Abstract
Purpose
Reduced retinal image contrast produced by accommodative lag is implicated with myopia development. Here, we measure accommodative error and retinal image quality from wavefront aberrations in myopes and emmetropes when they perform visually demanding and naturalistic tasks.
Methods
Wavefront aberrations were measured in 10 emmetropic and 11 myopic adults at three distances (100, 40, and 20 cm) while performing four tasks (monocular acuity, binocular acuity, reading, and movie watching). For the acuity tasks, measurements of wavefront error were obtained near the end point of the acuity experiment. Refractive state was defined as the target vergence that optimizes image quality using a visual contrast metric (VSMTF) computed from wavefront errors.
Results
Accommodation was most accurate (and image quality best) during binocular acuity whereas accommodation was least accurate (and image quality worst) while watching a movie. When viewing distance was reduced, accommodative lag increased and image quality (as quantified by VSMTF) declined for all tasks in both refractive groups. For any given viewing distance, computed image quality was consistently worse in myopes than in emmetropes, more so for the acuity than for reading/movie watching. Although myopes showed greater lags and worse image quality for the acuity experiments compared to emmetropes, acuity was not measurably worse in myopes compared to emmetropes.
Conclusions
Retinal image quality present when performing a visually demanding task (e.g., during clinical examination) is likely to be greater than for less demanding tasks (e.g., reading/movie watching). Although reductions in image quality lead to reductions in acuity, the image quality metric VSMTF is not necessarily an absolute indicator of visual performance because myopes achieved slightly better acuity than emmetropes despite showing greater lags and worse image quality. Reduced visual contrast in myopes compared to emmetropes is consistent with theories of myopia progression that point to image contrast as an inhibitory signal for ocular growth.
Keywords: myopia, image quality, accommodation, aberrations
Myopia has shown dramatic increase in the prevalence rate in many regions of the world,1 suggesting a strong role of environmental factors. Several environmental factors have been attributed to the development and progression of myopia. One of these hypotheses is related to accommodation. It has been suspected that myopic individuals have high levels of aberration2–5 and display larger accommodative lags6–9 than emmetropes. As a result, a markedly blurred optical image on the retina may lead to excessive axial growth in the eye, as well as myopia, due to either a moderate form deprivation or a hyperopically defocused retinal image. This hypothesis is largely based on evidence from animal models that suggest that the eye grows in a direction that eliminates blur.10,11 The animal model of lens-induced myopia suggests that the hyperopic blur induced by accommodative lags reduces image contrast, which signals the eyes to elongate axially to reduce the degree of hyperopic blur. According to Wallman and Winawer10 and Smith et al.,11 eyes have an intrinsic propensity for growth and will continue to grow unless actively inhibited by retinal activity triggered by image contrast.
An implicit assumption in the foregoing account is that increased accommodative lag reduces image contrast on the retina, thereby releasing the inhibition of axial elongation. This assumption is supported by evidence that myopes show reduced accommodation under monocular viewing conditions,12,13 when viewing thorough full correction,14,15 and when accommodation is stimulated using minus lenses.7–9 However, the implication that accommodative lag necessarily reduces image quality is less certain.
Depending on how accommodation is measured, and how accommodative lag is defined, large artifacts can lead to misleading results.16–20 This lingering uncertainty suggests a need to measure image quality directly using wavefront aberrometry to determine whether retinal image is indeed reduced in myopic eyes when accommodating to a near target and whether that reduction is larger than for emmetropic eyes. Moreover, the visual task used to elicit accommodative responses may influence the magnitude of that response21 and, therefore, would temper our attempt to generalize laboratory results into the real world of daily living. Collins et al.5 showed worse image quality in progressing myopic adults than emmetropes before a 2-hour “reading task” performed at each individual’s habitual reading distance (mean in myopes = 35 cm and emmetropes = 41 cm). The use of different reading distances, as acknowledged by the authors,5 makes between-group comparisons difficult. For all the above reasons, we investigated the accuracy of accommodation and foveal image quality during accommodation for adult populations of emmetropic and myopic individuals to both visually demanding and naturalistic targets. Our results confirm that visual task plays a significant role in determining the degree of accommodative error, and the resulting image quality, in both populations.
METHODS
Participants
Participants were recruited from the Indiana University student population. Ten emmetropes and 11 myopes between the ages of 18 and 25 years (mean age, 22 years) participated in the experiment. The mean spherical equivalent was −3.00D (range, −1.25D to −6.50D) for the myopic group and 0.30D (range, +0.75 to 0.00D) for the emmetropic group. Selection criteria for the study included best-corrected visual acuity of 20/20 or better in both eyes, with one line or less of difference in visual acuity between the eyes, refractive astigmatism <1D in both eyes, anisometropia <1D, and absence of any ocular disease. None of the participants had a history of refractive or other ocular surgery. Before data collection, subjective measures of accommodative amplitudes were collected to ensure normal values for age (range, 8D to 12D; push-up technique). Binocularity was also confirmed in all participants using the animals/circles subtest of the Titmus stereo fly test.
The experiment adhered to the tenets of the Declaration of Helsinki and conformed to a protocol approved by the institutional research board at Indiana University. Informed consent was obtained from all participants after verbal and written explanation of the procedures involved in the study.
Apparatus
A complete ophthalmic analysis system (COAS, Wavefront Sciences, Inc.) was used to measure on-axis wavefront aberrations of the right eye based on Shack-Hartmann principle.22 The instrument’s measurement axis was aligned with the patient’s line of sight using a real-time display of S-H pupil image provided by COAS. Aberrations of the corrected eye were measured for an 840-nm radiation that was reflected into the eye by a beam splitter. The instrument’s internal telescopic relay lenses made the wavefront sensor optically conjugate to the spectacle plane. Thus, our measurement of wavefront error refer to the combination of the eye + corrective lens, which is appropriate for computing retinal image quality in object space for an eye viewing through a corrective lens. Since our goal was to measure wavefront error of the corrected eye, rather than the naked eye, no compensation for spectacle lens magnification of the eye’s entrance pupil was necessary.
