Abstract
Background
There are limited data on the effectiveness of employer-sponsored financial incentives for employee weight loss.
Objective
To test the effectiveness of two financial incentive designs for promoting weight loss among obese employees.
Design
Randomized controlled trial. Allocation sequence was generated dynamically at the time of request of treatment assignment. Participants were unblinded to assignment; investigators were blinded to assignment until collection of primary outcome data.
Setting
Children’s Hospital of Philadelphia
Participants
105 employees with a body mass index between 30 and 40 kg/m2
Interventions
24 weeks of monthly weigh-ins (control)(n=35); individual incentive, designed as $100 per person per month for meeting or exceeding target weight loss (individual arm)(n=35); group incentive, designed as $500 per month split between any participant within groups of 5 who met or exceeded their target weight loss (group arm)(n=35).
Measurements
Weight loss after 24 weeks (primary outcome); weight loss after 36 weeks, changes in behavioral mediators of weight loss (secondary outcomes).
Results
Group incentive participants lost more weight than individual arm participants (between-group difference in weight loss favoring group = mean 9.7 pounds, 95% CI 4.4 to 14.9; P < 0.001). Twelve weeks after incentives ended and adjusting for 3-group comparisons, group arm participants maintained greater weight loss than control arm participants (between-group difference in weight loss = mean 6.5 pounds, 95% CI 1.2 to 11.7; P = 0.016) but not more than individual arm participants (difference = 5.9 pounds, 95% CI, 0.8 to 11.0; P = 0.024).
Limitations
Single employer, short follow-up
Conclusions
A group-based financial incentive was more effective than an individual incentive and monthly weigh-ins at promoting weight loss among obese employees at 24 weeks.
Primary Funding Source
National Institute on Aging
Trial Registration
ClinicalTrials.gov Identifier: NCT01208350
INTRODUCTION
Most adults in the United States are overweight or obese (1), a public health challenge associated with increased mortality (2–4) and higher costs for employers (5), private payers (6), and public health insurance programs (7–9). Despite the health and economic consequences of obesity, there has been limited success in alleviating the problem. Consequently there is broad interest in new approaches to combat obesity and change behaviors that contribute to it (10). One approach that has shown promise in promoting healthy behaviors is the use of financial incentives. An estimated 67% of large employers are using these strategies (11) in the hopes of decreasing the incidence of chronic disease and slowing the growth of health care costs (12, 13).
While previous studies have shown that financial incentives can produce short-term weight loss (14–16), prior interventions have focused more on incentivizing individuals and less on leveraging the group structure inherent in workplace settings to potentially achieve greater effectiveness (17). The goal of this study was to test the effectiveness of 2 incentive designs in promoting weight loss among obese employees. Both designs used the same upfront allocation of resources but delivered the incentive through an individually-targeted approach or a group-based approach.
METHODS
Design Overview
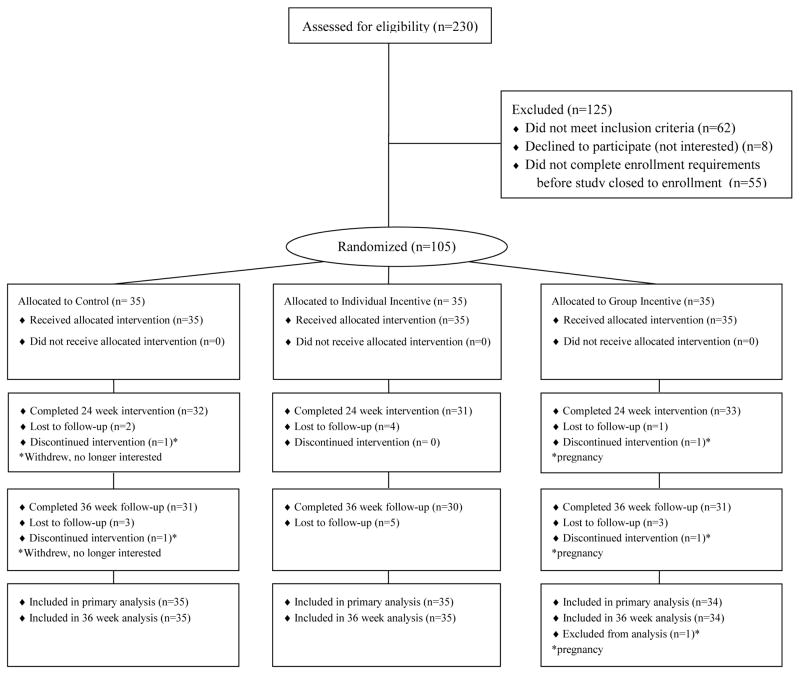
We conducted a 36-week parallel design randomized, controlled trial between March 17, 2011 and January 21, 2012. One hundred and five participants (Figure 1) gave their informed consent, were given the goal of losing 1 pound per week for 24 weeks (18, 19), and were randomly assigned to a monthly weigh-in control group or 1 of 2 monthly financial incentive groups. Weights were measured using incentaHEALTH™ workplace scales that provided precision to 0.2 pounds. All participants had access to a secure website to track their individual progress and complete questionnaires. The protocol was approved by the institutional review board of the University of Pennsylvania.
Figure 1.
Flow of Study Participants
Setting and Participants
Eligible participants were employees of the Children’s Hospital of Philadelphia who were between ages 18 and 70 and had a body mass index of 30 to 40 kilograms per meter squared. The upper age was set at 70 because there may be less benefit from weight reduction after age 70 than at younger ages (20). Individuals with a body mass index less than 30 kilograms per meter squared were excluded to ensure all participants could safely lose, and would be likely to benefit from losing, the target weight of 24 pounds over the 24-week intervention. Other exclusion criteria included conditions that would make participation infeasible (e.g., inability to consent or illiteracy) or potentially unsafe [e.g., current treatment for substance abuse, consumption of > 5 alcoholic drinks per day, addiction to prescription medications or street drugs, serious psychiatric diagnoses, myocardial infarction or stroke in the last 6 months, metastatic cancer, diabetes requiring treatment with medication other than metformin, currently pregnant or breastfeeding, or having a history of an eating disorder or unsafe weight loss behaviors (e.g., laxative or diuretic use)].
Participants were recruited through workplace flyers, posters, and email newsletters. Potential participants visited the study website to complete a screening questionnaire. Those who were eligible were required to complete a weigh-in on the workplace scale to confirm their body mass index. Individuals who met all eligibility criteria were then asked to complete an online informed consent document. All participants were recruited in March and April 2011.
