Summary
Binding of melanocortin peptide agonists to the melanocortin-1 receptor of melanocytes results in eumelanin production, whereas binding of the agouti signalling protein inverse agonist results in pheomelanin synthesis. Recently, a novel melanocortin-1 receptor ligand was reported. A β-defensin gene mutation was found to beresponsible for black coat colour in domestic dogs. Notably, the human equivalent, β-defensin 3, was found to bind with high affinity to the melanocortin-1 receptor; however, the action of β-defensin as an agonist or antagonist was unknown. Here, we use in vitro assays to show that β-defensin 3 is able to act as a weak partial agonist for cAMP signalling in human embryonic kidney (HEK) cells expressing human melanocortin-1receptor. β-defensin 3 is also able to activate MAPK signalling in HEK cells stably expressing either wild type or variant melanocortin-1 receptors. We suggest that β-defensin 3 may be a novel melanocortin-1 receptor agonist involved in regulating melanocyte responses in humans.
Keywords: cAMP, HBD3, MAPK, MC1R, NDP-MSH, pigmentation
Melanin synthesis is regulated by the melanocortin-1 receptor (MC1R), which is expressed on melanocytesfound in the skin and hair follicles. Eumelanin synthesis is stimulated by binding of alpha-melanocyte stimulating hormone (α-MSH) to MC1R, while binding of the inverse agonist agouti signalling protein (ASIP) results in pheomelanin synthesis [reviewed in (Beaumont et al., 2009,2011)]. Recently, one of the β-defensin peptides, known for their antimicrobial activity (Chen et al., 2006), was found to be a novel ligand for MC1R. A mutation in the canine CBD103 gene, which codes for β-defensin 103, was found to cause black coat colour in domestic dogs and the grey wolf (Anderson et al., 2009; Candille et al., 2007). CBD103 was able to bind to dog and mouse Mc1r, and the CBD103 black coat colour mutation was found to increase affinity for Mc1r (Candille et al., 2007). However, CBD103 was not able to increase cAMP levels in mouse melanocytes, suggesting that CBD103 may be able to activate cAMP-independent signalling pathways. Candille and co-workers suggested that CBD103 found at high concentrations in dog skin may prevent ASIP binding – and in the absence of melanocortin peptide agonists, CBD103 may raise ‘basal’ levels of MC1R signalling, thus explaining the fact that mutations in the melanocortin peptide precursor POMC do not always have large effects on pigmentation (Clementet al., 2008). Although high affinity binding of the human ortholog human β-defensin 3 (HBD3 – also known as DEFB103A) to MC1R was also demonstrated (Candille et al., 2007), whether HBD3 is relevant in human pigmentation is yet to be investigated, as is β-defensin action as a potential agonist or antagonist for MC1R.
We wished to investigate the effect of HBD3 on MC1R-mediated cAMP signalling and MAPK pathways and compare this to the effects of the MC1R super agonist NDP-MSH or the inverse agonist ASIP-YY. ASIP-YY is the cysteine-rich, C-terminal, MC1R binding domain of ASIP (McNulty et al., 2005). To ensure that any effect we see is mediated by MC1R, we have utilized the heterologous expression system of human embryonic kidney (HEK293) cells stably expressing human MC1R or the vector control. We first investigated the effect of HBD3 alone on cAMP signalling in two different MC1R wild type expressing HEK293 cell clones. Candille and co-workers have shown that despite high affinity for the mouse Mc1r (Ki 15.1 nM), concentrations of the dog version CBD103 up to 1uM did not induce cAMP in mouse melan-a melanocytes (Candille et al., 2007). Surprisingly, 100 and 300 nM HBD3 did induce a cAMP increase over basal levels in our human system; however, this response was small in comparison with the positive control 1 nM NDP-MSH (Figure 1A and C). ASIP-YY has been reported to act as an MC1R inverse agonist (Hida et al., 2009). We did not see a substantial change in basal cAMP by administration of ASIP-YY. Despite the relatively small induction of cAMP, 300 nM HBD3 caused a significant increase compared to 100 nM ASIP-YY (Figure S1E and F). In contrast, no HBD3 induction of cAMP was seen in the vector alone control (Figure S1G), indicating an MC1R-specific response. B16 mouse melanoma cells did not show any cAMP induction at 100 nM HBD3 (Figure 1E), possibly due to differences between the human and mouse receptors, or higher concentrations of HBD3 may be required to see an effect, of note, the binding affinity of HBD3 to mouse Mc1r has not been tested.
