Abstract
This paper discusses the use of 13C-based metabolism analysis for the assessment of intrinsic product yields — the actual carbon contribution from a single carbon substrate to the final product via a specific biosynthesis route — in the following four cases. First, undefined nutrients (such as yeast extract) in fermentation may contribute significantly to product synthesis, which can be quantified through an isotopic dilution method. Second, product and biomass synthesis may be dependent on the co-metabolism of multiple-carbon sources. 13C labeling experiments can track the fate of each carbon substrate in the cell metabolism and identify which substrate plays a main role in product synthesis. Third, 13C labeling can validate and quantify the contribution of the engineered pathway (versus the native pathway) to the product synthesis. Fourth, the loss of catabolic energy due to cell maintenance (energy used for functions other than production of new cell components) and low P/O ratio (Phosphate/Oxygen Ratio) significantly reduces product yields. Therefore, 13C-metabolic flux analysis is needed to assess the influence of suboptimal energy metabolism on microbial productivity, and determine how ATP/NAD(P)H are partitioned among various cellular functions. Since product yield is a major determining factor in the commercialization of a microbial cell factory, we foresee that 13C-isotopic labeling experiments, even without performing extensive flux calculations, can play a valuable role in the development and verification of microbial cell factories.
Keywords: Cell maintenance, Co-metabolism, Metabolic flux analysis, P/O ratio, Yeast extract
Introduction
Recent advances in metabolic engineering have enabled us to engineer microbial cell factories for the efficient synthesis of diverse products, including bulk chemicals, pharmaceutical drugs and biofuels [1,2]. For example, advanced biofuels produced by engineered microorganisms with properties similar to that of petroleum-based fuels, have been reported extensively [3-7]. The emergence of systems biology and synthetic biology has greatly increased the potential of microbial cell factories towards the production of value-added chemicals [8-10]. For economically viable manufacture of bulk and commodity chemicals [11], the product yield is an important consideration. Researchers often include either rich medium or multiple feedstocks in microbial fermentations. Thereby, estimation of the intrinsic product yield is difficult since undefined nutrients may also contribute to the product synthesis (Figure 1). Additionally, new enzymes are often employed to improve microbial productivity [4,12-14], and the separate contributions of the heterologous and native pathways to product synthesis needs further validation. Finally, the synthesis of high-energy products (such as biofuels) requires a large amount of ATP and NAD(P)H. Due to suboptimal energy metabolism (e.g., cell maintenance cost), the actual bacterial biosynthesis is often at least three-fold lower than the amount that would be predicted from reaction stoichiometry [15].
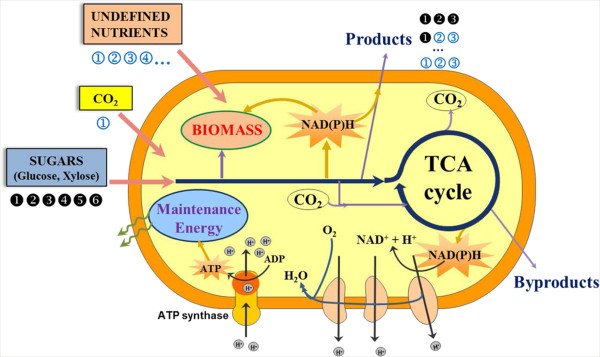
Figure 1.
Schematic description of microbial metabolism. Microbes have the ability to co-metabolize diverse feedstock. Dark circles indicate labeled carbon. The enrichment of labeling in the product acts as an indicator for the relative uptake of sugars.
Therefore, 13C-analysis is the recommended method to track the in vivo carbon fluxes from specific substrates to final products. Feeding microbial cultures with 13C-substrates results in unique isotopic patterns amongst the cell metabolites (13C-fingerprints) [16] to delineate metabolic pathways [17]. Integration of 13C-fingerprints with metabolic modeling can elucidate the intracellular metabolic fluxes (i.e., 13C-MFA). In the biotechnology field, 13C-MFA can reveal metabolic responses of microbial hosts to product synthesis and growth conditions [18-20], identify the rigid metabolic nodes that cause bottlenecks for further rational pathway engineering [21], and perform characterization of novel microbial physiologies [22-25]. In addition to these applications, 13C-MFA may reveal the effect of suboptimal energy metabolism on intrinsic product yields.