Randomization and Interventions
The study coordinator requested treatment assignment via a web-based platform, which assigned participants to the 3 study arms using 1:1:1 central computerized randomization with a block size of 15. The allocation sequence was generated dynamically by the randomization program, subject to the constraint that within each block of 15 participants, 5 were assigned to each of the 3 arms; research team members were thus unable to predict future assignments. Arm assignments were communicated to participants via an automated secure website message and email or text message. Neither the participants nor the study coordinator could be blinded to arm assignment due to the nature of the interventions. Data analysts and all investigators were blinded to arm assignment until collection of primary outcome data was complete.
Control arm participants were provided with a link to the Weight-control Information Network (http://win.niddk.nih.gov/) of the National Institute of Diabetes and Digestive and Kidney Diseases and were both scheduled for and reminded via automated email or text message to attend monthly weigh-ins on the workplace scales. All weights collected were displayed graphically to each participant through the secure website. After each monthly weigh-in, an automated message notified participants of whether they met or did not meet their weight loss goal for that 4-week period.
Individual arm participants received the information that control arm participants received but were also told that $100 would be set aside for them at baseline, 4 weeks, 8 weeks, 12 weeks, 16 weeks, and 20 weeks, and that the $100 would be electronically transmitted to them if they met or exceeded their target monthly weight loss as determined by their end-of-month weigh-in. After each monthly weigh-in, an automated message notified participants of their earnings, or for those not meeting the target, of what they would have earned if they had met their weight loss goal. The $100 per participant per month incentive allocation is comparable to the incentives used in previous studies (15, 16) and is within the range of what employers are allowed to offer as health outcomes-based incentives (21). The total upfront allocation of incentives for meeting weight loss goals in the individual arm was $21,000.
Group arm participants received the same information as control arm participants. Similar to the individual arm, the upfront allocation of incentives for meeting weight loss goals was $100 per participant per month (totaling $21,000). However, group arm participants were placed into groups of 5 and were told they would not learn the identities of the 4 other individuals in their group. At the end of each 4-week period during the 24-week intervention, $500 was split among participants in each group who were at or below their monthly target weight. If no participant met his/her weight loss goal, then no money was distributed. In cases where a group arm member withdrew from the study, the remaining members of that group were still eligible to receive the full $500 per month. After each monthly weigh-in, an automated message notified participants of their earnings or, if they failed to meet the target, what they would have earned if they had met their weight loss goal.
We used 2 strategies to maximize retention of study participants. First, participants received $20 for completing each monthly weigh-in, $50 for completing the 24-week weigh-in, and $50 for completing the 36-week weigh-in. These participation incentives brought the overall resource allocation for incentives (i.e., for both participation in scheduled weigh-ins and meeting weight loss goals) to $28,000 in each incentive arm. Second, the weight loss goal trajectory was adjusted every 4 weeks for participants who failed to attain their monthly goal. In these cases the slope of the trajectory was increased such that the overall weight loss goal of 24 pounds remained, but less successful participants would not have to immediately lose large amounts of weight to meet their monthly goals. The rate of weight loss when trajectories were adjusted was capped at 2 pounds per week to ensure a safe rate of weight loss. A similar approach was used in previous studies and resulted in participant loss to follow up rates of just 8.7% (15) and 9.1% (16).
Participants were monitored for excessive weight loss, defined as losing more than 5 pounds in one week, 8 pounds in two weeks, or 12 pounds in four weeks. If weight loss exceeded any of these thresholds, the study coordinator contacted the participant to inquire about their health status and use of diuretics, diet pills, purging, or excessive exercise.
Outcomes and Follow-up
Our primary outcome was weight loss at 24 weeks. We hypothesized that group arm participants would have greater weight loss that control arm participants, individual arm participants would have greater weight loss than control arm participants, and group arm participants would have more weight loss than individual arm participants.
Secondary outcomes included weight loss at 36 weeks (i.e., 12 weeks after incentives ended) and changes in physical activity, eating behaviors, and participation in weight-related wellness programs from baseline to primary outcome measurement at 24 weeks. Physical activity was measured at baseline, 24 weeks, and 36 weeks through online administration of the short form of the International Physical Activity Questionnaire (22) and was operationalized as metabolic equivalent of task (MET)-minutes of physical activity during the last 7 days. Eating behaviors were measured at baseline, 24 weeks, and 36 weeks through online administration of the Three Factor Eating Questionnaire-R18 (23, 24) and were operationalized as 0 to 100 scores in cognitive restraint, emotional eating, and uncontrolled eating. Participation in weight-related wellness programs (employer-sponsored health coaching, employer incentive for fitness club attendance, or a commercial weight loss program) was measured at baseline, 24 weeks, and 36 weeks through an online questionnaire.
We also conducted exploratory analyses of weight loss goal attainment by month.
Statistical Analysis
All participants who were randomized to a study arm were included in the analyses testing for differences between arms, with the exception of one participant who became pregnant during the intervention period and was excluded from all analyses because she no longer met study inclusion criteria. SAS software Version 9.3 (SAS Institute, Inc., Cary, NC) was used to analyze the data.
For the primary outcome of 24-week weight loss and the secondary outcome of 36-week weight loss, we performed multiple imputation using PROC MI to derive missing 24-week and 36-week weights (25). For each of the five imputed datasets we used PROC GLM to conduct t-tests for direct comparisons of outcomes by arm; we also assessed the impact of adjusting for demographic variables. The results were then combined using PROC MIANALYZE. More information about the multiple imputation procedures is shown in the Appendix.
In the remaining secondary outcome and exploratory analyses we used observed data only. For continuous variables, we used PROC GLM to conduct t-tests, with the exception of the exploratory outcome of number of months in which weight loss goals were met; in this case we used PROC NPAR1WAY to conduct Wilcoxon-Mann-Whitney tests. For categorical variables, we used PROC FREQ to conduct Pearson χ2 tests or Fisher’s exact tests.
All hypothesis tests were 2-sided. To maintain the Type I error rate while testing the three hypotheses of primary interest, we used a Bonferroni correction to define an α of 0.0167 as our threshold for statistical significance.
For power calculations we defined 11 pounds as a clinically significant amount of weight loss in this population (26, 27), assumed an 11 pound standard deviation for weight loss (16), and used a two-sided α of 0.0167. Based on these assumptions, 27 subjects per arm would provide 90% power to detect an 11 pound difference in weight loss between arms. We increased this number to 35 subjects per arm to allow for a 20% loss to follow-up rate and accommodate the need for groups of 5 participants in the group arm.