The commercial aberrometer was incorporated into an open-field apparatus for presenting external visual targets positioned at various physical distances along the primary line of sight of the measured (right) eye. This arrangement also permitted the left eye to view the target for binocular viewing, allowing for a naturalistic measurement of accommodation. A diagram of the apparatus and additional technical details are given in a previous article.16
Wavefront aberrations at 840 nm were adjusted to 552 nm for monocular and binocular acuity tasks using quasi-monochromatic light (at 552 nm). For the binocular reading and binocular movie tasks, wavefront aberrations were adjusted to 565 nm that used white light. These adjustments were based on an optical model of ocular chromatic aberration23 and primarily affected the Zernike coefficient C20 for defocus but also had a minor effect on other aberration coefficients. No correction was made for possible focus shifts that can occur if IR radiation used for measurements is reflected from the fundus layers other than the entrance apertures of the cone photoreceptors.
Targets and Experimental Conditions
Wavefront aberration of the right eyes were measured for four experimental conditions, which included two acuity tasks (monocular and binocular acuity) that are visually demanding and two naturalistic tasks (binocular reading and binocular movie) encountered in everyday life.
All measurements were performed with natural pupils without administering any mydriatic or cycloplegic drug. Room lights were dimmed to maintain large pupil diameters. The experimenter manually aligned the subject’s pupil with the optical axis of the instrument using the S-H image of the pupil.
Targets for each experimental condition were mounted on an optical rail that allowed positioning at three viewing distances (100, 40, and 20 cm) along the primary line of sight of the right eye, so no rotation was required for the right eye. Target vergence (TV: −1D, −2.5D, and −5D) is the negative inverse of the viewing distance from the target to the spectacle plane. For every experimental condition and each TV, aberrations were measured three times from the right eye. The order of experimental conditions and target vergences followed a random sequence. For each target vergence in each condition, a burst of five aberrometry measurements was taken within 1.5 seconds. Thus, aberrations at each target vergence was an average of 15 measurements (3 repeats × 5 measures per repeat). To obtain valid aberrometry data with our COAS instrument, as evidenced by a circular pattern of spots captured by the wavefront sensor, fixation errors must be less than 2.5 degrees for a 3.5-mm pupil. Fixation errors of this magnitude have negligible effect on measurements of ocular wavefront error.24 Thus, to avoid data contamination by eye movements, the experimenter monitored the spot pattern captured by the wavefront aberrometer and measurements were taken only when a full circular pattern of spots was present.
For all tasks, subjects viewed the visual stimulus through trial lenses implementing their habitual corrective lenses. Since the largest viewing distance was 1 m, subjects always accommodated to some extent when tested, and all subjects achieved 20/20 or better visual acuity in each eye through the nominal habitual correction.
Acuity Task
The goal of this task was to measure aberrations of the accommodated eye near the endpoint of subject’s visual acuity when visual acuity is maximized. For this task, subjects viewed Landolt “C” letters presented on a microcomputer display (LiteEye by eMagin, Inc.), with a 15-μm pixel width (852 × 600 pixels). Individual pixels in the display subtended 0.25 arcmin at the closest viewing distance tested (20 cm). The stroke in a 0 logMAR (20/20) letter was represented by at least 4 pixels at all target vergences tested. A narrow-band interference filter (552 nm) placed immediately in front of the display made the visual stimuli appear black letters on a green background with luminance of 32.5 cd/m2. Acuity was measured with the Freiburg forced choice staircase procedure25 using letters with four possible orientations. An experimenter monitored progress and captured aberrometry measurements of the corrected eye when the staircase was near the endpoint of visual acuity. The acuity task was performed under binocular and monocular fixation conditions, although aberrations were only measured for the right eye for both conditions. For monocular fixation, the left eye was occluded using an eye patch.
Binocular Reading and Binocular Movie Task
These naturalistic tasks were performed only under binocular viewing conditions with a goal of comparing image quality to that measured during the binocular acuity condition. Targets for the naturalistic tasks were displayed on a fourth-generation iPod touch (Apple Inc.) with “retina” display (resolution, 960 × 450 pixels). For the binocular reading task, the targets were high-contrast (>80%) words with black letters on a white background (204 cd/m2). Each letter subtended a constant angular subtense of 42 minutes of arc (20/165 equivalent) at all viewing distances. The task required subjects to read a story presented one word at a time at a fixed target distance, with each word appearing in the center of the iPod display to ensure that the center of the word coincided with the optical axis of the aberrometer. To encourage the subject’s close attention, some words were deliberately misspelled, and subjects were instructed to raise their thumb when spelling errors were detected. All subjects reported achieving clear vision of the words for all target vergences tested.
The “binocular movie” task simply required the subjects to passively watch a commercial black and white movie presented on the iPod display. Over a 30-second period, the experimenter positioned the display along the optical rail at the same target distances used in the other tasks. The movie was not scaled for each target distance and therefore had a larger angular subtense when viewed at a closer distance.