Product yield using rich medium
Engineered microbes have many metabolic burdens that can inhibit both biomass growth and product synthesis. Since rich media includes both primary carbon substrates (e.g., sugars) and large amounts of nutrients (such as yeast extract), it is commonly used in fermentations to provide diverse nutrients for cell growth and stabilize the production performance of microbes [9,10]. This reduces the culture lag phase and promotes their productivity. Multiple studies have revealed that supplementing culture medium with yeast extract or terrific broth — a highly enriched medium that contains yeast extract, tryptone and glycerol as carbon sources — to engineered microbes significantly improves their final biosynthesis yields [26,27]. Since nutrient supplements can provide undefined building blocks for both biomass and product synthesis, it is difficult to precisely calculate the intrinsic product yield from rich-medium fermentation. To overcome this problem, 13C-analysis can gain insights into the carbon contribution from the nutrients to product biosynthesis.
For example, two E. coli strains engineered for isobutanol production (i.e., a low performance strain with an Ehrlich pathway [28] and a high performance JCL260 strain with overexpression of both the keto-acid pathway and the Ehrlich pathway [29]) display an increase in isobutanol titer with the inclusion of yeast extract in their culture medium. Using fully labeled glucose and non-labeled yeast extract as carbon sources, 13C-experiments revealed that the low-performance strain derived ~50% of the carbons in the produced isobutanol from yeast extract (Figure 2). On the other hand, JCL260 synthesized isobutanol solely from 13C-glucose and used yeast extract mainly for biomass growth [28]. This observation confirms that overexpression of the keto-acid pathway overcomes bottleneck in the synthesis of isobutanol and effectively pulls the carbon flow from glucose to product. In another work, an E. coli strain was engineered for the conversion of acetate into free fatty acids via the overexpression of both acetyl-coA synthetase and the fatty acid pathways. During acetate fermentation, yeast extract significantly promoted fatty acid productivity, resulting in 1 g/L fatty acids from ~10 g/L acetate [30]. 13C-analysis of the culture with fully labeled acetate and yeast extract has shown that ~63% carbons in the free fatty acids were synthesized from 13C-acetate (Figure 2). Thereby, the intrinsic product yield from a primary substrate in a rich medium could be correctly estimated based on isotopomer analysis.
Figure 2.
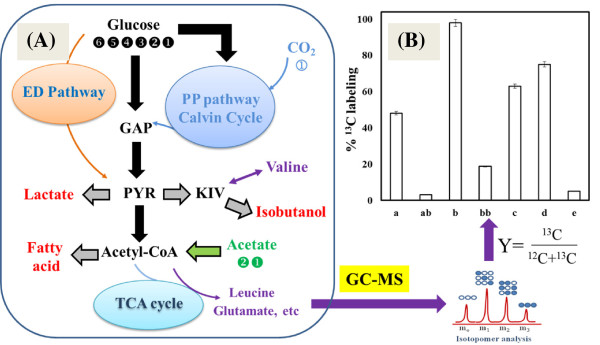
Schematic examples to demonstrate the use of 13C-analysis in elucidating the contributions of various carbon substrates towards the final product synthesis. (A) Biosynthesis yield analyzed by feeding cells with 13C-substrates (such as fully labeled glucose and acetate). Abbreviations: GAP, Glyceraldehyde-3-phosphate; PYR, pyruvate; KIV, ketoisovalerate. (B) Relative product yields from a primary substrate (a – Isobutanol from glucose in a low performance strain; ab – valine from glucose in a low performance strain; b – Isobutanol from glucose in JCL260; bb – valine from glucose in JCL260) [28]; c – Free fatty acids from acetate in an E. coli strain [30]; d - biomass from glucose in wild type Synechocystis 6803 [32]; e - D-lactate from acetate in engineered Synechocystis 6803 [33]).
Product yield during co-metabolism of multiple carbon substrates
Algal species are able to utilize both CO2 and organic carbon substrates. Such mixotrophic metabolisms can alleviate the dependence of algal hosts on light and CO2 limitations, and thus enable them to achieve high biomass growth rate and product titer [31]. 13C-metabolite analysis has been used to track their photomixotrophic metabolisms in different scenarios. For example, Synechocystis sp. PCC 6803 (blue-green algae) is capable of performing photomixotrophic growth. 13C-MFA has shown that CO2 contributes to 25% of Synechocystis biomass yield during its mixotrophic growth with 13C-glucose and 12CO2[32]. On the other hand, 13C-analysis has tracked D-lactate synthesis in an engineered Synechocystis 6803 [33]. In that study, the lactate production increased substantially during the co-metabolism of both CO2 and acetate. Experiments with fully labeled acetate and 12CO2 determined that nearly all of the lactate molecules were non-labeled and that only the acetyl-CoA-derived proteinogenic amino acids (leucine, glutamate and glutamine) were 13C-labeled. This observation suggests that acetate entered into TCA cycle and was involved only in biomass growth, while the yield of D-lactate was completely derived from CO2 (Figure 2). This result further indicates that acetate could inhibit the pyruvate decarboxylation reaction and thus direct more carbon flux from pyruvate to lactate. The above study shows the value of 13C-analysis in improving our understanding of pathway regulations for product synthesis. Since many microbial platforms (including both algal species and heterotrophs) may co-metabolize multiple carbon substrates simultaneously, isotopomer feeding can reveal the contributions of each substrate to the corresponding metabolite pools, and thus predict the potential bottlenecks in biomass or product formations.