Figure 1.
HBD3 acts as a partial agonist for melanocortin-1 receptor mediated (MC1R-mediated) cAMP signalling. Total intracellular cAMP was measured after 5–10 min stimulation with the indicated ligands. All values represent the stimulated levels over basal control (stimulated minus basal). Error bars indicate the mean ± SEM. Accumulation of cAMP per well in human embryonic kidney 293 (HEK293) cells stably expressing MC1R wild-type clone WTe (A) or clone WT#21 (C) stimulated with 1 nM NDP- melanocyte stimulating hormone MSH, 100 nM agouti signalling protein ASIP-YY, 100 nM HBD3 or 300 nM HBD3. n = 3–5 (A) or 3–4 (C) independent experiments. Accumulation of cAMP in WTe (B) or WT#21 (D) cells relative to1 nM NDP-MSH stimulation. Cells were stimulated with 1 nM NDP-MSH alone or in combination with 100 nM ASIP-YY, 100 nM HBD3 or 300 nM HBD3. n = 3 independent experiments. (E) Accumulation of cAMP per well in B16 cells stimulated with 1 nM NDP-MSH, 100 nM ASIP-YY or 100 nM HBD3. n = 2–3 independent experiments. (F) Accumulation of cAMP in B16 cells relative to 1 nM NDP-MSH stimulation alone. Cells were stimulated with 1 nM NDP-MSH alone or in combination with 100 nM ASIP-YY or 100 nM HBD3. n = 3–4 independent experiments.
HBD3 has been shown to bind to MC1R and displace NDP-MSH in ligand-binding assays (Candille et al., 2007). Here, we show that 100 or 300 nM concentrations of HBD3 are able to inhibit the level of 1 nM NDP-MSH-induced cAMP in two different MC1R-expressing HEK clones (Figure 1B and D), consistent with a role for HBD3 as a competitive antagonist. Initial experiments revealed that higher concentrations of NDP-MSH resulted in less inhibitory effect of HBD3 on cAMP activation (Figure S1I). However, ASIP-YY was a more potent antagonist in these experiments. ASIP-YY has been shown to have a slightly higher affinity for MC1R (Ki 0.95 nM) than HBD3 (13.8 nM Ki) (Candille et al., 2007); however, increased concentration of HBD3 did not significantly increase the level of inhibition. We used B16 cells to confirm that HBD3 acts as a competitive antagonist for NDP-MSH induction of cAMP in melanocytic cells (Figure 1F). In addition, we have also shown that HBD3 is able to act as a competitive antagonist for α-MSH induction of cAMP (Figure S1H).
In terms of MC1R-mediated cAMP signalling, relative to NDP-MSH, HBD3 appears to act as a weak agonist, and relative to ASIP-YY, HBD3 acts as a weaker competitive inhibitor to NDP-MSH. We, therefore, suggest that HBD3 is able to act as a partial agonist, with both agonistic and antagonistic effects on cAMP signalling.