Sensitivity Analyses
We conducted 2 sensitivity analyses to gauge the robustness of the primary outcome results to inclusion of the participant who withdrew after 10 weeks due to pregnancy and evaluation of longitudinal 24-week weight change instead of aggregate 24-week weight loss. In the first, we repeated the primary outcome analysis with an imputed 24-week weight for the participant who was withdrawn due to pregnancy (details in Appendix). In the second, we used PROC GENMOD to conduct a repeated measures analysis using observed data and generalized estimating equations.
Role of the Funding Source
This work was supported by grant RC2103282621 from the National Institute on Aging. Support was also provided by the Department of Veterans Affairs and the Robert Wood Johnson Foundation. Neither the sponsors nor the funders had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
RESULTS
The sample was predominantly female (89%) and White (63%) or African American (30%). The mean baseline body mass index was 34.6 kilograms per meter squared. Other sample characteristics are shown in Table 1.
Table 1.
Characteristics of the Study Sample
| Participant Characteristics | N (%) or Mean (SD)
|
||
|---|---|---|---|
| Control (n=35) | Individual (n=35) | Group (n=35) | |
| Female | 32 (91) | 30 (86) | 31 (89) |
| Age (years) mean (SD) | 44.5 (10) | 44.4 (11) | 47.0 (9) |
| Initial weight measurements, mean (SD) | |||
| Weight (pounds) | 209.1 (32) | 210.3 (26) | 217.8 (33) |
| Body mass index (kg/m2) | 34.13 (3) | 34.43 (2) | 35.09 (3) |
| Race/ethnicity* | |||
| White, non-Hispanic | 22 (63) | 19 (54) | 25 (71) |
| African American, non-Hispanic | 8 (23) | 15 (43) | 8 (23) |
| Other non-Hispanic | 2 (6) | 0 (0) | 1 (3) |
| Hispanic | 3 (9) | 1 (3) | 1 (3) |
| Education | |||
| Less than college | 2 (6) | 1 (3) | 5 (14) |
| Some college | 10 (29) | 14 (40) | 11 (31) |
| College graduate | 9 (26) | 11 (31) | 10 (29) |
| Post-college degree | 14 (40) | 9 (26) | 9 (26) |
| Household income, $† | |||
| < $50,000 | 6 (17) | 7 (21) | 5 (14) |
| $50,000 to < $100,000 | 12 (34) | 13 (38) | 18 (51) |
| >= $100,000 | 17 (49) | 14 (41) | 12 (34) |
| Physical activity (MET minutes in last 7 days), mean (SD)‡ | 1986 (1816) | 2027 (1826) | 1395 (1498) |
| Eating behaviors | |||
| Cognitive restraint, mean (SD)§ | 45.4 (18) | 44.6 (19) | 40.4 (17) |
| Uncontrolled eating, mean (SD)§ | 43.2 (20) | 42.9 (19) | 49.8 (16) |
| Emotional eating, mean (SD)§ | 49.5 (28) | 48.6 (30) | 65.7 (24) |
| Participation in any weight-related program|| | 14 (40) | 15 (43) | 18 (51) |
| Importance of controlling weight, mean (SD)** | 9.0 (1.5) | 8.8 (1.6) | 8.9 (1.6) |
| Confidence in controlling weight, mean (SD)** | 7.3 (2.1) | 7.7 (2.2) | 7.0 (2.4) |
Abbreviations: MET, metabolic equivalent of task.
Self-identified by participants on baseline questionnaire.
Income data were missing for 1 individual arm participant.
MET minutes are a quantification of physical activity that reflect both intensity (in METs) and duration (in minutes) of activity.
Measured on a 0 to 100 scale. Higher scores signify more of that behavior.
Employer-sponsored personal health coaching, employer financial incentive for fitness club attendance, or commercial weight loss program.
Measured on a 0 to 10 scale. Higher scores signify greater importance of controlling weight and more confidence in controlling weight.
Ninety-six participants (91%) completed the 24 week weigh-in. At 24 weeks there was a statistically significant difference in weight loss between participants in the group and control arms (between-group difference in weight loss favoring group incentive = mean 9.7 pounds, 95% CI, 4.4 to 14.9; P < 0.001) and between the group and individual arms (between-group difference favoring group incentive = mean 7.0 pounds, 95% CI, 1.9 to 12.2; P = 0.008) (Table 2). We refit the models adding demographic variables (age, sex, education, race and household income) as covariates along with treatment group and found nearly identical results (data not shown). The results were nearly identical with inclusion of the participant who withdrew due to pregnancy (Appendix Table 1) and results were qualitatively the same when modeling longitudinal weight change (Appendix Table 2).
Table 2.
Weight Loss
| Measure* | Within-Group Change | Between Group Difference in Change | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control (n=35) | Individual (n=35) | Group (n=34) | Individual vs. Control | Group vs. Individual | Group vs. Control | |
| Weight loss at 24 weeks, pounds | ||||||
| Mean | 1.1 | 3.7 | 10.7 | 2.6 | 7.0 | 9.7 |
| 95% CI | −2.8, 5.0 | 0, 7.4 | 7.3, 14.2 | −2.9, 8.2 | 1.9, 12.2 | 4.4, 14.9 |
| P value for comparison | 0.34 | 0.008 | < 0.001 | |||
| Weight loss at 36 weeks, pounds | ||||||
| Mean | 1.0 | 1.7 | 7.5 | 0.6 | 5.9 | 6.5 |
| 95% CI | −2.6, 4.6 | −2.0, 5.4 | 3.7, 11.3 | −4.5, 5.7 | 0.8, 11.0 | 1.2, 11.7 |
| P value for comparison | 0.81 | 0.024 | 0.016 | |||
Abbreviation: CI, confidence interval
Conversion factor: To convert pounds to kilograms, multiply by 0.45.
Measures use both observed and imputed data.
With Bonferroni correction for three-way comparison, threshold for statistical significance = 0.0167.
At 24 weeks, group arm participants experienced a greater increase in cognitive restraint around eating compared to control (difference in mean change 15.4, 95% CI, 5.9 to 24.8; P = 0.002) and individual (difference in mean change 12.6, 95% CI, 3.3 to 22.0; P = 0.009) arm participants (Table 3). There were no statistically significant differences in uncontrolled eating, emotional eating, physical activity, or weight-related wellness program participation at 24 weeks.