Product yield from alternative pathways
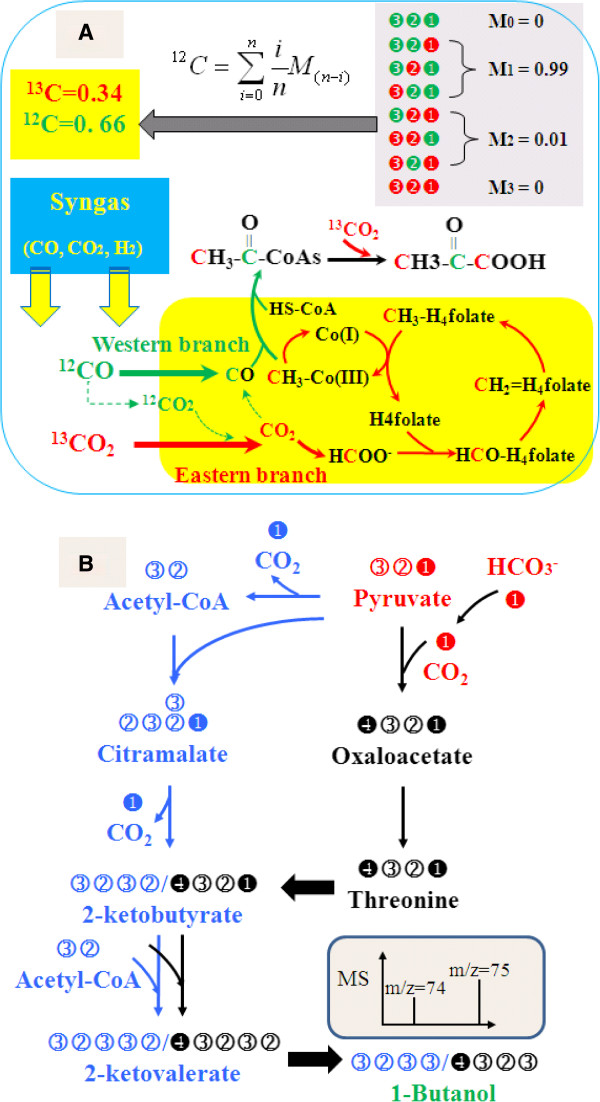
13C-analysis can decipher the yield of products with multiple biosynthesis routes. For example, the acetogenic bacterium Clostridium carboxidivorans uses syngas (H2, CO and CO2) to generate various chemicals (e.g., acetate, ethanol, butanol, and butyrate) [34]. It contains several routes for CO2 fixation, which includes the Wood-Ljungdahl pathway, the anaplerotic pathways, and the pyruvate synthase reactions. 13C-experiments can identify the relative contribution of each CO2 fixation pathways towards product synthesis. As a demonstration, cultivation of Clostridium with labeled 13CO2 and 12CO has been shown in Figure 3A. Analysis of the labeling patterns in either alanine or pyruvate could reveal the relative contributions of the different CO2 assimilation reactions to biomass and product synthesis.
Figure 3.
Schematic examples illustrate that 13C-analysis can be utilized to determine the contributions of various biosynthetic pathways towards final product yield. (A) 13C analysis to study the carbon assimilation during syngas fermentation (13CO2, 12CO and H2). Analysis of metabolite labeling patterns can determine CO2 and CO utilization for pyruvate production. The isotopomer data of pyruvate was used as a demonstration of 13C applications for product yield calculations. (B) Threonine and citramalate pathway for the synthesis of 1-butanol. The carbon rearrangement network shows the labeling of 1-butanol from the two biosynthesis pathways, when fed with 1-13C pyruvate and 13C bicarbonate.