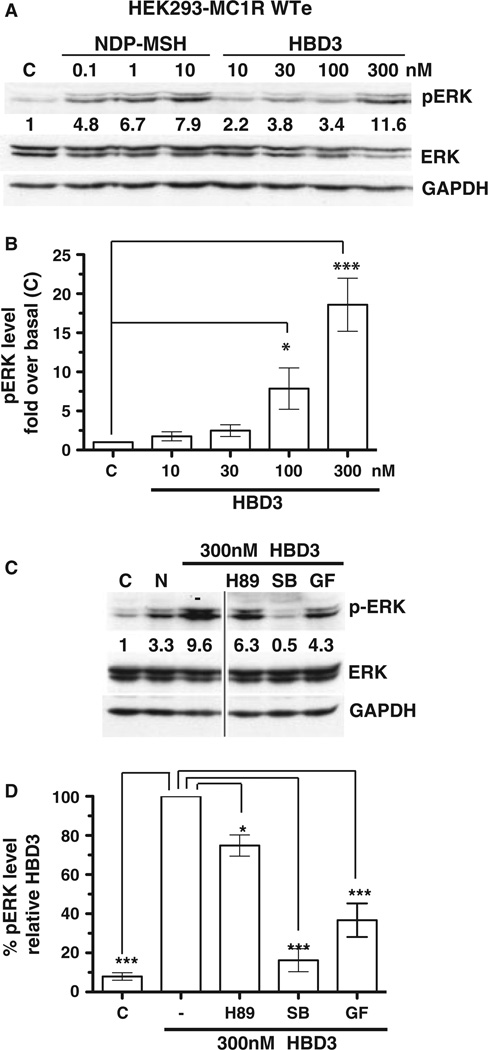
To determine the effect of HBD3 and NDP-MSH on MC1R-induced MAPK signalling, we assayed phosphorylation of ERK (pERK). α-MSH has previously been reported to induce MAPK signalling (Busca et al., 2000), and here we show a dose-dependant NDP-MSH-induced MAPK response in HEK293 cells expressing MC1R (Figure 2A). We also show a similar dose-dependant MAPK response to HBD3, with both 100 and 300 nM HBD3 inducing a significant increase in pERK over basal levels (Figure 2A and B). There was no induction observed in control cells (Figure S2B and E) indicating an MC1R-specific response. Interestingly, the kinetics of the HBD3 MAPK response was different to the NDP-MSH MAPK response. NDP-MSH induction of pERK was down-regulated after 5–10 min, confirming results from previous studies (Herraiz et al., 2011); however, HBD3 induction of pERK was sustained up to 1 hr after stimulation (Figure S2A). Stimulation of B16 cells with up to 300 nM HBD3 did not result in a pERK response (data not shown).
Figure 2.
HBD3 activates mediated melanocortin-1 receptor mediated (MC1R-mediated) MAPK signalling. Total cell lysates of human embryonic kidney 293 (HEK293) cells stably expressing WT MC1R (WTe) were analysed by western blot for the presence of phosphorylated ERK, or total ERK/GAPDH as a loading control. C = control unstimulated. (A) WTe cells were stimulated with increasing doses of NDP-MSH or HBD3 as indicated and numbers represent quantification of pERK band intensity normalized to GAPDH levels. (B) Quantification of pERK normalized to GAPDH levels and expressed as fold over basal (unstimulated) levels. Results are the mean ± SEM of n = 4–8 independent experiments. (C) WTe cells were stimulated with 300 nM HBD3 alone or in combination with the PKA inhibitor H89, p38 inhibitor SB203580(SB) or the PKC inhibitor GF109203X (GF). N = 10 nM NDP-MSH. Numbers represent quantification of pERK band intensity normalized to GAPDH levels. (D) Quantification of pERK normalized to GAPDH levels and expressed relative to the 300 nM HBD3 alone control. Results are the mean ± SEM of n = 3 independent experiments.
We wished to determine whether the HBD3 MAPK response was linked to cAMP signalling and PKA activation or mediated by other kinases implicated in non-canonical MC1R signalling pathways. Stimulation of MC1R-expressing HEK293 cells with HBD3 in the presence of different kinase inhibitors revealed that the MAPK activation was largely independent of PKA, although a small decrease in pERK levels in the presence of the PKA inhibitor occurred (Figure 2C and D). This was also the case for NDP-MSH activation of MAPK (Figure S2D), consistent with previous studies suggesting MSH-induced MAPK signalling occurs independent of cAMP (Herraiz et al., 2011), although in our system cKIT cannot be involved as HEK293 does not express cKIT. In addition, PKA-independent activation of ERK by cAMP has been shown previously (Busca et al., 2000), so we cannot formally rule out a role for cAMP in HBD3 activation of ERK. A PKC inhibitor did have a significant effect on HBD3 activation of pERK (Figure 2C and D), indicating this response is partially dependant on PKC. Most notably, the NDP-MSH and HBD3-MAPK response were almost completely abolished in the presence of a p38 inhibitor (Figure 2C, 2D and Figure S2D); furthermore, we demonstrate increased phosphorylation of p38 in HBD3-treated cells (Figure S2C). This suggests HBD3 activation of pERK in HEK cells occurs via p38. We have previously demonstrated p38 signalling occurs after MSH-induced MC1R activation in human melanocytes, with a synergistic effect of UVR (Newton et al., 2007), also PKC and p38 are involved in the NDP-MSH induction of the NR4A receptor family (Smith et al., 2008).
