Abstract
Although albuminuria has been used as a prognostic biomarker for progression of diabetic nephropathy, it fails in terms of both sensitivity and specificity. Hence, there is a need for better urinary or plasma biomarkers that can predict which diabetic patients are at highest risk for progression of their disease. In this issue, Bhensdadia, et al. report proteomic investigations that identified urinary haptoglobin as potential prognostic biomarker for progressive diabetic nephropathy. Although as a single marker urinary haptoglobin adds little to albuminuria, together the two markers appear to provide better diagnostic accuracy than albuminuria alone.
Despite some advances in care over the past 20 years, diabetic nephropathy remains the single largest cause of chronic kidney disease and end-stage renal disease (ESRD) in the United States with no clear evidence of abatement. The 2012 United States Renal Data Systems has recently reported that the numbers of patients with ESRD resulting from diabetic nephropathy accounted for almost 45% of all incident ESRD patients in the United States in 2010(1), the highest share of this population ever. Since, fortunately, nephropathy occurs in a minority of either type 1 or type 2 diabetic patients screening for the presence of diabetic kidney disease routinely has been performed by testing for microalbuminuria (spot urine albumin/creatinine ratio of 30–299 µg/mg). In both type 1 and type 2 diabetic patients it is rare to develop progressive loss of renal function without first developing microalbuminuria (2, 3) and some degree of diabetic glomerulopathy is found in virtually all type 1 and most type 2 diabetic patients with microalbuminuria (3). However, as a progression indicator or biomarker, albuminuria is much less helpful. While substantial levels of albuminuria (albumin/creatinine ratio >300 µg/mg) are associated with a higher risk of progression, many patients have progressive disease without increases in albuminuria and even in the presence of normalbuminuria (2). Thus, albuminuria fails as a definitive prognostic biomarker for progressive disease and we are left with uncertainty about prognosis as well as optimal treatment for any patient with diabetes, microalbuminuria and relatively normal kidney function.
Given albuminuria’s fall from grace as the definitive prognostic biomarker for progressive diabetic nephropathy a number of investigators have sought to find other more accurate biomarkers that reliably predict which patients will develop progressive nephropathy and decline in kidney function. Advances in high-throughput analytical mass spectrometry (MS) coupled with improved chromatography have enabled MS–based proteomics as a major discovery tool to aid biomarker discovery and validation. There has been intense interest as investigators have focused on urinary and plasma proteome patterns from healthy subjects and type 1 and type 2 diabetic patients with different degrees of albuminuria and ranges of renal function. Several recent studies using MS-based platforms highlight the power of this approach. Otu and colleagues were able to identify type 2 diabetic Pima Indians who developed diabetic nephropathy within a 10 year follow up period based on a 12–peak urine peptide signature (4). Similarly, Rossing and co–workers identified a comprehensive urinary polypeptide signature that corresponded with very high sensitivity and specificity to the presence of diabetic nephropathy (5). More recently they developed a chronic kidney disease classifier that organized data from 273 urinary peptides that appeared to predict development of progressive nephropathy in quite small cohorts of both type 1 and type 2 patients somewhat better than did the urinary albumin excretion rate (6). Other studies have also confirmed urinary peptides, such as collagen fragments, as predictors of diabetic nephropathy and its progression. While promising, one of the major limitations of these studies is that the majority of the identified urinary peptide fragments arise from abundant plasma proteins and may simply serve as relatively expensive indicators of non-selective proteinuria. Importantly, with the single exception noted above, none of these peptides or panels of peptides have been rigorously evaluated against albumin excretion rates for added prognostic value.
In this issue of Kidney International, Bhensdadia, et al. report a series of proteomic investigations designed to identify prognostic biomarkers for identification of type 2 diabetic patients likely to have progressive nephropathy (7). Starting with urine samples collected as part of a sub-study of the VA Diabetes Trial, the investigators performed a case-control mass spectrometry discovery trial to identify urinary proteins that were differentially excreted in baseline samples from diabetic subjects who went on to develop progressive disease compared to subjects who maintained stable kidney function. From this discovery phase they detected two candidate markers that were found at higher levels in progressors’ urine. These two markers and a few other candidates were taken forward into a small verification trial and haptoglobin was found to be the only marker that discriminated between progressors and non-progressors.