Table 3.
24-Week Change in Potential Weight Loss Mediators*
| Measure | Within-Group Change | Between Group Difference in Change | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control (n=28) | Individual (n=29) | Group (n=30) | Individual vs. Control | Group vs. Individual | Group vs. Control | |
| Physical activity in last 7 days, MET-minutes† | ||||||
| Mean | 368‡ | 489 | 1087 | 121 | 597 | 718 |
| 95% CI | −521, 1258 | −419, 1398 | 29, 2144 | −1215, 1458 | −704, 1898 | −607, 2043 |
| P value for comparison | 0.86 | 0.36 | 0.28 | |||
| Cognitive restraint in eating§ | ||||||
| Mean | 4.6 | 7.3 | 19.9|| | 2.7 | 12.6 | 15.4 |
| 95% CI | −1.2, 10.4 | 2.1, 12.4 | 11.0, 28.9 | −6.7, 12.2 | 3.3, 22.0 | 5.9, 24.8 |
| P value for comparison | 0.57 | 0.009 | 0.002 | |||
| Uncontrolled eating§ | ||||||
| Mean | −3.8 | −2.6 | −6.8|| | 1.3 | −4.2 | −2.9 |
| 95% CI | −7.8, 0.1 | −6.2, 1.1 | −13.1, −0.4 | −5.3, 7.9 | −10.8, 2.3 | −9.5, 3.7 |
| P value for comparison | 0.70 | 0.20 | 0.38 | |||
| Emotional eating§ | ||||||
| Mean | 0.4 | 0.4 | −2.2 | −0.0 | −2.6 | −2.6 |
| 95% CI | −8.6, 9.4 | −5.3, 6.1 | −9.9, 5.4 | −10.4, 10.4 | −12.9, 7.7 | −13.0, 7.7 |
| P value for comparison | 1.00 | 0.61 | 0.62 | |||
| Started weight-related program during study** | ||||||
| % | 7 | 7 | 13 | 0 | 6 | 6 |
| 95% CI | 0, 24 | 0, 23 | 4, 31 | −25, 25 | −19, 31 | −20, 31 |
| P value for comparison | 1.00 | 0.67 | 0.67 | |||
| Continued weight-related program during study** | ||||||
| % | 21 | 34 | 37 | 13 | 2 | 15 |
| 95% CI | 6, 37 | 17, 52 | 19, 54 | −10, 36 | −22, 27 | −8, 38 |
| P value for comparison | 0.27 | 0.86 | 0.20 | |||
Abbreviations: MET, metabolic equivalent of task; CI, confidence interval
The response rate for the 24-week survey was 84%.
MET minutes are a quantification of physical activity that reflect both intensity (in METs) and duration (in minutes) of activity.
n=27 for this measure.
Higher scores signify more of that behavior.
n=29 for this measure.
Employer-sponsored personal health coaching, employer financial incentive for fitness club attendance, or commercial weight loss program.
With Bonferroni correction for three-way comparison, threshold for statistical significance = 0.0167.
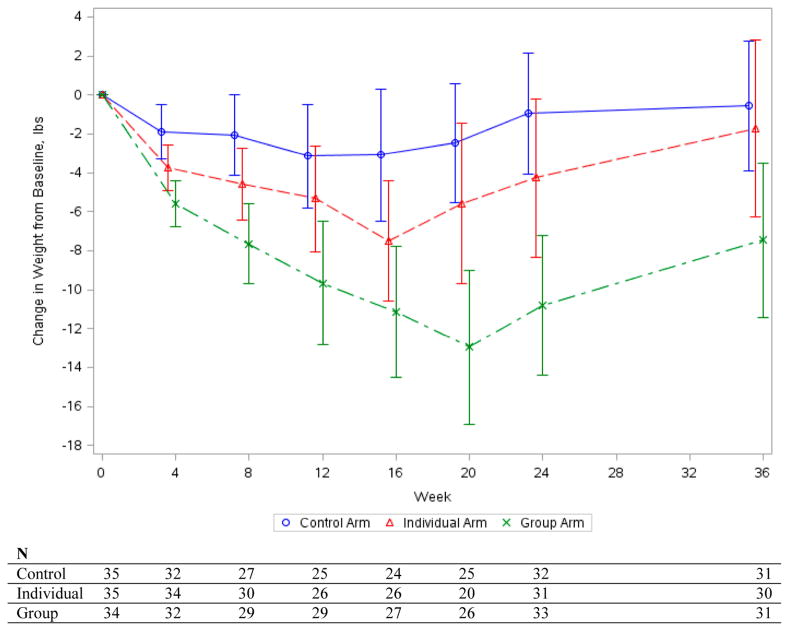
Ninety-two participants (88%) completed the 36 week weigh-in. At 36 weeks (Figure 3) there remained a statistically significant difference in weight loss relative to baseline between participants in group and control arms (between-group weight loss difference favoring the group incentive = mean 6.5 pounds, 95% CI, 1.2 to 11.7; P = 0.016) but not between the group and individual arms (between-group difference = mean 5.9 pounds, 95% CI, 0.8 to 11.0; P = 0.024) after the Bonferroni correction. There were no statistically significant differences in change in cognitive restraint around eating, uncontrolled eating, emotional eating, physical activity, or weight-related wellness program participation at 36 weeks (data not shown).
Figure 3. Weight Change through 24-Week Intervention and 36-Week Follow-up.
Error bars indicate 95% confidence intervals. 8 missing 24 week weights were imputed and 12 missing 36 week weights were imputed. Plots of mean weight change, the respective 95% confidence intervals, and individual weight change include both observed and imputed data.
