accumulated the greatest amount of MVPA; in general, this is when adolescents accumulate the majority of their PA.
Participants in our study were of average fitness; our sample differs from those in much of the previous literature, which indicates that adolescents with type 1 diabetes have lower levels of fitness (5,6). The participants also accumulated more daily MVPA than has been reported in previous studies (4,13); mean MVPA was 107.2 ± 57.5 min/day for males and 117.8 ± 49.4 min/day for females. Sixty-eight percent of the adolescents participated in organized sports. Given the study required a fitness and body composition test, more active and fit individuals may have volunteered. In agreement with the previous literature (5,14), participants had average adiposity levels, and adiposity did not have an effect on risk of hypoglycemia. Sex, however, was significant in the relationship between MVPA and overnight hypoglycemia, with males having a greater risk. Male participants had higher percentages of fat-free mass, on average, than the female participants, which may contribute to this difference in risk, due to increased skeletal muscle glucose transport.
We report that in adolescents with type 1 diabetes, a 30-min increase in afternoon/evening MVPA increases the risk of hypoglycemia by 43% overnight and 31% on the following day. The associations are independent of sex, fitness, and adiposity, and the following-day relationship is stronger when adjusted for concurrent MVPA. The associations are exponential; if an adolescent accumulates the recommended 60 min/day of MVPA, they have a 104 and 72% higher risk for hypoglycemia overnight and the following day, respectively, when compared with no MVPA. An increased risk of this magnitude warrants education for not only the adolescent, but also for coaches, parents, and school officials.
Organized sports often have practices of 90–120 min, which would more than double their risk of having a hypoglycemic event. When adjusting for minutes of MVPA, however, participants in organized sports did not differ in their hypoglycemia risk from those not in organized sports. This indicates that participation in organized sports does not increase hypoglycemia risk over independent activity of the same intensity and duration. The results also indicated that higher fitness is associated with an increased risk for hypoglycemia of 50% (for every 5 mL/kg/min increase in VO2max). The implication is that being fit or habitually active does not protect against bouts of hypoglycemia, but rather increases risk. Participants with higher fitness had lower %BF; higher amounts of lean body mass aid in transporting glucose into the muscles, leading to hypoglycemia. Additionally, in our study, the more fit adolescents had higher amounts of vigorous intensity PA within their minutes of MVPA, suggesting that absolute volume of PA (for example metabolic equivalent of task minutes) may also influence hypoglycemia risk.
In agreement with previous research completed by the DirecNet Study Group (9), the current study found an increased risk of overnight hypoglycemia with more MVPA. Additionally, we found an extended period of time in which adolescents should be cognizant of their blood glucose levels. This finding indicates that there is an increased window of time of 32 h post activity in which adolescents were at continued risk of hypoglycemia.
Strengths of the current study include the use of valid measures to obtain study values, including the measurement of VO2max using indirect calorimetry during a maximal exercise test, as well as using air-displacement plethysmography to assess %BF. Another strength was the use of CGM and 24-h accelerometry to assess the temporal associations between MVPA and hypoglycemia round the clock. Much of the research in PA and adolescents with type 1 diabetes has been completed in a controlled laboratory setting. The use of CGM and accelerometry allowed for the analysis of the PA and hypoglycemia relationship in a free-living setting, permitting the measurement of participants’ normal daily activity.
Use of the GENEActiv is a strength of this study; it was used to decrease compliance issues seen with other accelerometers. The accelerometer was worn at the wrist and is waterproof, ensuring participants were able to comfortably wear the accelerometer 24 h/day, resulting in full-day data collection. In our study, compliance was measured as the proportion of days with ≥600 min of wear time over the number of anticipated collection days; our compliance rate was 92%. Nineteen of the 20 participants wore the monitor at least 2 days for ≥600 min/day and contributed to the analysis. Another benefit of the GENEActiv is that data are collected in units of raw acceleration (21). The use of raw acceleration allows for more transparent data analyses than proprietary movement counts.
Limitations of the current study include a small convenience sample. Additionally, there were no dietary measures collected. Diet is an important aspect of glycemic control, and could affect hypoglycemic events. To partially address this limitation, analyses were completed adjusting for initial glucose values during both sleep and waking hours; however, they were not significant. Use of CGM also presents limitations, including loss of sensitivity during bouts of hypoglycemia and numerical errors (27). To address this limitation, CGMs were calibrated twice daily with patients’ home glucose meters. Future analyses should continue to assess patterns of hypoglycemia to better determine a time of highest risk of hypoglycemia.
We report that increasing afternoon/evening MVPA results in an increased risk of hypoglycemia overnight and during the day following activity. Additionally, higher fitness levels are associated with an increased risk of hypoglycemia. This indicates the importance of educating adolescents with type 1 diabetes, their parents, and their coaches on prevention of hypoglycemia following activity.
Article Information
Acknowledgments. The authors thank all of the study participants and the staff of the Institute for Clinical and Translational Science for the work completed.
Funding. This work was supported by a grant from the Doris Duke Charitable Foundation, the National Center for Advancing Translational Sciences, and the National Institutes of Health through grant 2-UL1-TR000442-06 for the Institute for Clinical and Translational Science.