To their credit, the investigators used an additional verification step in over 200 subjects to compare the haptoglobin/creatinine ratio to albumin/creatinine ratio in the ability to predict progressive early decline in kidney function. Here the results were somewhat disappointing as the haptoglobin/creatinine ratio was not significantly superior to the albumin/creatinine ratio in predicting kidney functional decline though there was some modest enhancement in discrimination when both tests were used together. While these data do not support institution of “haptoglobinuria” as a new, improved biomarker for progression in diabetic nephropathy, if these results are validated in another independent large cohort the haptoglobin/creatinine ratio may add some discriminatory power to albuminuria which remains the current, if clearly inadequate, standard prognostic indicator for type 2 diabetic patients with early nephropathy.
A major merit of this study is the careful and sequential analysis from state-of-the art discovery experiments, to initial validation, and finally to comparison to current best practices. An important strength of this work is the utilization of selected reaction monitoring to more accurately quantify peptides derived from proteins of interest as the shotgun discovery work is not reproducibly quantitative. This analytic scheme can well serve the diabetic nephropathy research community as a model for future biomarker investigations. We would recommend that all future biomarker analyses for diabetic nephropathy adhere to a similarly robust verification and quantification schema and precisely determine whether and to what extent the purported new biomarker adds to albuminuria as a prognostic indicator.
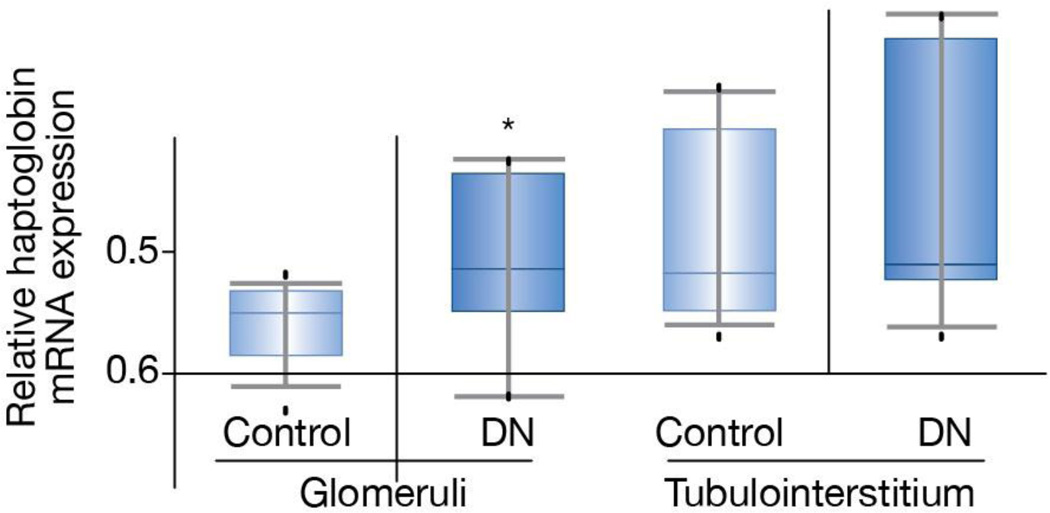
An interesting question that arises from this study is why haptoglobin might be a marker of progressive diabetic kidney disease. Although the authors used an untargeted screening method there is some evidence for haptoglobin as a participant in renal injury responses. For example, haptoglobin genotypes have been shown to be related to progressive kidney disease in diabetes with an approximately two-fold increased risk found for decline in GFR in type 1 diabetic patients with the Hp 2/2 compared with the Hp 1/1 genotype (8). Haptoglobin serves to prevent hemoglobin-induced oxidative tissue damage and there is evidence that the Hp 2/2 genotype encodes a haptoglobin molecule that is less effective in this capacity resulting in increased redox active iron and increased oxidative tissue damage (9). However, there has been no suggestion that the Hp 2/2 genotype would be associated with increased urinary levels of the molecule. Another interesting possibility is that higher haptoglobin expression may be a marker of stress or injury in either in glomerular or tubular cells from patients with more severe diabetic nephropathy before the progression becomes clinically evident otherwise. Indeed, haptoglobin gene and protein expression is markedly increased in several animal models as well as in patients with acute kidney injury (10). In analysis of published genome-wide expression data through the online reference database, Nephromine, there does appear to be some increase in haptoglobin mRNA expression in glomeruli and to a lesser extent in the tubulointerstitium of patients with progressive diabetic kidney disease (Fig. 1). The human diabetic nephropathy database used for this analysis is from a recently published Affymetrix expression array study (11) used to identify differentially regulated transcripts in microdissected human kidney samples, 22 of which were from subjects with diabetes. Diabetic nephropathy subjects were significant for their racial diversity and decreased glomerular filtration rate (~25–35 mL/min). The haptoglobin genotype is not known for these subjects.