Wavefront Calculation of Refractive State, Accommodative Error, and Retinal Image Quality
In this report, we define refractive state as the target vergence that optimizes retinal image quality.26,27 For each state of accom modation elicited by the visual target, functional accommodative error of the corrected eye was computed as the difference between the optimum target vergence and the actual target vergence. The visual Strehl ratio was used to quantify image quality using the metric VSMTF,28 which is a normalized metric equal to the volume under the neurally weighted modulation transfer function (MTF) divided by the corresponding volume for the perfect optical system free of all aberrations. This metric is a neurally weighted measure of visual contrast known to yield unbiased measures of refractive state.29 However, when pupil diameter varies during the experiment, VSMTF becomes difficult to interpret because the normalization also varies with pupil size. Thus, this definition was modified by normalizing the ocular MTF volume for the full pupil by the MTF volume for an optically perfect eye with a fixed pupil diameter of 3 mm.16(The choice of 3 mm is not critical since neural weighting of the MTF to compute VSMTF counteracts the increased optical bandwidth achieved by perfect optics and larger pupil.) This modified metric (designated VSMTF*) facilitates comparison of image quality despite changes in pupil size. The maximum possible value of VSMTF* for an eye with a 3 mm pupil is 1, which occurs for an optically perfect eye. To enhance comparisons over a large dynamic range, the base10 logarithm of VSMTF* is reported as logVSMTF*. To aid comparisons between visual tasks, we used monochromatic analysis for polychromatic as well as monochromatic stimuli.
RESULTS
Accuracy of Accommodation
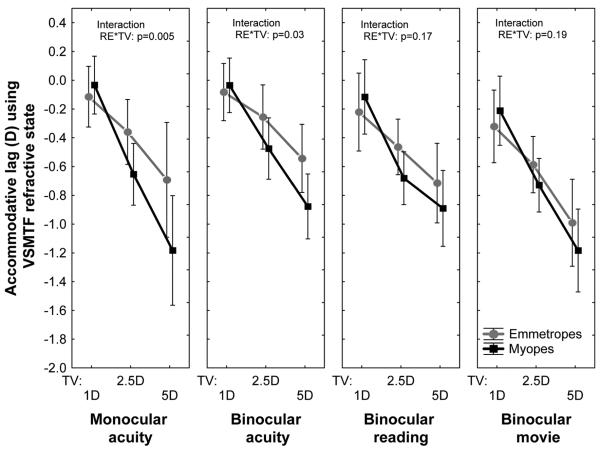
The primary aim of our study was to compare the quality of the retinal image during accommodation in myopic and emmetropic eyes. The first step toward this goal was to measure the accuracy of accommodation under four different viewing conditions: while performing a monocular acuity task or a binocular acuity task, when reading large text, and when viewing a movie. The mean lag of accommodation for each of these viewing conditions is shown as a function of target vergence in Fig. 1 for the two refractive groups. Both groups showed increased accommodative lags with closer target vergences (p < 0.001) for all four viewing conditions. Accommodative lag varied significantly between tasks in emmetropes (p < 0.001) and approached significance in myopes (p = 0.08). On average, accommodation was most accurate during the binocular acuity task and least accurate when viewing a movie (p < 0.001). More accommodative lag was measured in myopic eyes than in emmetropic eyes during the acuity experiment (Fig. 1) but not when reading the story or watching a movie.
FIGURE 1.
Population mean (± confidence interval) lag of accommodation for each target vergence in the four test conditions.
Image Quality during Accommodation
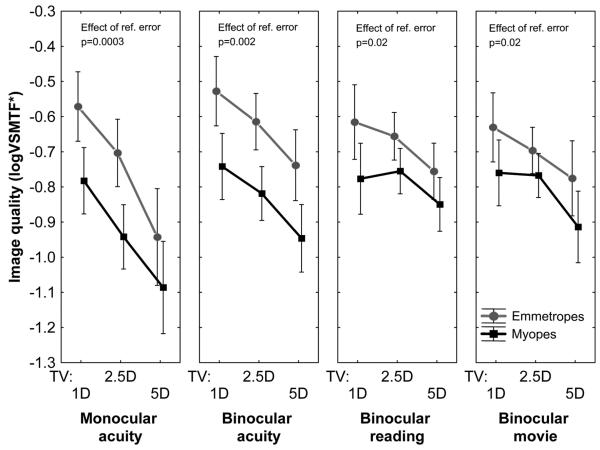
In an aberration-free eye, inaccurate accommodation will cause retinal image quality to suffer. For an aberrated eye, however, defocus caused by inaccurate accommodation interacts with the higher-order aberrations when producing the retinal image. As a result, the retinal image can be improved or degraded as a result of defocus depending on the magnitude and sign of the higher-order aberrations. To quantify the combined effects of all aberrations (including defocus due to accommodative lag), we computed the image quality metric VSMTF* as a function of target vergence in both refractive groups during the four visual tasks. As shown in Fig. 2, image quality was consistently worse in myopic eyes compared to emmetropic eyes for all target vergences and all visual tasks. This difference between refractive groups was greater for the binocular acuity task and for the monocular acuity task than for the reading or movie task. This result is consistent with the observations noted above that more accommodative lag was present in myopic eyes than in emmetropic eyes during the acuity experiment but not when reading the story or watching a movie.
FIGURE 2.
Mean (± confidence interval) image quality (VSMTF*) for each target vergence in the four test conditions.