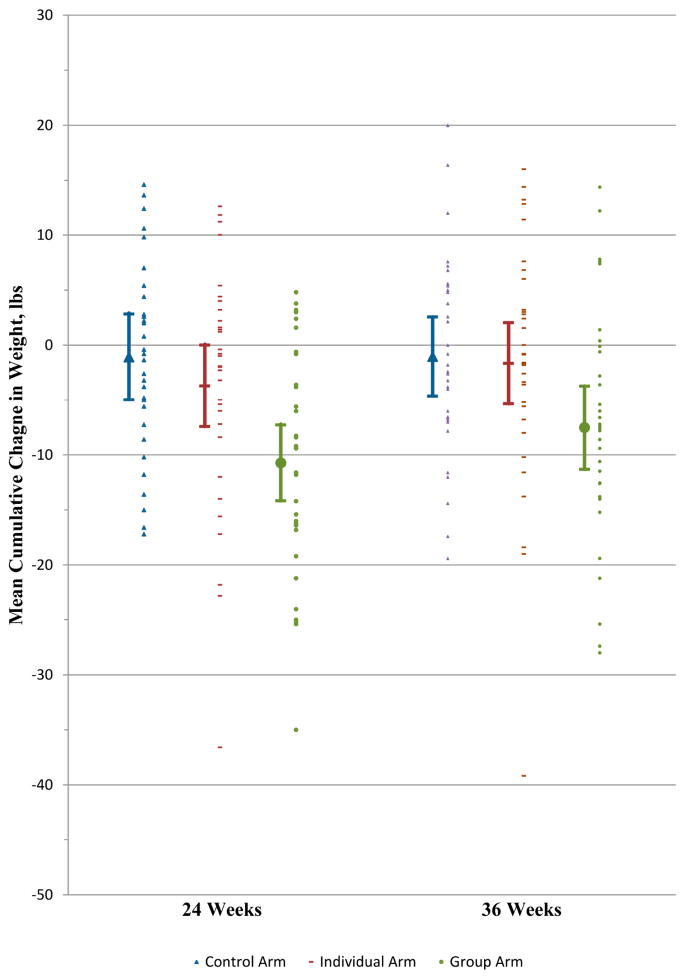
The monthly mean cumulative weight changes by arm are shown in Figure 2 and exploratory analyses of weight loss goal attainment by month and overall are presented in Appendix Table 3.
Figure 2. Mean Cumulative Weight Change by Month during 24-Week Intervention Period.
Error bars indicate 95% confidence intervals. Mean weight changes at each time point and the respective 95% confidence intervals include only observed data. The denominator for each arm in each month is shown at the bottom of the figure.
In exploratory analyses, we compared incentives earned for achieving weight loss goals, and the frequency of episodes of excessive weight loss over the 24-week intervention period. Participants in the group arm earned a mean $514.7 (SD $522.6); those in the individual arm earned a mean $128.6 (SD $165.5) (between-group difference = mean $386.1, 95% CI, $201.0 to $571.3; P < 0.001). There were 11 occurrences of excessive weight loss: 8 episodes among 6 group arm participants and 3 episodes in 1 individual arm participant. Investigation of these episodes did not reveal any unsafe weight loss strategies.
DISCUSSION
In this weight loss trial comparing 2 forms of financial incentive with an equal upfront allocation of resources, a group-based incentive was more effective than an individual incentive in promoting weight loss among obese employees at 24 weeks. The differences in weight loss between the group and control arm was sustained 12 weeks after the incentive intervention ended.
We searched the PubMed database using the terms financial incentives and weight loss to identify all trials published between January 1, 1980 and November 26, 2012 that evaluated the effects of financial incentives for weight loss. We identified 12 such studies (14–16, 28–36) that reported on weight loss but differed from our effort in that they either did not utilize a randomized design (28, 30, 33, 35, 36), did not include a follow-up period (28–30, 34, 36), were conducted outside of a workplace (15, 16, 28, 30–32, 35), offered incentives within a multifaceted intervention (31, 32, 34, 36), asked participants to put their own money at risk (16, 28–30), or did not compare an individual incentive to a group-based incentive (14–16, 27–34). Our study is the only randomized trial to have compared the effects of group-based and individual incentives, or to have demonstrated a statistically significant difference in weight loss between an incentive group and control group after incentives ended.
The greater effectiveness of the group incentive could be due to several factors. Above all, the opportunity to earn a reward larger than $100 for achieving a weight loss goal likely served as a strong motivator. Two factors would have augmented this motivation. First, people are often overly optimistic about their abilities relative to others (37) and thus might have expected greater success, and a larger reward, than fellow group members. Second, expectation of a larger reward would have been reinforced because most group members did not meet their weight loss goals in most months, leaving a larger reward for those who did meet goals.
The group arm also sought to introduce the threat of “social takeover” (knowledge that group members would acquire the incentive one had failed to earn) (38), competition, loss aversion (16, 39), and regret (40, 41). Though these factors may have also motivated weight loss, we are unable to determine their relative impact since we did not ask participants about their perceptions.
These results have important implications for future incentive design. First, more dollars were earned in the group arm than in the individual arm despite offering the same amount of incentives ex ante to participants. Second, although we did not design this study to compare daily versus monthly rewards, the amount of weight loss in the individual arm, when contrasted with prior studies that offered daily rewards (15, 16), suggests that more frequent rewards may be a key ingredient to the success of individually-targeted incentives. While frequent rewards require frequent weigh-ins and thus can be administratively complex, these results suggest there may be a tradeoff between the effectiveness and administrative simplicity of different incentive designs. Although administrative complexity could be reduced through technologies that provide “automated hovering” (42), future studies should explicitly examine reward frequency to inform program planners of the implications of different approaches.
Although we were not powered to detect differences in potential mediators of weight loss, our findings provide insight into how incentives for weight loss may impact obesity-related behaviors. Among group arm participants, we observed a statistically significant 24-week increase in cognitive restraint around eating, a key factor in weight management (43).
We tested one group-based incentive design, though other group-based designs are possible. Approaches like “The Biggest Loser,” for example, have received popular attention as a way to harness group dynamics to encourage weight loss. Since the winner-take-all nature of such approaches could be demotivating for all but the most successful person, however, we provided a reward to all participants who achieved monthly weight loss goals. Given the range of possible group-based incentive designs, more data are needed on their comparative effectiveness.
Our study has limitations. We tested these approaches in one group of employees that may not be generalizable to all settings. We were not able to collect data on all mechanisms through which incentives may motivate weight loss, though our measures provide some insight into processes. Our follow-up data are limited to 12 weeks after incentives (44). Although the adjustment of weight loss goal trajectories produced a retention rate that exceeded those of many weight loss studies (45–47), the larger monthly weight loss goals when trajectories adjusted could have diminished motivation to achieve these goals in subsequent months. Finally, our use of a Bonferroni correction for the three pairwise comparisons may be overly conservative.