Yield of a product form a biosynthesis pathway may suffer losses from side reactions and intermediate degradation/secretion. A statistical analysis on previous metabolic engineering works observed 20% ~ 30% yield reduction per engineered enzymatic reaction step (“Rule of Thumb”) [26,27]. To reduce the carbon loss, novel pathways are constantly proposed and engineered into microbial hosts to create a “short-cut” or carbon efficient route from the feedstock to the final product. Whenever heterologous pathways are engineered into a microbe, the actual contribution to the final product of the new pathway versus the native pathway is often difficult to be estimated [35]. In the following example, we demonstrate that 13C-experiments can determine the relative fluxes through multiple pathways by measuring product labeling. Specifically, 1-butanol could be produced simultaneously from a threonine pathway and a citramalate pathway (a short-cut keto acid-mediated pathway) in E. coli[36]. If 1st position 13C-pyruvate and 13C-bicarbonate were fed to 1-butanol producing cultures, labeling patterns in 1-butanol can reveal the fluxes through both the routes (Figure 3B). Recently, a non-oxidative glycolytic cycle (NOG) was designed to increase biofuel yield [12]. This NOG pathway starts with fructose 6-phosphate and undergoes three metabolic cycles to generate acetyl-CoA without losing any carbon. To probe the contribution of NOG pathway to overall cell metabolism, this study has presented a carbon rearrangement map for 13C-analysis of the NOG pathway function. These examples illustrate the potential of 13C-analysis to examine the in vivo activity of various novel pathways towards product synthesis.
Product yield influenced by bioenergetic efficiency
The theoretical product yield is generally calculated based on the stoichiometry of product synthesis from a carbon substrate. However, microbial energy metabolism also affects product yield because the synthesis of high-energy chemicals is energetically expensive, consuming large amounts of ATP/NAD(P)H. Cell maintenance (i.e., energy consumed for functions other than the production of new cell material) strongly competes for energy molecules and limits product synthesis. The maintenance energy involves regeneration of macromolecules, futile metabolic cycles, energy spilling reactions, proofreading, cell motility, preservation of chemical gradients, and repairing of cell damage caused by environmental stresses [37,38]. For example, non-growth-associated maintenance in wild type E. coli consumes 7.6 mmol of ATP per gram dry weight per hour [39]. Moreover, oxidative phosphorylation of NADH is a major source for ATP generation (theoretical maximum P/O ratio: 1 NADH ➔ 3 ATPs) [40]. Cytochrome oxidase is transmembrane protein complex that transfers electrons to O2 and translocate protons across the membrane to establish a proton gradient to power ATP synthase. However, proton translocation through membrane is not always coupled with electron transfer from NADH to O2, which reduces the contribution of oxidative phosphorylation to the establishment of the proton motive force for ATP synthesis [41,42]. Thereby, the actual P/O ratio, which is still in debate, is observed to be below 2.5 [43]. Under metabolic stresses, the respiration efficiency can be further reduced because trans-membrane proton gradients for ATP synthesis leak over time, resulting in loss of catabolic energy capture [37,44]. For example, the riboflavin producing Bacillus subtilis has a P/O ratio of 1.3, and a small increase in P/O ratio (from 1.3 to 1.5) could increase riboflavin yields by 20% [45].
The amount of energy from substrate catabolism diverted to non-growth functions varies dramatically depending on different organisms and growth conditions (e.g., during E. coli growth, its energy yield of substrate catabolism could be one-third of the theoretical maximum) [37]. To illustrate the impact of energy efficiency on product yield [46], a small-scale flux balance model related to fatty acid-overproducing strain was built exclusively for this report. This small-scale model employs eight reactions (Table 1) to demonstrates free fatty acid production as a function of non-growth associated ATP maintenance and P/O ratio [47]. The fluxes were resolved by the function below:
Table 1.
Simplified biochemical reactions considered in the model
| Flux, v | Reactions | Note |
|---|---|---|
| v(1) |
Glucose ➔ 2AceCoA + 2ATP + 4NADH |
Glycolysis |
| v(2) |
AceCoA + 1.75NADPH + 0.875ATP ➔ 0.125 C16:0 fatty acid |
Fatty acid synthesis |
| v(3) |
AceCoA ➔ 2NADH + NADPH + ATP + FADH2 |
TCA cycle |
| v(4) |
NADH ➔ NADPH |
Transhydrogenation |
| v(5) |
NADH ➔ P/O ATP |
Oxidative phosphorylation |
| v(6) |
FADH2 ➔ 0.67(P/O)ATP |
Oxidative phosphorylation |
| v(7) |
ATP ➔ ATP_maintenance |
ATP maintenance (non-growth associated) |
| v(8) | 6.6Glucose + 37.6ATP + 9.5NADPH + 2.5AceCoA ➔ 39.7Biomass + 3.1NADH | Biomass formation |
Note: Glucose consumption for both biomass growth and product synthesis is normalized to 100. The linear optimizer ‘linprog’ function in MATLAB is used for the optimization. The final yield (g fatty acid/g glucose) is calculated as follows: Y = (v(2)/8∙256)/(100∙180) g C16:0 fatty acid/g glucose.