Effect of Accommodative Error and Reduced Image Quality on Visual Acuity
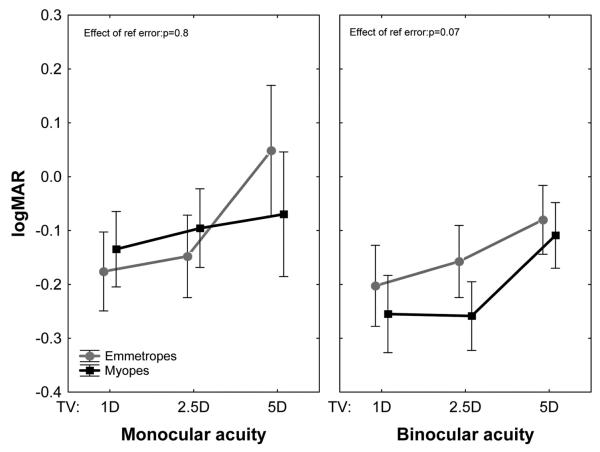
Loss of retinal image quality (Fig. 2) caused by inaccurate accommodation (Fig. 1) should cause visual acuity to suffer. This prediction was confirmed by measurements of binocular and monocular visual acuity, both of which declined as the target approached the eye as shown in Fig. 3. Mean binocular acuity was slightly worse for emmetropes than for myopes, but the rate of change in logMAR with TV was the same for both populations.
FIGURE 3.
Mean visual acuity changes for each target vergence in the binocular and monocular acuity tasks.
A causal link between accommodative error, loss of retinal image quality, and loss of acuity is illustrated graphically in Fig. 4 for binocular viewing. The format of this four-quadrant graph is designed to reveal the association between two variables (logMAR and VSMTF), both of which are functions of a third variable (accommodative error). To accomplish this goal, the variation of VSMTF with accommodative error is plotted in quadrant Q4 and the variation of logMAR with accommodative error is plotted in quadrant Q2. Quadrant Q3 is occupied by a 1:1 line that enables the graphical elimination of accommodative error to reveal the association between logMAR and VSMTF* in quadrant Q1. Thus, each data point in Q4 has a corresponding point in Q1 and Q2. In constructing Fig. 4, every subject contributed three points (one for each TV) to each quadrant plot.
FIGURE 4.
Summary of the effect of accommodative error on retinal image quality and visual acuity during binocular viewing in both study groups. Each symbol in each quadrant represents data for one target vergence for one participant. Open symbols indicate emmetropic eyes; closed symbols indicate myopic eyes. Target vergence is indicated by different symbol types (circle, TV = −5D; triangle, TV = −2.5D; square, TV = −1D). Positive accommodative error indicates accommodative lead; negative error indicates lag. The maximum possible value of logVSMTF* is 0, indicating an optically perfect eye. Larger negative values of logVSMTF* indicate worse image quality. The correspondence between the 4 quadrants is shown for one point by the dashed rectangle.
The scatterplot in Q4 shows that increased accommodative lag (indicated by a more negative dioptric value of accommodative error) reduces image quality (indicated by the more negative value of logVSMTF*). This relationship appears monotonic and approximately linear. As image quality declines, visual acuity declines (logMAR is more positive) as shown in Q1. Thus, acuity suffers when lag increases as shown in Q2. Although there is more scatter evident in Q1 than in Q2, possibly due to the interaction of higher-order aberrations with defocus due to accommodative lag, the relationship between logVSMTF and logMAR becomes clearer when the two refractive populations are considered separately. Emmetropic subjects tended to have worse acuity than myopic subjects even when retinal image quality is the same. Some possible explanations for this result are considered in the Discussion section.
A similar graph in Fig. 5 shows the sequential link between accommodative error, loss of retinal image quality, and loss of acuity for monocular viewing. The difference in acuity for the two refractive populations is less obvious in Q1 and Q2 for monocular viewing (Fig. 5) than for binocular viewing (Fig. 4).
FIGURE 5.
Summary of the effect of accommodative error on retinal image quality and visual acuity during monocular viewing in both study groups. Each symbol in each quadrant represents data for one target vergence for one participant. Symbol conventions are the same as for Fig. 4.
Effect of Task Type on Accommodative Lag and Image Quality
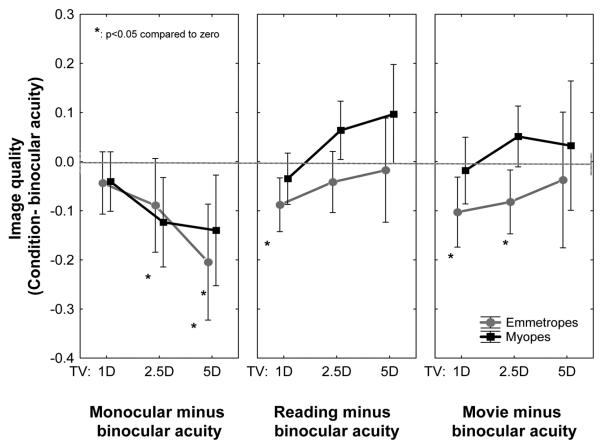
Binocular viewing of small letters while performing an acuity task is expected to elicit the most accurate accommodation and the best retinal image quality of the four test conditions studied. By comparison, monocular viewing lacks the additional drive to accommodate normally produced ocular convergence. Reading large text and watching a movie are less demanding visual tasks that do not necessarily require accurate accommodation and optimum image quality. To test these expectations experimentally, we measured accommodative error and computed image quality for all four visual tasks and the results are presented in Fig. 6 for accommodation accuracy and Fig. 7 for image quality. In these two figures, binocular viewing is the standard against which we compare the other three conditions.
FIGURE 6.
Mean (± confidence interval) accommodative error in monocular acuity, reading, and movie watching conditions compared against binocular viewing. More negative values indicate greater accommodative lag compared to binocular condition.
FIGURE 7.
Mean (± confidence interval) image quality in the monocular acuity, reading, and movie watching tasks compared against binocular viewing. More negative values indicate worse image quality compared to binocular acuity.
As anticipated, more accommodative lag was measured for the monocular acuity experiment and the two naturalistic tasks of reading and movie viewing as indicated by the negative values of the relative refractive state in Fig. 6. This was true for both refractive groups. Monocular viewing produced the least amount of additional lag compared to binocular viewing, and movie viewing elicited the greatest additional lag in emmetropes. Although the population mean values were negative for every test condition in both groups, a considerable amount of between-subject variation was present, especially for the closest target location (TV = −5D).