In summary, this weight loss trial comparing 2 forms of financial incentive with an equal upfront allocation of resources found that a group-based incentive was more effective than an individual incentive in promoting weight loss among obese employees at 24 weeks. Most large employers are offering financial incentives to promote healthy lifestyle activities among employees (11, 48), and the Patient Protection and Affordable Care Act will allow health outcome-based incentives to grow to 30% of total health insurance premiums in 2014 (49, 50). As employers’ use of financial incentives to motivate healthy behaviors accelerates, this study demonstrates that varying features of incentive design can lead to important differences in the costs of incentives and their effects on health outcomes.
Acknowledgments
Funding/support: This work was supported by grant RC2103282621 from the National Institute on Aging. Support was also provided by the Department of Veterans Affairs and the Robert Wood Johnson Foundation. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
We thank Dana Gatto, BS and Lin Yang, MS of the Leonard Davis Institute of Health Economics Center for Health Incentives and Behavioral Economics, Penn CMU Roybal P30 Center on Behavioral Economics and Health, and the Department of Medicine in the Perelman School of Medicine of the University of Pennsylvania, and Robert Croner, MS and Daniel Buckalew, MS, both of the Children’s Hospital of Philadelphia, for their assistance.
APPENDIX
Multiple Imputation Methods
Multiple imputation was implemented using PROC MI in the SAS software package Version 9.3. The following variables were included as covariates to predict 24-week and 36-week weights: treatment arm, age, sex, race, education, household income, baseline weight, importance of controlling weight, and confidence in controlling weight. Importance of controlling weight and confidence in controlling weight were both measured in the online baseline survey on a 0 to 10 scale in which higher scores indicate greater importance of controlling weight and more confidence in controlling weight, respectively. The EM algorithm (51) was used to produce maximum likelihood estimates; because we had monotone missing data patterns, we utilized the parametric regression imputation procedure assuming multivariate normality and missing at random (MAR) data (52). After the five imputed datasets were obtained, we used PROC GLM to conduct t-tests for each dataset separately; results from these analyses were combined using the standard formulae presented by Rubin (52), as implemented in PROC MIANALYZE in the SAS software package Version 9.3.
Attainment of Weight Loss Goals (Appendix Table 3)
In the first month, more group arm participants met their weight loss goals as compared with control arm participants (difference in percentages 42, 95% CI, 19 to 62; P < 0.001). This pattern continued through the fourth month, when more group arm participants met their monthly goals as compared with control arm participants (difference in percentages 32, 95% CI, 9 to 53; P < 0.001).
Overall, there was a statistically significant difference in the median number of monthly weight loss goals met between group arm participants and both control (difference in medians 2, 95% CI, 1 to 3; P < 0.001) and individual arm (difference in medians 1, 95% CI, 0 to 2; P = 0.010) participants. However, there were no statistically significant differences in the proportion of participants in each arm who met the overall 24-pound weight loss goal.
Appendix Table 1.
24-Week Weight Loss Including the Participant Excluded Due to Pregnancy*
| Measure | Within-Group Change | Between Group Difference in Change | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control (n=35) | Individual (n=35) | Group (n=35) | Individual vs. Control | Group vs. Individual | Group vs. Control | |
| Weight loss, pounds† | ||||||
| Mean | 1.0 | 3.7 | 10.6ठ| 2.6 | 7.0 | 9.6 |
| 95% CI | −2.5, 4.5 | 0.0, 7.3 | 7.1, 14.1 | −2.3, 7.6 | 2.0, 11.9 | 4.6, 14.5 |
| P value for comparison | 0.30 | 0.006 | < 0.001 | |||
Abbreviation: CI, confidence interval
Conversion factor: To convert pounds to kilograms, multiply by 0.45.
This participant discontinued the study after 10 weeks.
Measure uses both observed and imputed data.
Appendix Table 2.
24-Week Longitudinal Weight Loss
| Measure | Within-Group Change | Between Group Difference in Change | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control (n=35) | Individual (n=35) | Group (n=35) | Individual vs. Control | Group vs. Individual | Group vs. Control | |
| Weight loss, pounds* | ||||||
| Mean | 2.3 | 4.7 | 13.0 | 2.5 | 8.3 | 10.7 |
| 95% CI | −1.3, 5.8 | 0.3, 9.1 | 9.1, 16.9 | −3.2, 8.1 | 2.4, 14.2 | 5.5, 16.0 |
| P value for comparison | 0.39 | 0.006 | < 0.001 | |||
Abbreviation: CI, confidence interval
Conversion factor: To convert pounds to kilograms, multiply by 0.45.
Measure uses only observed data. Every participant is included, utilizing only observed weight measurements.
Appendix Table 3.
Attainment of Weight Loss Goals
| Measure* | Within-Group* | Between Group Difference | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control (n=35) | Individual (n=35) | Group (n=35) | Individual vs. Control | Group vs. Individual | Group vs. Control | |
| Met monthly weight loss goal | ||||||
| Week 4 | ||||||
| % | 34 | 54 | 76 | 20 | 22 | 42 |
| 95% CI | 19, 52 | 37, 71 | 59, 89 | −5, 43 | −1, 45 | 19, 62 |
| P value for comparison | 0.148 | 0.077 | < 0.001 | |||
| Week 8 | ||||||
| % | 17 | 29 | 47 | 11 | 18 | 30 |
| 95% CI | 7, 34 | 15, 46 | 30, 65 | −13, 35 | −6, 40 | 6, 50 |
| P value for comparison | 0.39 | 0.140 | 0.010 | |||
| Week 12 | ||||||
| % | 6 | 20 | 47 | 14 | 27 | 41 |
| 95% CI | 1, 19 | 8, 37 | 30, 65 | −11, 38 | 3, 48 | 18, 60 |
| P value for comparison | 0.151 | 0.022 | < 0.001 | |||
| Week 16 | ||||||
| % | 3 | 14 | 35 | 11 | 21 | 32 |
| 95% CI | 0, 15 | 5, 30 | 20, 54 | −13, 35 | −3, 42 | 9, 53 |
| P value for comparison | 0.198 | 0.054 | < 0.001 | |||
| Week 20 | ||||||
| % | 3 | 9 | 18 | 6 | 9 | 15 |
| 95% CI | 0, 15 | 2, 23 | 7, 35 | −19, 30 | −15, 31 | −9, 37 |
| P value for comparison | 0.61 | 0.31 | 0.055 | |||
| Week 24 | ||||||
| % | 0 | 3 | 9 | 3 | 6 | 9 |
| 95% CI | 0, 10 | 0, 15 | 2, 24 | −22, 37 | −18, 29 | −15, 31 |
| P value for comparison | 1.00 | 0.36 | 0.114 | |||
| Met overall 24-pound weight loss goal | ||||||
| % | 0 | 3 | 15 | 3 | 12 | 15 |
| 95% CI | 0, 11 | 0, 17 | 5, 32 | −21, 27 | −13, 35 | −9, 38 |
| P value for comparison | 0.49 | 0.198 | 0.053 | |||
| Number of months met goal | ||||||
| Median | 0 | 1 | 2.5 | 0 | 1 | 2 |
| 95% CI | 0, 1 | 0, 1 | 1, 3 | 0, 1 | 0, 2 | 1, 3 |
| P value for comparison | 0.141 | 0.010 | < 0.001 | |||
Abbreviation: CI, confidence interval
Conversion factor: To convert pounds to kilograms, multiply by 0.45.
n is the number of participants in each arm at the start of the intervention. Since all measures in this table use observed data only, the n in each arm decreases over time due to missing data. The n’s by week by arm are shown in Figure 2.