where the objective function is to maximize v(2) (i.e., the relative flux of fatty acid). A is the reaction stoichiometry. lb and ub are upper and lower bound for each reaction flux, v(i). Figure 4A shows the relationship between maximum yield, P/O ratio and ATP maintenance without constraining biomass growth (v(8) ≥ 0) (Table 1). A higher P/O ratio makes the microbial system less sensitive to the increased demand for ATP loss. When the ATP maintenance is low and the P/O ratio is close to 3, the fatty acid yield can reach the theoretical value of 0.36 g fatty acid/g glucose (Figure 4A). In such conditions, eliminating competing pathways or engineering new pathways to avoid carbon loss may be effective to achieve a yield close to the theoretical maximum [48-50]. When ATP consumption for maintenance increases, cells need to use extra carbon substrates for energy generation, thereby decreasing the fatty acid yield significantly. Under these circumstances, one should consider strategies that will either reduce cell maintenance or increase the flux towards ATP synthesis.
Figure 4.
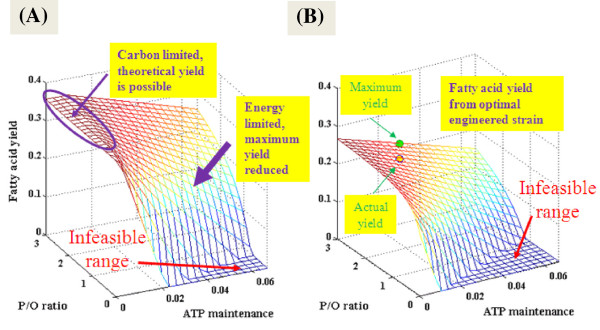
3D illustrations of relationships among theoretical yield, P/O ratio and non-growth associated ATP maintenance. (A) Theoretical Yield as a function of P/O ratio and non-growth associated ATP maintenance without constraining biomass growth (v(8) ≥ 0). (B) Theoretical Yield as a function of P/O ratio and non growth associated ATP maintenance at growth rate v(8) = 3.6. The units of yield and ATP maintenance are ‘g C16:0 fatty acid/g glucose’ and ‘mol ATP /g glucose’ respectively. Under certain circumstances, the energy cannot be balanced for fatty acid or biomass production, resulting zero yield [47].
In a recent study of an engineered E. coli for fatty acid overproduction [47], 13C-MFA showed that the theoretical ATP/NADPH generation (assuming P/O ratio = 3) from glucose catabolism was much higher than ATP/NADPH consumption for biomass growth and fatty acid synthesis. After optimization of biosynthesis pathway via 'push-pull-block' strategies, this engineered strain had a fatty acid yield of only 0.17 g fatty acid/g glucose (Figure 4B) because a substantive fraction of energy yield from glucose catabolism was lost due to the suboptimal energy metabolism. Such high cell maintenance and low P/O ratio in the engineered E. coli are likely caused by the various physiological stresses during biofuel overproduction (e.g., changed cell membrane integrity and compositions [51]). Thereby, 13C-MFA not only applies for a better understanding of carbon flux distribution, but also provides a diagnostic analysis of the energy-dependent metabolic capability for product yields. If the microbial metabolism demands a considerable amount of ATP/NAD(P)H for both biosynthesis and cell maintenance, optimal product yield is unlikely to be achieved by overexpressing biosynthesis pathways or by redirecting metabolic fluxes to avoid carbon losses. A more promising approach would be to improve energetic prosperity or respiration efficiency, thereby allowing the cells to “burn” substrates more efficiently to satisfy the energy requirement [52,53].
Conclusions
Product yield is one of the main considerations of microbial cell factories [54]. Microbial productivity is not only associated with the efficiency of biosynthesis enzymes, but is also intertwined with the energy metabolism [55]. Simple 13C analysis can characterize the hosts’ intrinsic production yields under different carbon sources, and determine the contributions of the different pathways to biosynthesis. In addition, 13C-MFA can profile microbial fluxomes and determine the amount of extra substrates that the cell consumes to compensate for ATP losses from diverse cellular processes, which is essential to understand metabolic capability of a microbial host for maximal product yields. In the end, 13C-analysis, using the labeled product as internal standards, can also be employed to correct product measurement noises in fermentation processes due to water loss, product evaporation or degradation [56]. This review paper aims to emphasize the indispensable value of 13C-labeling techniques to the metabolic engineering field as we foresee an extended use of 13C-experiments for the development of microbial cell factories.
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
AMV/YJT wrote the introduction, product yield from rich medium, multiple carbon substrates and parts of yield from alternative pathways. LH wrote the bioenergetic efficiency. LY contributed to the section of product yield from alternative pathways. WH polished the paper. All the authors approved the final manuscript.