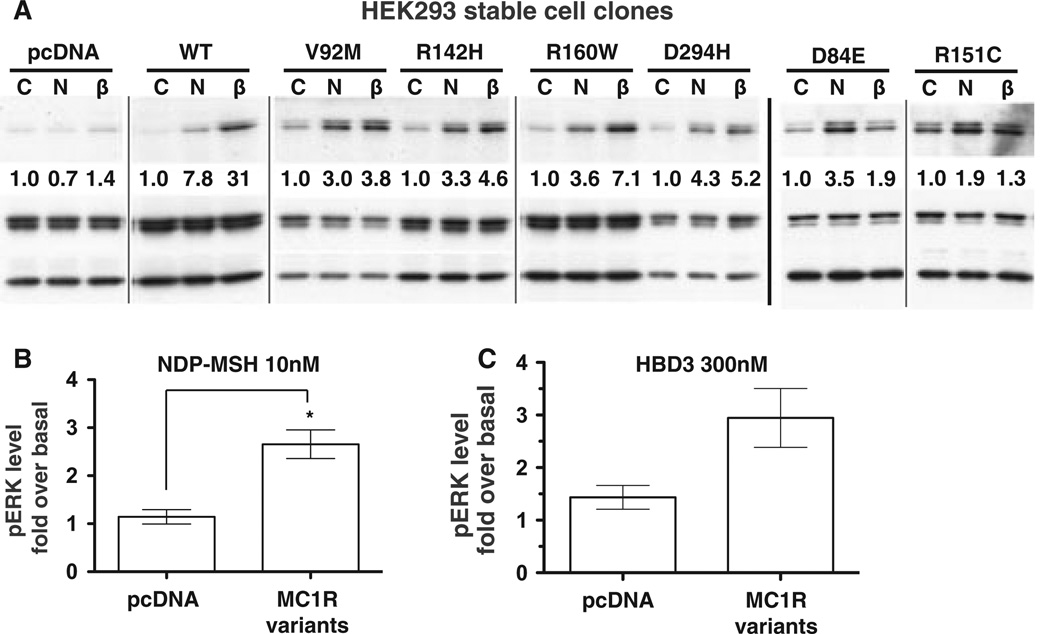
MC1R polymorphisms are linked to red hair and fair skin colour phenotypes (Beaumont et al., 2009). In our previous studies, MC1R variant receptors showed impaired cAMP responses to NDP-MSH because of impaired functional coupling or reduced cell surface expression (Beaumont et al., 2005, 2007). Despite this impaired cAMP signalling, it was recently found that variant receptors stimulated with NDP-MSH were able to activate MAPK as efficiently as wild-type MC1R (Herraizet al., 2009, 2011). To determine whether MC1R variants respond to NDP-MSH and HBD3 similar to the wild-type receptor, we utilized HEK293 cells stably expressing MC1R wild type or six different variant receptors. After stimulation with NDP-MSH, all variant receptors show an induction of pERK over the basal levels (Figure 3A and Figure S3A). However, this induction is lower than the MAPK response induced by the wild type MC1R–expressing cells, although variant receptor induction was more equivalent to a second wild-type MC1R stable clone expressing lower levels of MC1R protein (Figure S3C). With the exception of R151C and D84E, HBD3 stimulation produced a similar activation of ERK by variants (Figure 3A and Figure S3B). The level of pERK activation after NDP-MSH or HBD3 roughly correlated with the level of cAMP activation previously demonstrated after stimulation with NDP-MSH (Beaumontet al., 2007), suggesting variant receptors are impaired in both cAMP and MAPK signalling. However, the level of impairment for MAPK induction is not as severe as cAMP. This is not entirely consistent with the study of Herraiz and co-workers, possibly due to the use of a different cell type, we use heterologous expression in HEK293 cells, while the previous study used HBL human melanoma cells stably expressing MC1R variants (Herraiz et al., 2009, 2011). Signalling intermediates in HEK cells may differ to melanocytic cells, thus altering MC1R responses. Despite this difference compared to wild type MC1R–expressing cells, when NDP-MSH-mediated pERK activation from all variant receptor cell clones is combined, there is a significant increase compared to the levels of pERK in control cells (Figure 3B), with an average of 2.7-fold increase over basal levels. HBD3 induction of pERK was an average of threefold increase over basal levels for the combined variant receptor expressing cells (Figure 3C).