Duality of Interest. M.J.T. is a consultant for Daiichi Sankyo. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.M.M. wrote the manuscript and researched data. A.S., E.T., M.J.T., and D.W.E. researched data and reviewed and edited the manuscript. M.B.Z. researched data and reviewed the manuscript. K.F.J. designed the study, researched data, and reviewed and edited the manuscript. K.M.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Portions of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
References
- 1.Loprinzi PD, Cardinal BJ, Loprinzi KL, Lee H. Benefits and environmental determinants of physical activity in children and adolescents. Obes Facts 2012;5:597–610 [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services 2008 Physical Activity Guidelines for Americans. Washington, D.C., U.S. Govt Printing Office, 2008 [Google Scholar]
- 3.Centers for Disease Control and Prevention Youth risk behavior surveillance -United States, 2011. MMWR Surveill Summ 2012;61:1–162 [PubMed] [Google Scholar]
- 4.Maggio ABR, Hofer MF, Martin XE, Marchand LM, Beghetti M, Farpour-Lambert NJ. Reduced physical activity level and cardiorespiratory fitness in children with chronic diseases. Eur J Pediatr 2010;169:1187–1193 [DOI] [PubMed] [Google Scholar]
- 5.Lukács A, Mayer K, Juhász E, Varga B, Fodor B, Barkai L. Reduced physical fitness in children and adolescents with type 1 diabetes. Pediatr Diabetes 2012;13:432–437 [DOI] [PubMed] [Google Scholar]
- 6.Komatsu WR, Gabbay MA, Castro ML, et al. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr Diabetes 2005;6:145–149 [DOI] [PubMed] [Google Scholar]
- 7.Bernardini AL, Vanelli M, Chiari G, et al. Adherence to physical activity in young people with type 1 diabetes. Acta Biomed 2004;75:153–157 [PubMed] [Google Scholar]
- 8.Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008;31:2108–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsalikian E, Mauras N, Beck RW, et al. Diabetes Research In Children Network Direcnet Study Group Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr 2005;147:528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson K, Adolfsson P, Riddell MC, Scheiner G, Hanas R. Exercise in children and adolescents with diabetes. Pediatr Diabetes 2008;9:65–77 [DOI] [PubMed] [Google Scholar]
- 11.Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 2012;55:542–551 [DOI] [PubMed] [Google Scholar]
- 12.Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 2010;95:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuenca-García M, Jago R, Shield JP, Burren CP. How does physical activity and fitness influence glycaemic control in young people with Type 1 diabetes? Diabet Med 2012;29:e369–e376 [DOI] [PubMed] [Google Scholar]
- 14.Herbst A, Bachran R, Kapellen T, Holl RW. Effects of regular physical activity on control of glycemia in pediatric patients with type 1 diabetes mellitus. Arch Pediatr Adolesc Med 2006;160:573–577 [DOI] [PubMed] [Google Scholar]
- 15.Åman J, Skinner TC, de Beaufort CE, Swift PG, Aanstoot HJ, Cameron F, Hvidoere Study Group on Childhood Diabetes Associations between physical activity, sedentary behavior, and glycemic control in a large cohort of adolescents with type 1 diabetes: the Hvidoere Study Group on Childhood Diabetes. Pediatr Diabetes 2009;10:234–239 [DOI] [PubMed] [Google Scholar]
- 16.Schweiger B, Klingensmith G, Snell-Bergeon JK. Physical activity in adolescent females with type 1 diabetes. Int J Pediatr 2010;2010:328318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westerterp KR. Assessment of physical activity: a critical appraisal. Eur J Appl Physiol 2009;105:823–828 [DOI] [PubMed] [Google Scholar]
- 18.Maddalozzo GF, Cardinal BJ, Snow CA. Concurrent validity of the BOD POD and dual energy x-ray absorptiometry techniques for assessing body composition in young women. J Am Diet Assoc 2002;102:1677–1679 [DOI] [PubMed] [Google Scholar]
- 19.Moon JR, Tobkin SE, Costa PB, et al. Validity of the BOD POD for assessing body composition in athletic high school boys. J Strength Cond Res 2008;22:263–268 [DOI] [PubMed] [Google Scholar]
- 20.Nemeth BA, Carrel AL, Eickhoff J, Clark RR, Peterson SE, Allen DB. Submaximal treadmill test predicts VO2max in overweight children. J Pediatr 2009;154:677–681 [DOI] [PubMed] [Google Scholar]
- 21.Esliger DW, Rowlands AV, Hurst TL, Catt M, Murray P, Eston RG. Validation of the GENEA Accelerometer. Med Sci Sports Exerc 2011;43:1085–1093 [DOI] [PubMed] [Google Scholar]
- 22.Phillips LR, Parfitt G, Rowlands AV. Calibration of the GENEA accelerometer for assessment of physical activity intensity in children. J Sci Med Sport 2013;16:124–128 [DOI] [PubMed] [Google Scholar]
- 23.Esliger DW, Tremblay MS. Physical activity and inactivity profiling: the next generation. Can J Public Health 2007;98(Suppl. 2):S195–S207 [PubMed] [Google Scholar]
- 24.Esliger DW, Tremblay MS, Copeland JL, Barnes JD, Huntington GE, Bassett DR., Jr Physical activity profile of Old Order Amish, Mennonite, and contemporary children. Med Sci Sports Exerc 2010;42:296–303 [DOI] [PubMed] [Google Scholar]
- 25.Shvartz E, Reibold RC. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat Space Environ Med 1990;61:3–11 [PubMed] [Google Scholar]
- 26.Ogden C, Li Y, Freedman D, Borrud L, Flegal K. Smoothed percentage body fat percentiles for US children and adolescents, 1999-2004. Natl Health Stat Report 2011;43:1–7 [PubMed] [Google Scholar]
- 27.Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care 2008;31:1160–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]