Contributor Information
Arul M Varman, Email: varmanarul@gmail.com.
Lian He, Email: l.he@go.wustl.edu.
Le You, Email: leyou@go.wustl.edu.
Whitney Hollinshead, Email: whollinshead@wustl.edu.
Yinjie J Tang, Email: yinjie.tang@seas.wustl.edu.
Acknowledgement
We thank Prof. Maciek Antoniewicz from University of Delaware for his advice. The authors also thank Katrina Leyden for her close reading of this manuscript. This work was supported in parts by the funding from National Science Foundation (MCB0954016) and the Bridge Funding from Washington University in St. Louis.
References
- Peralta-Yahya PP, Zhang F, del Cardayre SB, Keasling JD. Microbial engineering for the production of advanced biofuels. Nature. 2012;488:320–328. doi: 10.1038/nature11478. [DOI] [PubMed] [Google Scholar]
- Woolston BM, Edgar S, Stephanopoulos G. Metabolic engineering: past and future. Annu Rev Chem Biomol Eng. 2013;4:259–288. doi: 10.1146/annurev-chembioeng-061312-103312. [DOI] [PubMed] [Google Scholar]
- Atsumi S, Hanai T, Liao JC. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature. 2008;451:86–89. doi: 10.1038/nature06450. [DOI] [PubMed] [Google Scholar]
- Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R. Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature. 2011;476:355–359. doi: 10.1038/nature10333. [DOI] [PubMed] [Google Scholar]
- Zhang F, Carothers JM, Keasling JD. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat Biotech. 2012;30:354–359. doi: 10.1038/nbt.2149. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Lee SY. Microbial production of short-chain alkanes. Nature. 2013. advance online publication. [DOI] [PubMed]
- Xu P, Gu Q, Wang W, Wong L, Bower AGW, Collins CH, Koffas MAG. Modular optimization of multi-gene pathways for fatty acids production in E. coli. Nat Commun. 2013;4:1409. doi: 10.1038/ncomms2425. [DOI] [PubMed] [Google Scholar]
- Keasling JD, Mendoza A, Baran PS. Synthesis: a constructive debate. Nature. 2012;492:188–189. doi: 10.1038/492188a. [DOI] [PubMed] [Google Scholar]
- Bastian S, Liu X, Meyerowitz JT, Snow CD, Chen MMY, Arnold FH. Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli. Metab Eng. 2011;13:345–352. doi: 10.1016/j.ymben.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Huffer S, Roche CM, Blanch HW, Clark DS. Escherichia coli for biofuel production: bridging the gap from promise to practice. Trends Biotechnol. 2012;30:538–545. doi: 10.1016/j.tibtech.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos G. Challenges in engineering microbes for biofuels production. Science. 2007;315:801–804. doi: 10.1126/science.1139612. [DOI] [PubMed] [Google Scholar]
- Bogorad IW, Lin T-S, Liao JC. Synthetic non-oxidative glycolysis enables complete carbon conservation. Nature. 2013;502:693–697. doi: 10.1038/nature12575. [DOI] [PubMed] [Google Scholar]
- Shen CR, Lan EI, Dekishima Y, Baez A, Cho KM, Liao JC. Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli. Appl Environ Microbiol. 2011;77:2905–2915. doi: 10.1128/AEM.03034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond-Watts BB, Bellerose RJ, Chang MC. Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways. Nat Chem Biol. 2011;7:222–227. doi: 10.1038/nchembio.537. [DOI] [PubMed] [Google Scholar]
- Russell JB. The energy spilling reactions of bacteria and other organisms. J Mol Microbiol Biotechnol. 2007;13:1–11. doi: 10.1159/000103591. [DOI] [PubMed] [Google Scholar]
- Tang JK-H, You L, Blankenship RE, Tang YJ. Recent advances in mapping environmental microbial metabolisms through 13C isotopic fingerprints. J Roy Soc Interface. 2012;9:2767–2780. doi: 10.1098/rsif.2012.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Mouttaki H, Lin L, Huang R, Wu B, Hemme CL, He Z, Zhang B, Hicks LM, Xu J, Zhou J, Tang YJ. Characterization of the central metabolic pathways in Thermoanaerobacter sp. Strain X514 via isotopomer-assisted metabolite analysis. Appl Environ Microbiol. 2009;75:5001–5008. doi: 10.1128/AEM.00715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanopoulos GN, Aristidou AA, Nielsen J. Metabolic engineering principles and methodologies. San Diego: Academic; 1998. [Google Scholar]