Figure 3.
NDP-MSH and HBD3 activate MAPK signalling in melanocortin-1 receptor (MC1R) variant receptor expressing cells. Total cell lysates of human embryonic kidney 293 (HEK293) cells stably expressing MC1R WT (WTe), MC1R variants or the pcDNA3.1 vector as indicated were analysed by western blot for the presence of phosphorylated ERK, or total ERK/GAPDH as a loading control. (A) Cells were stimulated for 5 min in the presence of the indicated ligands. C = control unstimulated cells, N = 10 nM NDP-MSH, β = 300 nM HBD3. Grey lines indicate cropped regions from bands in the same blot or different blots from the same experiment with the same exposure time. The black line indicates different blots from a second experiment. The blots as shown are representative of the level of activation that was achieved over 2–3 independent experiments as summarized in Figure S3A and B. (B) HEK cell stably expressing pcDNA3.1 vector or anyMC1R variant including D84E, V92M, R142H, R151C, R160W or D294H stimulated with (B) 10 nM NDP-MSH or (C) 300 nM HBD3 for 5 min. Quantification of pERK levels normalized to GAPDH loading control and expressed as fold over basal (unstimulated) levels. Results are the mean ± SEM, n ≥ 3 (B) or n ≥ 2 (C) independent experiments. A t-test was performed to find significance.
Given that the β-defensin peptide is important for MC1R regulation of pigmentation in dogs and mice (Candille et al., 2007), HBD3 is present at high levels in human skin (Harder et al., 2001), and human keratinocytes produce HBD3 in response to UVR (Glaser et al., 2009), we propose that HBD3 signalling, either via MC1R-activation of cAMP or MAPK, may be important in human pigmentation and responses to UVR. Future studies are needed to determine whether HBD3 is able to induce cAMP and/or MAPK signalling in human melanocytes.
Supplementary Material
Significance.
The melanocortin-1 receptor (MC1R) expressed by melanocytes is a key determinant of skin and hair colour phenotypes. The β-defensin 3 peptide was recently shown to be a novel MC1R ligand and regulator of pigment-type switching in both mice and dogs. Although there is as yet no demonstration of an equivalent role in human pigmentation, here we provide in vitro evidence for MC1R-dependent β-defensin 3 (HBD3) activation of both mitogen-activated protein kinase (MAPK) and cAMP signalling pathways. Given that HBD3 is present at high levels in human skin, we propose that HBD3 may be a hitherto overlooked regulator of human melanocyte responses.
Acknowledgements
We thank Amjad Yousuf for his contribution to the cAMP assays. This work was funded in part by grants from the NHMRC (APP1023884) and ARC (ARC-DP0771169) of Australia to RAS, and from the National Institutes of Health (NIH-R01DK064265) to GM. RAS is an NHMRC SRF (NHMRC-511029).
Footnotes
Supporting information
Additional Supporting information may be found in the online version of this article:
Figure S1. cAMP signalling in stably transfected HEK293, transiently transfected MM96L and B16 cells.
Figure S2. MAPK signalling in stably transfected HEK293 cells.
Figure S3. MAPK signalling in HEK293 cells stably expressing MC1R variants.