The increased accommodative lag shown in Fig. 6 for emmetropic observers was reflected in a loss of image quality as shown in Fig. 7. The surprising result was that image quality was slightly better in myopic observers when reading and watching the movie despite having larger accommodative lag. Some possible explanations for this unanticipated result are considered in the Discussion section. Although the trends are clear, the increased variance in accommodative lag and retinal image quality for the nearest target prevented the mean results from reaching statistical significance (Fig. 7).
DISCUSSION
The goal of our study was to investigate the accuracy of accommodation and foveal image quality when adult myopes and emmetropes accommodate to various visual stimuli. These measurements are important in the context of current theories of myopia development because inaccuracies of accommodation are assumed to reduce retinal image quality, which releases the inhibition to axial elongation.10,11 While some studies show evidence for increased accommodative lags in myopes,7–9,12 few recent longitudinal studies show that accommodative lags are not associated with myopic progression.13,30 However, large spurious results can occur depending on how accommodation is measured and how accommodative lag is defined.17 If myopes do show large accommodative lags, it is uncertain if the interaction of these errors with higher-order aberrations indeed reduces image quality on the retina.
We calculated accommodative refractive state and retinal image quality directly from wavefront aberrations using an image quality metric (VSMTF) and showed that myopes exhibit greater accommodative errors than emmetropes, specifically for the visually demanding acuity tasks that require maximal performance. Accommodative lags of up to −1.75D (monocularly) and −1.5D (binocularly) occurred for the closest target vergence tested (circle symbols; Figs. 4 and 5). The general trend of higher accommodative lags producing reduced image quality, thereby reducing visual acuity, was observed under both binocular and monocular viewing conditions, similar to a previous study.16 Overall, myopes consistently displayed poorer image quality for all target vergences compared to emmetropes (e.g., Fig. 2). However, visual acuity was slightly better in myopes compared to emmetropes (Fig. 4) for a given level of retinal image quality. How might we explain why myopes have better acuity than emmetropes when image quality (which takes lag and other aberrations into account) is the same? This is the central puzzle we must solve to understand the causal link from increased lag to reduced IQ to reduced visual acuity.
One might argue that higher-order aberrations (including spherical aberration) are greater in myopes,4,5 but this argument fails to account for superior acuity compared to emmetropes when IQ is the same (hence, the effect of aberrations are the same) for both groups. Another unconvincing argument is that the retinal image will be physically larger (in mm2) on the myopic retina, but it is more important to note that the angular size of the retinal image will be the same. Moreover, the density of cone photoreceptors (number of cones per square degree) is constant for a simple model of an expanding globe, which makes an increase in acuity with axial elongation unlikely.31,32 More likely, acuity of the corrected myope should decline, not improve, due to spectacle magnification. So, this explanation also fails.
Perhaps the simplest explanation is that VSMTF* of the corrected eye is an imperfect (or inappropriate) measure of image quality that fails to capture functionally important differences between emmetropic and myopic eyes. Another possibility is that the myopic eyes have a functionally greater depth of field for the acuity task due to higher spherical aberration. Even though different eyes have different-sized pupils, we estimated the sign of C40 during accommodation and natural pupils for each subject. Table 1 shows that myopes consistently show greater percentage of positive spherical aberration for each target vergence. The literature also shows evidence for larger depth of focus seen in myopes using both subjective33 as well as objective techniques that used image quality metrics5,18 Charman34,35 proposed that greater levels of higher-order aberrations seen in myopes2,3,5,36 might increase depth of focus, which would in turn reduce the sensitivity to retinal image blur. The decreased sensitivity consequently allows the accommodative system to exert minimum necessary accommodation to bring the target into focus.
TABLE 1.
Percentage of participants with positive spherical aberration (C40) for each target vergence in binocular and monocular acuity tasks
| Binocular |
Monocular acuity |
|||||
|---|---|---|---|---|---|---|
| Target vergence | 1D | 2.5D | 5D | 1D | 2.5D | 5D |
| Emmetropes | 70% | 50% | 10% | 60% | 40% | 30% |
| Myopes | 92% | 75% | 25% | 83% | 75% | 25% |
A larger depth of focus may also result in greater tolerance to defocus induced blur on acuity. This is evident from studies that show better visual performance during lens-induced defocus in myopes compared to emmetropes.37–39 Radhakrishnan et al.39 showed higher visual acuity in the presence of minus lens–induced defocus in myopes (similar to hyperopic defocus due to accommodative lags) than in emmetropes but showed similar visual acuity for positive lens–induced defocus. On the other hand, Poulere et al.38 tested the effect of defocus (+2D) on monocular blur adaptation and visual acuity in emmetropes and myopes. The decrease in visual acuity through defocus was less in myopes than in emmetropes, suggesting higher blur tolerance. Blur adaptation was not significantly different in the study by Poulere et al.38 but other studies33 suggest greater blur adaptation in myopes. Further, many studies demonstrate that myopes show higher levels of experience-based blur compensation, probably due to the habitual adaptation to uncorrected levels of blur.40,41 Thus, our observation of good visual acuity, despite large accommodative lags in myopes, appears to be supported by these above studies and could be a result of several optical and neural factors.
An alternative possibility may be related to the dynamic nature of accommodation. It is possible that the subject put in a burst of accommodative effort just long enough to clear the image to perform the acuity task without our notice. There was a small time lapse (~1 to 2 seconds) between letter presentation and aberration measurement, so a better experimental design might be to present the letter only briefly, synchronized with aberration measurement, to avoid that possibility. Further, it is known that accommodation exhibits increased accommodative microfluctuations especially for closer target vergences.42–44 Recently, Plainis et al.20 showed that fluctuations during steady-state accommodation may preserve image quality, at least temporally even when accommodative errors are moderate. Since myopes show larger accommodative microfluctuations compared to emmetropes,45–47 this may also explain the good visual acuity observed in this group, despite large accommodative lags.