- Misra A, Conway MF, Johnnie J, Qureshi TM, Derrick AM, Agbo EC, Sriram G. Metabolic analyses elucidate nontrivial gene targets for amplifying dihydroartemisinic acid production in yeast. Front Microbiol. 2013;4(200) doi: 10.3389/fmicb.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers PF, Burgard AP, Dasika MS, Nowroozi F, Van Dien S, Keasling JD, Maranas CD. Metabolic flux elucidation for large-scale models using 13C labeled isotopes. Metab Eng. 2007;9:387–405. doi: 10.1016/j.ymben.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanopoulos G. Metabolic fluxes and metabolic engineering. Metab Eng. 1999;1:1–11. doi: 10.1006/mben.1998.0101. [DOI] [PubMed] [Google Scholar]
- Zamboni N, Fendt SM, Rühl M, Sauer U. 13C-based metabolic flux analysis. Nat Protoc. 2009;4:878–892. doi: 10.1038/nprot.2009.58. [DOI] [PubMed] [Google Scholar]
- Zamboni N, Sauer U. Novel biological insights through metabolomics and 13C-flux analysis. Curr Opin Microbiol. 2009;12:553–558. doi: 10.1016/j.mib.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Wiechert W. 13C Metabolic flux analysis. Metab Eng. 2001;3:195–206. doi: 10.1006/mben.2001.0187. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR, Kraynie DF, Laffend LA, Gonzalez-Lergier J, Kelleher JK, Stephanopoulos G. Metabolic flux analysis in a nonstationary system: fed-batch fermentation of a high yielding strain of E. coli producing 1,3-propanediol. Metab Eng. 2007;9:277–292. doi: 10.1016/j.ymben.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varman AM, Xiao Y, Leonard E, Tang Y. Statistics-based model for prediction of chemical biosynthesis yield from Saccharomyces cerevisiae. Microb Cell Fact. 2011;10:45. doi: 10.1186/1475-2859-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletti P, Goyal Y, Varman AM, Feng X, Wu B, Tang Y. Evaluating factors that influence microbial synthesis yields by linear regression with numerical and ordinal variables. Biotechnol Bioeng. 2011;108:893–901. doi: 10.1002/bit.22996. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Feng X, Varman AM, He L, Yu H, Tang YJ. Kinetic modeling and isotopic investigation of isobutanol fermentation by two engineered Escherichia coli strains. Ind Eng Chem Res. 2012;51:15855–15863. doi: 10.1021/ie202936t. [DOI] [Google Scholar]
- Baez A, Cho K-M, Liao JC. High-flux isobutanol production using engineered Escherichia coli: a bioreactor study with in situ product removal. Appl Microbiol Biotechnol. 2011;90:1681–1690. doi: 10.1007/s00253-011-3173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Ruan Z, Liu Z, Wu SG, Varman AM, Liu Y, Tang YJ. Engineering Escherichia coli to convert acetic acid to free fatty acids. Biochem Eng J. 2013;76:60–69. [Google Scholar]
- Goodson C, Roth R, Wang ZT, Goodenough U. Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost. Eukaryot Cell. 2011;10:1592–1606. doi: 10.1128/EC.05242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Hua Q, Shimizu K. Metabolic flux analysis in Synechocystis using isotope distribution from 13C-labeled glucose. Metab Eng. 2002;4:202–216. doi: 10.1006/mben.2002.0226. [DOI] [PubMed] [Google Scholar]
- Varman AM, Yu Y, You L, Tang YJ. Photoautotrophic production of D-lactic acid in an engineered cyanobacterium. Microb Cell Fact. 2013;12:117. doi: 10.1186/1475-2859-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou JS-C, Balkwill DL, Drake GR, Tanner RS. Clostridium carboxidivorans sp. nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int J Syst Evol Microbiol. 2005;55:2085–2091. doi: 10.1099/ijs.0.63482-0. [DOI] [PubMed] [Google Scholar]
- Mattozzi M, Ziesack M, Voges MJ, Silver PA, Way JC. Expression of the sub-pathways of the Chloroflexus aurantiacus 3-hydroxypropionate carbon fixation bicycle in E. coli: Toward horizontal transfer of autotrophic growth. Metab Eng. 2013;16:130–139. doi: 10.1016/j.ymben.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Atsumi S, Liao J. Directed evolution of Methanococcus jannaschii citramalate synthase for biosynthesis of 1-propanol and 1-butanol by Escherichia coli. Appl Environ Microbiol. 2008;74:7802–7808. doi: 10.1128/AEM.02046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehler TM, Jorgensen BB. Microbial life under extreme energy limitation. Nat Rev Micro. 2013;11:83–94. doi: 10.1038/nrmicro2939. [DOI] [PubMed] [Google Scholar]