With Bonferroni correction for three-way comparison, threshold for statistical significance = 0.0167.
Footnotes
Reproducible Research Statement
Protocol: Available to interested readers by contacting Dr. Kullgren at jkullgre@med.umich.edu.
Statistical Code: Available to interested readers by contacting Dr. Kullgren at jkullgre@med.umich.edu.
Data: Not available.
Author Contributions:
Conception and design: J. T. Kullgren, G. Loewenstein, D. A. Asch, K. G. Volpp
Analysis and interpretation of data: J. T. Kullgren, A. B. Troxel, Y. Tao, J. Zhu, K. G. Volpp
Drafting of the manuscript: J. T. Kullgren, A. B. Troxel, K. G. Volpp
Critical revision of the article for important intellectual content: J. T. Kullgren, A. B. Troxel, G. Loewenstein, D. A. Asch, L. A. Norton, L. Wesby, Y. Tao, J. Zhu, K. G. Volpp
Final approval of the article: J. T. Kullgren, A. B. Troxel, G. Loewenstein, D. A. Asch, L. A. Norton, L. Wesby, Y. Tao, J. Zhu, K. G. Volpp
Statistical expertise: A. B. Troxel, Y. Tao, J. Zhu
Obtaining of funding: D. A. Asch, K. G. Volpp
Administrative, technical, or logistic support: J. T. Kullgren, L. A. Norton, L. Wesby, K. G. Volpp
Collection and assembly of data: L. A. Norton, L. Wesby, Y. Tao, J. Zhu
Financial Disclosures: Dr. Volpp has served as a consultant to CVS Caremark and VAL Health and receives research funding from Humana, Horizon BCBS, Mckinsey, and CVS Caremark, none of which is related directly to the subject of this manuscript. Dr. Asch has served as a consultant to VAL Health. Dr. Loewenstein also has served as a consultant to CVS Caremark and VAL Health and receives research funding from Humana and CVS Caremark.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361(23):2252–60. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkelstein EA, Brown DS, Wrage LA, Allaire BT, Hoerger TJ. Individual and Aggregate Years-of-life-lost Associated With Overweight and Obesity. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.253. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein E, Fiebelkorn C, Wang G. The costs of obesity among full-time employees. Am J Health Promot. 2005;20(1):45–51. doi: 10.4278/0890-1171-20.1.45. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28(5):w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein EA, Fiebelkorn IC, Wang G. State-level estimates of annual medical expenditures attributable to obesity. Obes Res. 2004;12(1):18–24. doi: 10.1038/oby.2004.4. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein EA, Trogdon JG, Brown DS, Allaire BT, Dellea PS, Kamal-Bahl SJ. The lifetime medical cost burden of overweight and obesity: implications for obesity prevention. Obesity (Silver Spring) 2008;16(8):1843–8. doi: 10.1038/oby.2008.290. [DOI] [PubMed] [Google Scholar]
- 9.Cai L, Lubitz J, Flegal KM, Pamuk ER. The predicted effects of chronic obesity in middle age on medicare costs and mortality. Med Care. 2010;48(6):510–7. doi: 10.1097/MLR.0b013e3181dbdb20. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder SA. Shattuck Lecture. We can do better--improving the health of the American people. N Engl J Med. 2007;357(12):1221–8. doi: 10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]
- 11.16th Annual Towers Watson/National Business Group on Health Employer Survey on Purchasing Value in Health Care. Towers Watson/National Business Group on Health; 2011. [Google Scholar]
- 12.Claxton G, DiJulio B, Whitmore H, Pickreign J, McHugh M, Finder B, et al. Job-based health insurance: costs climb at a moderate pace. Health Aff (Millwood) 2009;28(6):w1002–12. doi: 10.1377/hlthaff.28.6.w1002. [DOI] [PubMed] [Google Scholar]
- 13.Heinen L, Darling H. Addressing obesity in the workplace: the role of employers. Milbank Q. 2009;87(1):101–22. doi: 10.1111/j.1468-0009.2009.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelstein EA, Linnan LA, Tate DF, Birken BE. A pilot study testing the effect of different levels of financial incentives on weight loss among overweight employees. J Occup Environ Med. 2007;49(9):981–9. doi: 10.1097/JOM.0b013e31813c6dcb. [DOI] [PubMed] [Google Scholar]
- 15.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631–7. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John LK, Loewenstein G, Troxel AB, Norton L, Fassbender JE, Volpp KG. Financial incentives for extended weight loss: a randomized, controlled trial. J Gen Intern Med. 2011;26(6):621–6. doi: 10.1007/s11606-010-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffery RW. Financial incentives and weight control. Prev Med. 2011 doi: 10.1016/j.ypmed.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aim for a Healthy Weight. U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; 2005. [Google Scholar]
- 19.Talking With Patients About Weight Loss: Tips for Primary Care Professionals. National Institute of Diabetes and Digestive and Kidney Diseases; 2005. [Google Scholar]
- 20.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 21.Nondiscrimination and Wellness Programs in Health Coverage in the Group Market. Federal Register. 2006;71(239):75013–55. [Google Scholar]
- 22.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 23.Angle S, Engblom J, Eriksson T, Kautiainen S, Saha MT, Lindfors P, et al. Three factor eating questionnaire-R18 as a measure of cognitive restraint, uncontrolled eating and emotional eating in a sample of young Finnish females. Int J Behav Nutr Phys Act. 2009;6:41. doi: 10.1186/1479-5868-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Lauzon B, Romon M, Deschamps V, Lafay L, Borys JM, Karlsson J, et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr. 2004;134(9):2372–80. doi: 10.1093/jn/134.9.2372. [DOI] [PubMed] [Google Scholar]