Refractive group differences between visual acuity were more apparent for the binocular acuity task than the monocular acuity task (Figs. 4 and 5). In this study, we only measured wavefront aberrations from the right eye, even under binocular viewing conditions. An obvious possibility is that the fellow eye had a clearer image that facilitated better visual acuity for binocular fixation. Thus, binocular visual acuity may have been better than would have been expected from monocular image quality, which is more evident for myopic eyes than for emmetropic eyes.
Effect of Task Type on Accommodation and IQ
Reading large text and watching a movie are less demanding visual tasks that do not necessarily require accurate accommodation and optimum image quality. For that reason, we hypothesized that visual task will significantly influence accommodative response and image quality, such that less demanding tasks will elicit greater accommodative error and subsequently poor retinal image quality. Our results confirmed in both refractive groups that the binocular movie task, which simply required participants to passively watch a movie, elicited the greatest accommodative lags compared to binocular acuity task that required maximal visual performance (Fig. 6). The differences might be attributed to the effect of cognitive demand on accommodation,48 with the acuity task requiring greater cognitive and visual demand and hence greater accommodative response compared to watching a movie. Another possibility may be related to differing spatial frequency characteristics of the stimuli, since the accuracy of accommodation response is known to depend on spatial frequency.21 Larger stimuli increase the depth of focus,49 which may also result in greater accommodative errors. Further, in the presence of aberrations, the optimal focus for high and low spatial frequency is different, contributing to greater differences in accommodative errors.50,51
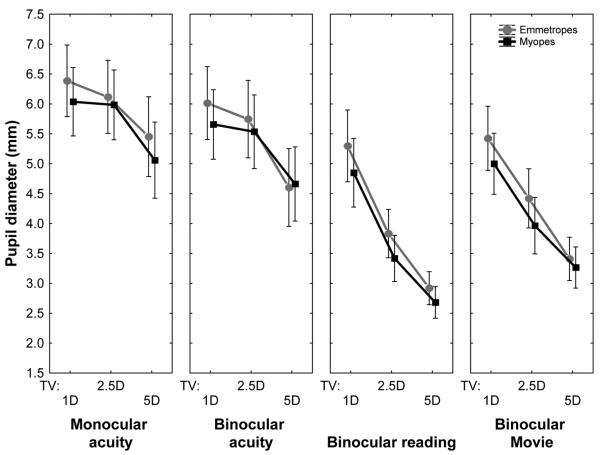
It is interesting to note that image quality for reading and movie targets did not show the monotonic decrease with closer target vergences as observed for the acuity task (Figs. 1 and 2, see also “Mono” in Figs. 6 and 7). What could explain the improvement in IQ for closest viewing target in these naturalistic tasks? One possible explanation is the pupillary diameter (Fig. 8). Pupil sizes were significantly smaller for the reading and movie tasks compared to the acuity tasks. This may be due to the large screen area and bright screen luminance of the iPod display (200 cd/m2) used for the naturalistic tasks compared to the microdisplay (32.5 cd/m2) used for the acuity experiments. Greater pupillary constriction may reduce optical blur caused by accommodative error and other higher-order aberrations, thus allowing for a better image quality.52 We did not attempt to match the screen luminance between conditions because we wanted to study accommodative response and image quality to everyday stimuli. Although we measured visual acuity with nearly monochromatic light (narrow-band interference filter, 552 nm) while the reading and movie tasks used polychromatic light, we used monochromatic analysis for polychromatic as well as monochromatic stimuli to aid comparisons.
FIGURE 8.
Mean (± confidence interval) pupil diameter for each target vergence in the four test conditions.
The increased accommodative lag (Fig. 6) for reading and movie tasks was reflected in a loss of image quality (Fig. 7) for emmetropic observers but not for myopes, despite having a larger accommodative lag. If myopes show smaller pupil size compared to emmetropes, then this may reduce monochromatic higher-order aberrations, resulting in better image quality compared to emmetropes. Fig. 8 indicates that this is not the case. Myopes and emmetropes show statistically similar pupil sizes for all conditions, thus refractive group differences cannot be explained by differences in pupil diameter. Complex interactions between aberrations and pupil size53 could have been different between myopes and emmetropes and may have resulted in the observed changes in image quality.
In summary, we find that accommodative lags increased and image quality declined with closer target vergence for all tasks in both refractive groups. Accommodative lag was associated with reduced retinal image quality, which was consistently worse in myopic eyes compared to emmetropic eyes for all target vergences and all visual tasks. This difference between refractive groups was greater for the binocular and monocular acuity tasks than for the reading or movie task. Despite these differences, myopes achieved better visual acuity than emmetropes for the same level of image quality, suggesting greater tolerance to retinal blur. The type of visual task significantly influenced accommodative response, such that accommodation was most accurate (and image quality was best) during the binocular acuity task, whereas accommodation was least accurate (and image quality worst, especially in emmetropes) while watching a movie.
On the basis of the results of our study, we conclude that the retinal image quality present when performing a visually demanding task (e.g., during clinical examination) may not be representative of image quality for less demanding tasks in everyday living. Although visual acuity is correlated with image quality, it is not necessarily an absolute indicator since myopes achieved slightly better visual acuity than emmetropes despite showing greater accommodative lags and worse image quality. The reduced image contrast in myopic eyes that we observed for all target vergences and all visual tasks is consistent with theories of myopia progression that point to image contrast as an inhibitory signal for ocular growth.10
ACKNOWLEDGMENTS
The authors wish to thank Dr. Arthur Bradley, Dr. Rowan Candy, and Dr. Ivan-Marin-Franch for useful discussions.