Figure 1.
Haptolglobin mRNA levels in microdissected glomeruli and tubulointerstitium from subjects with diabetic nephropathy compared to those from living transplant donors. Haptoglobin levels were 2.4-fold higher in the diabetic nephropathy subjects than in controls (p < 0.04). Levels in the tubulointerstitium were not statistically different though there were several diabetic subjects with substantially higher haptoglobin levels than in the controls. Nephromine™ was used for analysis and visualization. Nephromine is an online database of all published and some unpublished genome-wide expression data from human kidney studies and is freely available to all academic investigators (www.nephromine.org; Compendia Bioscience, Ann Arbor, MI) and is supported by the NIH O’Brien Kidney Core Center at the University of Michigan).
In a double publication earlier this year, the Joslin Clinic group showed that serum levels of tumor necrosis factor (TNF) receptors 1 and 2 could serve as accurate biomarkers of progressive diabetic nephropathy in studies of type 1 and type 2 diabetic patients followed in their institution(12, 13). Importantly, TNF receptor levels were additive to those of albuminuria in predicting outcomes, at least in type 2 diabetic patients. Thus it appears that we are haltingly but inexorably moving to an era in which high risk patients can be identified more accurately and where such patients can serve as the best subjects for focused randomized controlled trials that assess new approaches to forestall progression of this most common cause of kidney failure.
Acknowledgements
The authors acknowledge support from grants from the National Institutes of Health (P30 DK081943, DP3 DK094292, and DK082841)
Footnotes
Disclosures: Neither author has relevant conflicts. Dr. Brosius is participating in a clinical trial with Lilly and Co. and has consulting agreements through the University of Michigan with Lilly and Co. and Merck, Sharp and Dohme Research Laboratories.
References
- 1.U.S. Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 2.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 3.Nosadini R, Velussi M, Brocco E, Bruseghin M, Abaterusso C, Saller A, Dalla Vestra M, Carraro A, Bortoloso E, Sambataro M, et al. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes. 2000;49:476–484. doi: 10.2337/diabetes.49.3.476. [DOI] [PubMed] [Google Scholar]
- 4.Otu HH, Can H, Spentzos D, Nelson RG, Hanson RL, Looker HC, Knowler WC, Monroy M, Libermann TA, Karumanchi SA, et al. Prediction of Diabetic Nephropathy Using Urine Proteomic Profiling 10 Years Prior to Development of Nephropathy. Diabetes Care. 2007;30:638–643. doi: 10.2337/dc06-1656. [DOI] [PubMed] [Google Scholar]
- 5.Rossing K, Mischak H, Dakna M, Zurbig P, Novak J, Julian BA, Good DM, Coon JJ, Tarnow L, Rossing P, et al. Urinary Proteomics in Diabetes and CKD. Journal of the American Society of Nephrology. 2008;19:1283–1290. doi: 10.1681/ASN.2007091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zurbig P, Jerums G, Hovind P, Macisaac RJ, Mischak H, Nielsen SE, Panagiotopoulos S, Persson F, Rossing P. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes. 2012;61:3304–3313. doi: 10.2337/db12-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhensdadia NM, Hunt KJ, Lopes-Virella MF, Tucker JM, Mataria MR, Alge JL, Neely BA, Janech MG, Arthur JM. Urine haptoglobin predicts early renal functional decline in patients with type 2 diabetes. Kidney Int. 2013 doi: 10.1038/ki.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costacou T, Ferrell RE, Ellis D, Orchard TJ. Haptoglobin genotype and renal function decline in type 1 diabetes. Diabetes. 2009;58:2904–2909. doi: 10.2337/db09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ Res. 2005;96:435–441. doi: 10.1161/01.RES.0000156653.05853.b9. [DOI] [PubMed] [Google Scholar]
- 10.Zager RA, Vijayan A, Johnson AC. Proximal tubule haptoglobin gene activation is an integral component of the acute kidney injury "stress response". Am J Physiol Renal Physiol. 2012;303:F139–F148. doi: 10.1152/ajprenal.00168.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60:2354–2369. doi: 10.2337/db10-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]