- 25.Little RJA, Rubin D. Statistical Analysis with Missing Data. 2. Hoboken: John Wiley & Sons; 2002. [Google Scholar]
- 26.Summary: weighing the options--criteria for evaluating weight-management programs. Committee to Develop Criteria for Evaluating the Outcomes of Approaches to Prevent and Treat Obesity Food and Nutrition Board, Institute of Medicine, National Academy of Sciences. J Am Diet Assoc. 1995;95(1):96–105. [PubMed] [Google Scholar]
- 27.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 (Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 28.Butsch WS, Ard JD, Allison DB, Patki A, Henson CS, Rueger MM, et al. Effects of a reimbursement incentive on enrollment in a weight control program. Obesity (Silver Spring) 2007;15(11):2733–8. doi: 10.1038/oby.2007.325. [DOI] [PubMed] [Google Scholar]
- 29.Forster JL, Jeffery RW, Sullivan S, Snell MK. A work-site weight control program using financial incentives collected through payroll deduction. J Occup Med. 1985;27(11):804–8. doi: 10.1097/00043764-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Hubbert KA, Bussey BF, Allison DB, Beasley TM, Henson CS, Heimburger DC. Effects of outcome-driven insurance reimbursement on short-term weight control. Int J Obes Relat Metab Disord. 2003;27(11):1423–9. doi: 10.1038/sj.ijo.0802403. [DOI] [PubMed] [Google Scholar]
- 31.Jeffery RW, Wing RR. Long-term effects of interventions for weight loss using food provision and monetary incentives. J Consult Clin Psychol. 1995;63(5):793–6. doi: 10.1037//0022-006x.63.5.793. [DOI] [PubMed] [Google Scholar]
- 32.Jeffery RW, Wing RR, Thorson C, Burton LR, Raether C, Harvey J, et al. Strengthening behavioral interventions for weight loss: a randomized trial of food provision and monetary incentives. J Consult Clin Psychol. 1993;61(6):1038–45. doi: 10.1037//0022-006x.61.6.1038. [DOI] [PubMed] [Google Scholar]
- 33.Lahiri S, Faghri PD. Cost-effectiveness of a workplace-based incentivized weight loss program. J Occup Environ Med. 2012;54(3):371–7. doi: 10.1097/JOM.0b013e318247a394. [DOI] [PubMed] [Google Scholar]
- 34.Morgan PJ, Collins CE, Plotnikoff RC, Cook AT, Berthon B, Mitchell S, et al. Efficacy of a workplace-based weight loss program for overweight male shift workers: the Workplace POWER (Preventing Obesity Without Eating like a Rabbit) randomized controlled trial. Prev Med. 2011;52(5):317–25. doi: 10.1016/j.ypmed.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Relton C, Strong M, Li J. The ‘Pounds for Pounds’ weight loss financial incentive scheme: an evaluation of a pilot in NHS Eastern and Coastal Kent. J Public Health (Oxf) 2011;33(4):536–42. doi: 10.1093/pubmed/fdr030. [DOI] [PubMed] [Google Scholar]
- 36.Seidman LS, Sevelius GG, Ewald P. A cost-effective weight loss program at the worksite. J Occup Med. 1984;26(10):725–30. doi: 10.1097/00043764-198410000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Swenson O. Are we all less risky and more skillful than our fellow drivers? Acta Psychologica. 1981;47(12):143–8. [Google Scholar]
- 38.Hoelzl E, Loewenstein G. Wearing out your shoes to prevent someone else from stepping into them: Anticipated regret and social takeover in sequential decisions. Organizational Behavior and Human Decision Processes. 2005;98:15–27. [Google Scholar]
- 39.Kahneman D, Tversky A. Prospect Theory: An Analysis of Decision under Risk. Econometrica. 1979;47(2):263–91. [Google Scholar]
- 40.Connolly T, Butler D. Regret in economic and psychological theories of choice. Journal of Behavioral Decision Making. 2006;19(2):139–54. [Google Scholar]
- 41.Zeelenberg M, Pieters R. Consequences of regret aversion in real life: The case of the Dutch postcode lottery. Organizational Behavior and Human Decision Processes. 2004;93:155–68. [Google Scholar]
- 42.Asch DA, Muller RW, Volpp KG. Automated hovering in health care--watching over the 5000 hours. N Engl J Med. 2012;367(1):1–3. doi: 10.1056/NEJMp1203869. [DOI] [PubMed] [Google Scholar]
- 43.Johnson F, Pratt M, Wardle J. Dietary restraint and self-regulation in eating behavior. Int J Obes (Lond) 2012;36(5):665–74. doi: 10.1038/ijo.2011.156. [DOI] [PubMed] [Google Scholar]
- 44.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–67. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Davis MJ, Addis ME. Predictors of attrition from behavioral medicine treatments. Ann Behav Med. 1999;21(4):339–49. doi: 10.1007/BF02895967. [DOI] [PubMed] [Google Scholar]
- 46.Honas JJ, Early JL, Frederickson DD, O’Brien MS. Predictors of attrition in a large clinic-based weight-loss program. Obes Res. 2003;11(7):888–94. doi: 10.1038/oby.2003.122. [DOI] [PubMed] [Google Scholar]
- 47.Teixeira PJ, Going SB, Houtkooper LB, Cussler EC, Metcalfe LL, Blew RM, et al. Pretreatment predictors of attrition and successful weight management in women. Int J Obes Relat Metab Disord. 2004;28(9):1124–33. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- 48.Health Care Changes Ahead. Towers Watson; 2011. [Google Scholar]
- 49.Volpp KG, Asch DA, Galvin R, Loewenstein G. Redesigning employee health incentives--lessons from behavioral economics. N Engl J Med. 2011;365(5):388–90. doi: 10.1056/NEJMp1105966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madison KM, Volpp KG, Halpern SD. The law, policy, and ethics of employers’ use of financial incentives to improve health. J Law Med Ethics. 2011;39(3):450–68. doi: 10.1111/j.1748-720X.2011.00614.x. [DOI] [PubMed] [Google Scholar]
- 51.Dempster AP, Laird NM, Rubin D. Maximum likelihood from incomplete data vis the EM algorithm. Journal of the Royal Statistical Society. 1977;(39):1–38. Series B. [Google Scholar]
- 52.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]