REFERENCES
- 1.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32:3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 2.He JC, Sun P, Held R, Thorn F, Sun X, Gwiazda JE. Wavefront aberrations in eyes of emmetropic and moderately myopic school children and young adults. Vision Res. 2002;42:1063–70. doi: 10.1016/s0042-6989(02)00035-4. [DOI] [PubMed] [Google Scholar]
- 3.Paquin MP, Hamam H, Simonet P. Objective measurement of optical aberrations in myopic eyes. Optom Vis Sci. 2002;79:285–91. doi: 10.1097/00006324-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Buehren T, Collins MJ, Carney LG. Near work induced wavefront aberrations in myopia. Vision Res. 2005;45:1297–312. doi: 10.1016/j.visres.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Collins MJ, Buehren T, Iskander DR. Retinal image quality, reading and myopia. Vision Res. 2006;46:196–215. doi: 10.1016/j.visres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 6.McBrien NA, Millodot M. The effect of refractive error on the accommodative response gradient. Ophthalmic Physiol Opt. 1986;6:145–9. [PubMed] [Google Scholar]
- 7.Abbott ML, Schmid KL, Strang NC. Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic Physiol Opt. 1998;18:13–20. [PubMed] [Google Scholar]
- 8.Allen PM, O’Leary DJ. Accommodation functions: co-dependency and relationship to refractive error. Vision Res. 2006;46:491–505. doi: 10.1016/j.visres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34:690–4. [PubMed] [Google Scholar]
- 10.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Smith EL, 3rd, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010;51:3864–73. doi: 10.1167/iovs.09-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Accommodative lag before and after the onset of myopia. The CLEERE Study Group. Invest Ophthalmol Vis Sci. 2006;47:837–46. doi: 10.1167/iovs.05-0888. [DOI] [PubMed] [Google Scholar]
- 13.Berntsen DA, Sinnott LT, Mutti DO, Zadnik K. Accommodative lag and juvenile-onset myopia progression in children wearing refractive correction. Vision Res. 2011;51:1039–46. doi: 10.1016/j.visres.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakatsuka C, Hasebe S, Nonaka F, Ohtsuki H. Accommodative lag under habitual seeing conditions: comparison between myopic and emmetropic children. Jpn J Ophthalmol. 2005;49:189–94. doi: 10.1007/s10384-004-0175-7. [DOI] [PubMed] [Google Scholar]
- 15.Berntsen DA, Mutti DO, Zadnik K. The effect of bifocal add on accommodative lag in myopic children with high accommodative lag. Invest Ophthalmol Vis Sci. 2010;51:6104–10. doi: 10.1167/iovs.09-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Gil N, Martin J, Liu T, Bradley A, Diaz-Munoz D, Thibos LN. Retinal image quality during accommodation. Ophthalmic Physiol Opt. 2013;33:497–507. doi: 10.1111/opo.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thibos LN, Bradley A, Lopez-Gil N. Modelling the impact of spherical aberration on accommodation. Ophthalmic Physiol Opt. 2013;33:482–96. doi: 10.1111/opo.12047. [DOI] [PubMed] [Google Scholar]
- 18.Tarrant J, Roorda A, Wildsoet CF. Determining the accommodative response from wavefront aberrations. J Vis. 2010;10:4. doi: 10.1167/10.5.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buehren T, Collins MJ. Accommodation stimulus-response function and retinal image quality. Vision Res. 2006;46:1633–45. doi: 10.1016/j.visres.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Plainis S, Ginis HS, Pallikaris A. The effect of ocular aberrations on steady-state errors of accommodative response. J Vis. 2005;5:466–77. doi: 10.1167/5.5.7. [DOI] [PubMed] [Google Scholar]
- 21.Charman WN, Tucker J. Dependence of accommodation response on the spatial frequency spectrum of the observed object. Vision Res. 1977;17:129–39. doi: 10.1016/0042-6989(77)90211-5. [DOI] [PubMed] [Google Scholar]
- 22.Thibos LN. Principles of Hartmann-Shack aberrometry. J Refract Surg. 2000;16:S563–5. doi: 10.3928/1081-597X-20000901-14. [DOI] [PubMed] [Google Scholar]
- 23.Nam J, Rubinstein J, Thibos L. Wavelength adjustment using an eye model from aberrometry data. J Opt Soc Am (A) 2010;27:1561–74. doi: 10.1364/JOSAA.27.001561. [DOI] [PubMed] [Google Scholar]
- 24.Cheng X, Himebaugh NL, Kollbaum PS, Thibos LN, Bradley A. Test-retest reliability of clinical Shack-Hartmann measurements. Invest Ophthalmol Vis Sci. 2004;45:351–60. doi: 10.1167/iovs.03-0265. [DOI] [PubMed] [Google Scholar]
- 25.Bach M. The Freiburg Visual Acuity test—automatic measurement of visual acuity. Optom Vis Sci. 1996;73:49–53. doi: 10.1097/00006324-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Gil N, Fernández-Sánchez V, Thibos L, Montȩs-Micó R. Objective amplitude of accommodation computed from optical quality metrics applied to wavefront outcomes. J Optom. 2009;2:223–34. [Google Scholar]
- 27.Cheng X, Bradley A, Thibos LN. Predicting subjective judgment of best focus with objective image quality metrics. J Vis. 2004;4:310–21. doi: 10.1167/4.4.7. [DOI] [PubMed] [Google Scholar]