Appendix S1. Methods and supporting figure legends.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Anderson TM, Vonholdt BM, Candille SI, et al. Molecular and evolutionary history of melanism in North American gray wolves. Science. 2009;323:1339–1343. doi: 10.1126/science.1165448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont KA, Newton RA, Smit DJ, Leonard JH, Stow JL, Sturm RA. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum. Mol. Genet. 2005;14:2145–2154. doi: 10.1093/hmg/ddi219. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Shekar SL, Newton RA, James MR, Stow JL, Duffy DL, Sturm RA. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum. Mol. Genet. 2007;16:2249–2260. doi: 10.1093/hmg/ddm177. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Liu YY, Sturm RA. Chapter 4 The Melanocortin-1 Receptor Gene Polymorphism and Association with Human Skin Cancer. Prog. Mol. Biol. Trans. Sci. 2009;88C:85–153. doi: 10.1016/S1877-1173(09)88004-6. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Wong SS, Ainger SA, Liu YY, Patel MP, Millhauser GL, Smith JJ, Alewood PF, Leonard JH, Sturm RA. Melanocortin MC(1) receptor in human genetics and model systems. Eur. J. Pharmacol. 2011;660:103–110. doi: 10.1016/j.ejphar.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busca R, Abbe P, Mantoux F, Aberdam E, Peyssonnaux C, Eychene A, Ortonne JP, Ballotti R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000;19:2900–2910. doi: 10.1093/emboj/19.12.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candille SI, Kaelin CB, Cattanach BM, Yu B, Thompson DA, Nix MA, Kerns JA, Schmutz SM, Millhauser GL, Barsh GS. A -defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–1423. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xu Z, Peng L, Fang X, Yin X, Xu N, Cen P. Recent advances in the research and development of human defensins. Peptides. 2006;27:931–940. doi: 10.1016/j.peptides.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Clement K, Dubern B, Mencarelli M, Czernichow P, Ito S, Wakamatsu K, Barsh GS, Vaisse C, Leger J. Unexpected endocrine features and normal pigmentation in a young adult patient carrying a novel homozygous mutation in the POMC gene. J. Clin. Endocrinol. Metab. 2008;93:4955–4962. doi: 10.1210/jc.2008-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schroder JM, Schwarz A, Schwarz T. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J. Allergy Clin. Immunol. 2009;123:1117–1123. doi: 10.1016/j.jaci.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Herraiz C, Jimenez-Cervantes C, Zanna P, Garcia-Borron JC. Melanocortin 1 receptor mutations impact differentially on signalling to the cAMP and the ERK mitogen-activated protein kinase pathways. FEBS Lett. 2009;583:3269–3274. doi: 10.1016/j.febslet.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Herraiz C, Journe F, Abdel-Malek Z, Ghanem G, Jimenez-Cervantes C, Garcia-Borron JC. Signaling from the human melanocortin 1 receptor to ERK1 and ERK2 mitogen-activated protein kinases involves transactivation of cKIT. Mol. Endocrinol. 2011;25:138–156. doi: 10.1210/me.2010-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida T, Wakamatsu K, Sviderskaya EV, et al. Agouti protein, mahogunin, and attractin in pheomelanogenesis and melanoblast-like alteration of melanocytes: a cAMP-independent pathway. Pigment Cell Melanoma Res. 2009;22:623–634. doi: 10.1111/j.1755-148X.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty JC, Jackson PJ, Thompson DA, Chai B, Gantz I, Barsh GS, Dawson PE, Millhauser GL. Structures of the agouti signaling protein. J. Mol. Biol. 2005;346:1059–1070. doi: 10.1016/j.jmb.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Newton RA, Roberts DW, Leonard JH, Sturm RA. Human melanocytes expressing MC1R variant alleles show impaired activation of multiple signaling pathways. Peptides. 2007;28:2387–2396. doi: 10.1016/j.peptides.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Smith AG, Luk N, Newton RA, Roberts DW, Sturm RA, Muscat GE. Melanocortin-1 receptor signaling markedly induces the expression of the NR4A nuclear receptor subgroup in melanocytic cells. J. Biol. Chem. 2008;283:12564–12570. doi: 10.1074/jbc.M800480200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.