- Bodegom P. Microbial maintenance: a critical review on its quantification. Microb Ecol. 2007;53:513–523. doi: 10.1007/s00248-006-9049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A, Palsson BO. Stoichiometric flux balance models quantitatively predict growth and metabolic by-product secretion in wild-type Escherichia coli W3110. Appl Environ Microbiol. 1994;60:3724–3731. doi: 10.1128/aem.60.10.3724-3731.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumori F, Rees D, Brindle KM, Radda GK, Campbell ID. 31P NMR saturation transfer studies of aerobic Escherichia coli cells. Biochim Biophys Acta (BBA) - Mol Cell Res. 1988;969:185–193. doi: 10.1016/0167-4889(88)90074-2. [DOI] [PubMed] [Google Scholar]
- Bekker M, de Vries S, Ter Beek A, Hellingwerf KJ, de Mattos MJT. Respiration of Escherichia coli can be fully uncoupled via the nonelectrogenic terminal cytochrome bd-II oxidase. J Bacteriol. 2009;191:5510–5517. doi: 10.1128/JB.00562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Hellingwerf KJ, de Mattos MJ T, Bekker M. Uncoupling of substrate-level phosphorylation in Escherichia coli during glucose-limited growth. Appl Environ Microbiol. 2012;78:6908–6913. doi: 10.1128/AEM.01507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 4. New York: W H Freeman; 1995. [Google Scholar]
- Varela CA, Baez ME, Agosin E. Osmotic stress response: quantification of cell maintenance and metabolic fluxes in a lysine-overproducing strain of Corynebacterium glutamicum. Appl Environ Microbiol. 2004;70:4222–4229. doi: 10.1128/AEM.70.7.4222-4229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer U, Bailey JE. Estimation of P-to-O ratio in Bacillus subtilis and its influence on maximum riboflavin yield. Biotechnol Bioeng. 1999;64:750–754. doi: 10.1002/(SICI)1097-0290(19990920)64:6<750::AID-BIT15>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Lennen RM, Pfleger BF. Engineering Escherichia coli to synthesize free fatty acids. Trends Biotechnol. 2012;30:659–667. doi: 10.1016/j.tibtech.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Xiao Y, Gebreselassie N, Zhang F, Antoniewicz MR, Tang YJ, Peng L. Central metabolic responses to the overproduction of fatty acids in Escherichia coli based on 13C-metabolic flux analysis. Biotechnol Bioeng. 2014;111:575–585. doi: 10.1002/bit.25124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keasling JD. Manufacturing molecules through metabolic engineering. Science. 2010;330:1355–1358. doi: 10.1126/science.1193990. [DOI] [PubMed] [Google Scholar]
- Mainguet SE, Gronenberg LS, Wong SS, Liao JC. A reverse glyoxylate shunt to build a non-native route from C4 to C2 in Escherichia coli. Metab Eng. 2013;19:116–127. doi: 10.1016/j.ymben.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee KH, Kim TY, Lee SY. Metabolic engineering of Escherichia coli for the production of L-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci U S A. 2007;104:7797–7802. doi: 10.1073/pnas.0702609104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennen RM, Kruziki MA, Kumar K, Zinkel RA, Burnum KE, Lipton MS, Hoover SW, Ranatunga DR, Wittkopp TM, Marner WD, Pfleger BF. Membrane stresses induced by overproduction of free fatty acids in Escherichia coli. Appl Environ Microbiol. 2011;77:8114–8128. doi: 10.1128/AEM.05421-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Zhao H. Investigating xylose metabolism in recombinant Saccharomyces cerevisiae via 13C metabolic flux analysis. Microb Cell Fact. 2013;12:114. doi: 10.1186/1475-2859-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Reinefeld J, Stellmacher R, Schäfer R, Lange A, Meyer H, Lalk M, Zelder O, von Abendroth G, Schröder H, Haefner S, Wittmann C. Systems-wide analysis and engineering of metabolic pathway fluxes in bio-succinate producing Basfia succiniciproducens. Biotechnol Bioeng. 2013;110:3013–3023. doi: 10.1002/bit.24963. [DOI] [PubMed] [Google Scholar]
- Chen G-Q. New challenges and opportunities for industrial biotechnology. Microb Cell Fact. 2012;11:111. doi: 10.1186/1475-2859-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajikumar PK, Xiao W-H, Tyo KEJ, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science. 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Yuan J, Kimball EH, Rabinowitz JD. Absolute quantitation of intracellular metabolite concentrations by an isotope ratio-based approach. Nat Protocol. 2008;3:1299–1311. doi: 10.1038/nprot.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]