- 28.Thibos LN. The optics of wavefront sensing. Ophthalmol Clin North Am. 2004;17:111–7. doi: 10.1016/j.ohc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Martin J, Vasudevan B, Himebaugh N, Bradley A, Thibos L. Unbiased estimation of refractive state of aberrated eyes. Vision Res. 2011;51:1932–40. doi: 10.1016/j.visres.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weizhong L, Zhikuan Y, Wen L, Xiang C, Jian G. A longitudinal study on the relationship between myopia development and near accommodation lag in myopic children. Ophthalmic Physiol Opt. 2008;28:57–61. doi: 10.1111/j.1475-1313.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 31.Strang NC, Winn B, Bradley A. The role of neural and optical factors in limiting visual resolution in myopia. Vision Res. 1998;38:1713–21. doi: 10.1016/s0042-6989(97)00303-9. [DOI] [PubMed] [Google Scholar]
- 32.Chui TY, Yap MK, Chan HH, Thibos LN. Retinal stretching limits peripheral visual acuity in myopia. Vision Res. 2005;45:593–605. doi: 10.1016/j.visres.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfield M, Abraham-Cohen JA. Blur sensitivity in myopes. Optom Vis Sci. 1999;76:303–7. doi: 10.1097/00006324-199905000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic Physiol Opt. 1999;19:126–33. doi: 10.1046/j.1475-1313.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- 35.Charman WN. Aberrations and myopia. Ophthalmic Physiol Opt. 2005;25:285–301. doi: 10.1111/j.1475-1313.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 36.Collins MJ, Wildsoet CF, Atchison DA. Monochromatic aberrations and myopia. Vision Res. 1995;35:1157–63. doi: 10.1016/0042-6989(94)00236-f. [DOI] [PubMed] [Google Scholar]
- 37.Thorn F, Cameron L, Arnel J, Thorn S. Myopia adults see through defocus better than emmetropes. In: Tokoro T, editor. Proceedings of the 6th International Conference on Myopia; Tokyo, Japan: Springer-Verlag; 1998. pp. 368–74. [Google Scholar]
- 38.Poulere E, Moschandreas J, Kontadakis GA, Pallikaris IG, Plainis S. Effect of blur and subsequent adaptation on visual acuity using letter and Landolt C charts: differences between emmetropes and myopes. Ophthalmic Physiol Opt. 2013;33:130–7. doi: 10.1111/opo.12020. [DOI] [PubMed] [Google Scholar]
- 39.Radhakrishnan H, Pardhan S, Calver RI, O’Leary DJ. Unequal reduction in visual acuity with positive and negative defocusing lenses in myopes. Optom Vis Sci. 2004;81:14–7. doi: 10.1097/00006324-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Pesudovs K, Brennan NA. Decreased uncorrected vision after a period of distance fixation with spectacle wear. Optom Vis Sci. 1993;70:528–31. doi: 10.1097/00006324-199307000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Cufflin MP, Mankowska A, Mallen EA. Effect of blur adaptation on blur sensitivity and discrimination in emmetropes and myopes. Invest Ophthalmol Vis Sci. 2007;48:2932–9. doi: 10.1167/iovs.06-0836. [DOI] [PubMed] [Google Scholar]
- 42.Denieul P. Effects of stimulus vergence on mean accommodation response, microfluctuations of accommodation and optical quality of the human eye. Vision Res. 1982;22:561–9. doi: 10.1016/0042-6989(82)90114-6. [DOI] [PubMed] [Google Scholar]
- 43.Charman WN, Heron G. Fluctuations in accommodation: a review. Ophthalmic Physiol Opt. 1988;8:153–64. doi: 10.1111/j.1475-1313.1988.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 44.Stark LR, Atchison DA. Pupil size, mean accommodation response and the fluctuations of accommodation. Ophthalmic Physiol Opt. 1997;17:316–23. [PubMed] [Google Scholar]
- 45.Harb E, Thorn F, Troilo D. Characteristics of accommodative behavior during sustained reading in emmetropes and myopes. Vision Res. 2006;46:2581–92. doi: 10.1016/j.visres.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Day M, Strang NC, Seidel D, Gray LS, Mallen EA. Refractive group differences in accommodation microfluctuations with changing accommodation stimulus. Ophthalmic Physiol Opt. 2006;26:88–96. doi: 10.1111/j.1475-1313.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 47.Seidel D, Gray LS, Heron G. Retinotopic accommodation responses in myopia. Invest Ophthalmol Vis Sci. 2003;44:1035–41. doi: 10.1167/iovs.02-0264. [DOI] [PubMed] [Google Scholar]
- 48.Kruger PB. The effect of cognitive demand on accommodation. Am J Optom Physiol Opt. 1980;57:440–5. doi: 10.1097/00006324-198007000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Atchison DA, Charman WN, Woods RL. Subjective depth-of-focus of the eye. Optom Vis Sci. 1997;74:511–20. doi: 10.1097/00006324-199707000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Xu R, Bradley A, Thibos LN. Impact of primary spherical aberration, spatial frequency and Stiles Crawford apodization on wavefront determined refractive error: a computational study. Ophthalmic Physiol Opt. 2013;33:444–55. doi: 10.1111/opo.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Gil N, Peixoto-de-Matos SC, Thibos LN, Gonzalez-Meijome JM. Shedding light on night myopia. J Vis. 2012;12:4. doi: 10.1167/12.5.4. [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Gil N, Iglesias I, Artal P. Retinal image quality in the human eye as a function of the accommodation. Vision Res. 1998;38:2897–907. doi: 10.1016/s0042-6989(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 53.van den Brink G. Measurements of the geometrical aberrations of the eye. Vis Res. 1962;2:233–44. [Google Scholar]